Abstract
Functional and ultrastructural investigations support the concept that altered brain connectivity, exhausted neural plasticity, and synaptic loss are the strongest correlates of cognitive decline in age-related neurodegenerative dementia of Alzheimer’s type. We have previously demonstrated that in transgenic mice, expressing amyloid-β precursor protein-Swedish mutation active caspase-3 accumulates in hippocampal postsynaptic compartments leading to altered postsynaptic density (PSD) composition, increased long-term depression (LTD), and dendritic spine loss. Furthermore, we found strong evidence that dendritic spine alteration is mediated by calcineurin activation, a calcium-dependent phosphatase involved in synapse signaling. In the present work, we analyzed the molecular mechanism linking alteration of synaptic plasticity to the increase of calcineurin activity. We found that acute treatment of young and plaque-free transgenic mice with the calcineurin inhibitor FK506 leads to a complete rescue of LTD and PSD composition. Our findings are in agreement with other results reporting that calcineurin inhibition improves memory function and restores dendritic spine density, confirming that calcineurin inhibition may be explored as a neuroprotective treatment to stop or slowdown synaptic alterations in Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical and experimental evidence (D’Amelio and Rossini 2012) supports that in the brain of patients suffering from Alzheimer’s disease (AD), amyloid-β (Aβ) and neurofibrillary tangles target synaptic terminals of functionally connected neuronal networks, affecting brain connectivity well before the neuronal death (D’Amelio et al. 2011, 2012; de Calignon et al. 2010, 2012). Changes in synapse structure and function contribute to neural system dysfunction causing cognitive deficits.
We have recently investigated (D’Amelio et al. 2011) the molecular mechanism causing early synaptic dysfunction in Tg2576 mice, a frequently used model of AD overexpressing the human amyloid-β precursor protein (AβPP) harboring the Swedish mutation (Hsiao et al. 1996). We demonstrated that an uncontrolled increase of caspase-3 activity in hippocampal synapses corresponds with the onset of alteration of postsynaptic density (PSD) composition, impairment of synaptic plasticity, and memory decline. These results support a previous study that found that caspase-3 is activated during physiological long-term depression (LTD) (Li et al. 2010). In the setting of Aβ-driven signaling cascade, a crucial mediator appears to be the caspase-3-dependent cleavage of calcineurin auto-inhibitory domain (D’Amelio et al. 2011; Mukerjee et al. 2000). The resulting increase of calcineurin activity causes dephosphorylation of AMPA receptor (AMPAR) GluA1 subunit on Ser845 triggering an increased LTD, expressed by long-lasting decrease in AMPAR function (D’Amelio et al. 2011; He et al. 2009; Esteban et al. 2003).
Recently, it has been reported that pharmacological inhibition of calcineurin reverses Aβ-dependent associative learning and memory impairment in the Tg2576 model of AD (Dineley et al. 2007, 2010) and improves Aβ-induced spine loss in a mouse model expressing AD-associated AβPP and presenilin 1, PS1 (Rozkalne et al. 2011).
Despite this array of data indicating calcineurin as a crucial molecular player in AD-related synaptic dysfunction, the molecular mechanism linking alteration of synaptic plasticity to calcineurin activity remains unexplored.
Here, we report that application of the calcineurin inhibitor FK506 to acute hippocampal slices from plaque-free memory-impaired Tg2576 mice leads to a complete rescue of the chemical form of LTD induced by a brief bath application of the group I glutamate metabotropic receptor (mGluR) agonist, (S)-3,5-dihydroxyphenylglycine (DHPG).
Furthermore, we find that in vivo administration of the calcineurin inhibitor results in the recovery of PSD composition, according to previous results demonstrating that the same treatment improves memory function (Dineley et al. 2007, 2010).
Since FK506, a commonly used immunosuppressant, might cause side effects in over 10 % of patients including hallucinations, aphasia, and memory impairment (Lee et al. 2008; Wijdicks et al. 1994), we also evaluated the effect of FK506 on age-matched non-transgenic control. Remarkably, we do not observe any significant changes in synaptic plasticity and PSD composition in control mice after pharmacological treatment.
Results
Calcineurin Inhibition Prevents DHPG-Induced LTD Impairment in Tg2576 Hippocampal Slices
Previous studies from our group (D’Amelio et al. 2011) and others (Dineley et al. 2007) have demonstrated that calcineurin activity is upregulated in the hippocampus of young and memory-impaired Tg2576 (TG) mice. Furthermore, we have found that the magnitude of LTD, induced with a low-frequency stimulation protocol, was significantly enhanced in young TG mice compared with age-matched wild-type (WT) control mice (D’Amelio et al. 2011).
We recorded field excitatory postsynaptic potentials (fEPSPs) from the stratum radiatum of the CA1 area upon stimulation of the Shaffer collaterals pathway every 30 s, at a test stimulation intensity attaining a half-maximal response. In agreement with our previous report indicating a similar efficacy of the basal synaptic transmission in WT and TG mice (D’Amelio et al. 2011), the test fEPSP amplitude in WT and TG slices was not significantly different (0.13 ± 0.01 mV; n = 15 and 0.12 ± 0.01 mV, n = 19, respectively; p > 0.05). However, a chemical form of LTD produced by 10 min perfusion of 50 μM DHPG (DHPG-LTD) was increased in TG mice when compared with WT controls (Fig. 1).
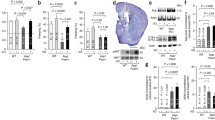
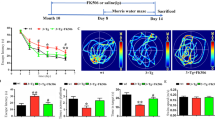
a Superimposed traces of the CA1 fEPSPs recorded immediately before DHPG (50 μM, 10 min) and at 60 min washout (smaller amplitude traces), in WT and TG slices in control (Ctrl) conditions (top traces) and pretreated for 20 min with 0.1 μM FK506 (bottom traces). b Histogram showing the degree of DHPG-LTD expressed as reduction (%) of fEPSP mean amplitude at 50–60 min from DHPG washout, in WT and TG slices in control conditions (Ctrl) and pretreated with FK506. DHPG-LTD of TG slices (n = 11 slices, N = 7 mice) in control conditions was significantly higher than that of both WT slices (n = 8, N = 5) in control conditions (*p < 0.05) and TG slices (n = 8, N = 4) pretreated with FK506 (**p < 0.01). No significant difference was observed between WT slices in control conditions and WT slices pretreated with FK506 (n = 7, N = 4). c Running plots of the normalized fEPSP mean amplitude (±s.e.m. displayed every 2.5 min) recorded from the CA1 area of wild-type (WT) and Tg2576 (TG) hippocampal slices exposed to 50 μM DHPG for 10 min, in control conditions (left panel) and following a 20 min pretreatment with 0.1 μM FK506 (right panel)
To test the hypothesis that pharmacological inhibition of calcineurin rescues synaptic plasticity impairment, we measured DHPG-LTD in acute TG hippocampal slices after 20 min of preincubation with calcineurin inhibitor FK506 (0.1 μM), or vehicle.
We found that the DHPG-LTD in FK506-treated TG hippocampal slices was significantly decreased with respect to vehicle-incubated TG slices (Fig. 1), restoring DHPG-LTD to the WT value. Interestingly, the degree of synaptic depression in WT slices was independent from the presence of FK506 (Fig. 1), indicating that calcineurin inhibition does not affect synaptic plasticity in physiological conditions. These data suggest that reducing calcineurin activity in TG neurons is sufficient to restore synaptic plasticity to WT levels.
Calcineurin Inhibition Prevents Calcineurin-Dependent reduction in pS845-GluA1 Levels in TG Hippocampal Slices and Blocks GluA1 Internalization
DHPG-LTD is similar to homosynaptic LTD induced by low-frequency stimulation, and importantly, this stimulation method induces selective dephosphorylation of tyrosine residues of GluA2 AMPAR subunit, but not GluA1 Ser845, through activation of tyrosine phosphatases (Gladding et al. 2009; Moult et al. 2006).
Since we have reported an increase of DHPG-LTD in TG hippocampal slices (Fig. 1) and we have demonstrated that GluA1 pSer845 levels were reduced in TG hippocampus at the onset of memory decline (D’Amelio et al. 2011), we asked whether the mechanism by which inhibition of calcineurin restores LTD expression involves preservation of GluA1 phosphorylation at Ser845. Immunoblot analysis of total homogenate of TG slices treated with 0.1 μM FK506 for 20 min showed an increase of GluA1pSer845/total GluA1 ratio, as compared with the vehicle-treated controls. By contrast, calcineurin inhibition had no effect on GluA1 phosphorylation at Ser831 and PSD-95 expression levels (Fig. 2a).
a Left panel representative immunoblots of protein extracted from WT and TG hippocampal slices after incubation with vehicle (Ctrl) or calcineurin inhibitor (FK506; 0.1 μM). Right panel graph showing GluA1pSer845/GluA1 ratio expressed as WT Ctrl percentage (mean ± s.d; n = 5 per group, *p < 0.05). b Surface level of GluA1 determined by slice biotinylation assay. Left panel representative immunoblots of biotinylated and total proteins from WT and TG hippocampal slices after incubation with vehicle (Ctrl) or calcineurin inhibitor (FK506; 0.1 μM). Absence of actin in surface samples indicates specificity of the biotinylation assay. Right panel: The relative ratio of surface to total GluA1 expression (WT Ctrl percentage). Data are expressed as mean ± S.D. (n = 5 per group. *p < 0.05)
It has been reported that phosphorylation of AMPAR GluA1 subunit on Ser845 increases channel open probability and promotes receptor recycling (Middei et al. 2013; He et al. 2009; Esteban et al. 2003). Thus, to verify whether GluA1 localization is involved in the rescuing effect of FK506 on DHPG-LTD, we monitored GluA1 subunit surface exposition by biotinylation assay. Whereas the surface levels of GluA1 subunit were reduced in TG slices compared with WT, following FK506 treatment, GluA1 subunit surface expression returned to control levels, without any change in NMDA receptor subunit GluN1 and in total protein level of GluA1 (Fig. 2b).
Collectively, these results prove that calcineurin inhibition prevents GluA1 dephosphorylation at Ser845 and blocks its internalization.
Calcineurin Inhibition In Vivo Rescues PSD Composition
Once demonstrated the capability of FK506 to rescue GluA1 phosphorylation at Ser845 and GluA1 surface expression in acute TG hippocampal slices, we analyzed the effect of in vivo calcineurin inhibition on PSD composition in WT and TG mice. Mice were treated with FK506 (10 mg/kg, i.p.) or saline solution, and hippocampal synaptic fractionation was performed 16 h after the injection.
Proteins were isolated from synaptic fractions and accurately quantified spectrophotometrically by Bradford method. PSD (TxP) and microsome (P3) fractions from all samples collected were blotted and probed with specific antibodies to check their purity (Supplementary Fig. S1a), and also separated by SDS-PAGE and stained with Coomassie Brilliant Blue to check pattern distribution and total amount of proteins (Supplementary Fig. S1b). The accuracy in the determination of amount of synaptic proteins is particularly relevant because the subcellular fractions are very dynamic, and for this reason, no synaptic marker might be used as adequate loading control.
As previously demonstrated (D’Amelio et al. 2011), the subcellular distribution of GluA1 was changed in saline-injected TG mice compared with saline-injected WT mice, with a decrease in the PSD-enriched fraction (TxP) and an increase in the microsome-enriched fraction (P3). Importantly, a single injection of FK506 restored GluA1 synaptic distribution in TG mice without any considerable effect on WT mice (Fig. 3a). Moreover, the analysis of synaptic markers in isolated hippocampal PSD revealed that in vivo treatment with FK506 was able to restore GluA1 phosphorylation at Ser845 in TG mice. By contrast, calcineurin inhibition had no effect on GluA1 phosphorylation at Ser831, and the relative amount of postsynaptic components analyzed remained unchanged in PSD preparation from both genotypes (Fig. 3b).
a Left panel representative immunoblots of PSD (TxP) and microsomal (P3) proteins from WT and TG hippocampus. Synaptic fractionation was performed 16 h after i.p. injection of saline solution or FK506 (10 mg/kg). Right panel: GluA1 expression (WT Ctrl percentage) in TxP and P3 fraction. Data are expressed as mean ± S.D. (n = 5 per group, *p < 0.05). b Left panel representative immunoblots of PSD (TxP) proteins probed with indicated antibodies. Right panel: GluA1pSer845 levels (WT Ctrl percentage) in TxP fraction. Data are expressed as mean ± S.D. (n = 5 per group, *p < 0.05)
Molecular modifications of PSD composition precede dendritic spine alterations and cognitive decline. The observation that i.p. injection of FK506 is able to restore PSD composition in TG mice is in line with previous results demonstrating that calcineurin inhibition improves memory function in Tg2576 mice (Dineley et al. 2007) and recovers spine density in APP/PS1 mouse model of AD (Rozkalne et al. 2011).
Discussion
Despite the relevance of intracellular Aβ in AD onset and progression has been demonstrated in many experimental models of disease (Cavallucci et al. 2012), the characterization of the molecular mechanisms by which Aβ impairs synaptic plasticity and contributes to progressive synapse impairment remains to be clarified, and very few treatments have been tested as strategies to slowdown synaptic dysfunction and spine loss (Nisticò et al. 2012a, b; Balducci et al. 2011). It has been demonstrated that calcineurin expression, by gene transfer, causes dendritic spine loss and dystrophic neurite swelling; on the other hand, expression of a peptide-encoding calcineurin inhibitor reverses these morphological alterations (Wu et al. 2010). More recently, it has been observed (Rozkalne et al. 2011) that the calcineurin inhibitor FK506 reverses many dendritic spine alterations in mice expressing AD-associated AβPP and PS1. Moreover, a single injection of the same calcineurin inhibitor (10 mg/kg, i.p.) improves the memory deficits in plaque-free Tg2576 (Dineley et al. 2007) and in wild-type mice injected with oligomeric Aβ (Dineley et al. 2010). Despite these experimental evidences indicating calcineurin as a crucial molecular player in AD-related synaptic dysfunction, the molecular mechanism linking alteration of synaptic plasticity to calcineurin activity remains unexplored. Here, we demonstrate that pharmacological inhibition of calcineurin in acute slices from young plaque-free Tg2576 mice displaying both early memory deficits, biochemical, and morphological alterations (D’Amelio et al. 2011; Cimini et al. 2009) is able to rescue aberrant LTD. The effect of calcineurin inhibitor FK506 on DHPG-LTD is due to its capability to prevent calcineurin-mediated dephosphorylation (Schreiber and Crabtree 1992) of GluA1 AMPAR subunit. The blockade of GluA1 dephosphorylation at Ser845 prevents AMPAR removal from PSD. Relevantly, we also demonstrated that a single injection of FK506, by using the same treatment schedule previously employed to improve memory function (Dineley et al. 2007), is able to restore PSD composition.
Our combined ex vivo and in vivo analyses suggest a model for the beneficial effect of calcineurin inhibition on PSD composition and, consequently, on synaptic plasticity (Fig. 4). Synaptic localization and function of AMPAR depend on the phosphorylation state of its subunits. As previously demonstrated (D’Amelio et al. 2011), Aβ overload in TG hippocampus causes mitochondria-dependent caspase-3 overactivation specifically at synapse, where it cleaves calcineurin eliminating its auto-inhibitory domain with consequent increase of phosphatase activity. The resulting dephosphorylation of GluA1 subunit at Ser845 triggers AMPAR removal from postsynaptic site. Both in WT and TG hippocampus, the mGluR agonist DHPG induces the activation of a protein tyrosine phosphatase which dephosphorylates tyrosine residues of GluA2 subunit, leading to the AMPAR internalization and LTD expression (Gladding et al. 2009; Moult et al. 2006). In TG hippocampus, the reduced level of AMPAR expression at postsynaptic site is reflected by the stronger LTD expression. Pretreatment of TG hippocampal slices with calcineurin inhibitor FK506 restores postsynaptic AMPAR levels and rescues LTD to the WT level.
Model of FK506 effect on synaptic plasticity in Tg2576 hippocampus. Aβ overload in Tg2576 (TG) hippocampus causes mitochondria-dependent caspase-3 (casp-3) overactivation at synapse. Casp-3 cleaves and activates calcineurin (CN) which, in turn, dephosphorylates Ser845 of GluA1 subunit, with consequent AMPAR removal from postsynaptic site. Both in wild-type (WT) and in TG hippocampus, the mGluR agonist DHPG induces the activation of a protein tyrosine phosphatase (PTP) which dephosphorylates tyrosine residues of GluA2 subunit, leading to the AMPAR internalization and long-term depression (LTD) expression. In TG hippocampus, the basal reduced level of AMPAR expression at postsynaptic site is reflected by the stronger LTD induction. Pretreatment of TG hippocampal slices with calcineurin inhibitor FK506 restores postsynaptic AMPAR levels and reduces LTD to the WT level
These results are in line with previous data showing beneficial effects of calcineurin inhibition on synapse function and integrity, thus suggesting new pharmacological strategies to restore memory/cognitive decline in AD patients.
Methods
Animals
Heterozygous male Tg2576 mice (Hsiao et al. 1996) and wild-type littermates were used. All experiments were performed on independent groups of 6-month-old mice. Experiments have been carried out in accordance with the Declaration of Helsinki and follow international guidelines on the ethical use of animals from the European Communities Council Directive of November 24, 1986 (86/609/EEC). Formal approval of these experiments was obtained from the Italian Ministry of Health (D.L.vo 116/92).
In Vitro Slice Preparation and Electrophysiology
Mice were anaesthetized by inhalation of 2-Bromo-2-Chloro-1,1,1-trifluoroethane and killed by decapitation. Hippocampal slices were prepared as previously described by Nisticò et al. (2012a, b). The brain was rapidly removed from the skull, and slices (400 μm) from both hemispheres were cut on the parasagittal plane in ice-cold artificial cerebrospinal fluid, ACSF (124 mM NaCl, 3 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, saturated with 95 % O2, and 5 % CO2, pH 7.4).
The slices left to recover for 1 h in ACSF at room temperature and then individually placed over an 8 × 8 array of planar electrodes, each 50 × 50 μm in size, with an interpolar distance of 150 μm (MED-P5155; Alpha MED Sciences, Kadoma, Japan). The slices were kept submerged in ACSF (5 ml/min; 31 °C) with a nylon mesh glued to a platinum ring. Voltage signals were acquired using the MED64 System (Alpha MED Sciences, Kadoma, Japan), digitized at 20 kHz and filtered (0.1–1 Hz) with a 6071E Data Acquisition Card (National Instruments, Austin, USA), using Conductor software (Alpha MED Sciences, Kadoma, Japan).
Field excitatory postsynaptic potentials were recorded from the dendritic region of the CA1 area, upon stimulation through one the 64 electrodes positioned in the stratum radiatum of the recorded slice. Stimulation intensity was set to a value attaining a half-maximal postsynaptic response and then delivered at 30 s intervals throughout the whole experimental session. After assessment of fEPSP amplitude stability, the slice was challenged with DHPG (50 μM) for 10 min and then washed out for at least 60 min. The degree of DHPG-LTD was evaluated by the fEPSP mean amplitude at 50–60 min from DHPG washout and normalized to the mean fEPSP amplitude recorded during the 10 min preceding DHPG perfusion. The values obtained in the different experimental conditions were compared with the Student’s t test for unpaired data, using p < 0.05 as limit for statistical significance.
Protein Extraction From Hippocampal Slices
Hippocampal slices were homogenized in lysis buffer (320 mM sucrose, 50 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1 % Triton X-100, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, and 5 mM NaF, protease inhibitor cocktail), incubated on ice for 30 min and centrifuged at 13,000×g for 10 min. The total protein content of resulting supernatant was determined by Bradford method.
Slice Biotinylation
Surface biotinylation was performed as previously described (Gladding et al. 2009), with little modifications. Hippocampal slices (200 μm) were incubated in standard ACSF containing 0.5 mg/ml sulfo-NHS-LC-Biotin (Pierce) for 1 h on ice and bubbled with 95 % O2 and 5 % CO2. After the biotinylation, the slices were washed once with ice-cold ACSF, followed by two washes in ice-cold TBS (50 mM Tris–HCl (pH 7.5) 150 mM NaCl). Slices were homogenized in homogenization buffer (50 mM Tris–HCl (pH 7.5), 0.3 M sucrose, 5 mM EDTA, 1 % Triton X-100, 0.1 % SDS, 0.5 mM sodium orthovanadate, 5 mM β-glycerophosphate, and protease inhibitor cocktail) with a pellet pestle and then briefly sonicated. Samples were centrifuged at 13,000×g for 10 min, and the total protein content of resulting supernatant was determined. For each sample, 200 μg of proteins were incubated with NeutrAvidin agarose (Pierce) in a 1:1 ratio for 16 h at 4 °C. Biotinylated proteins attached to NeutrAvidin coated beads were separated by centrifugation (1,000×g for 1 min). The beads were washed two times with wash buffer (Pierce), and the bound proteins were eluted with SDS sample buffer by boiling it for 10 min at 95 °C.
Sub-Synaptic Fractionation
Sixteen hours before sub-synaptic fractionation mice were treated with FK506. FK506 injection solution (Prograf 5 mg/ml, Astellas Pharma S.p.A., Italy) was diluted to 2 mg/ml with sterile 0.9 % saline and administered at 10 mg/kg i.p. Vehicle injections consisted of 0.9 % sterile saline.
Hippocampus was homogenized in homogenization buffer (320 mM sucrose, 10 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM NaHCO3, 1 mM PMSF, 1 mM sodium orthovanadate, 5 mM NaF, 20 mM β-glycerophosphate, and protease inhibitor cocktail) with ten strokes of a tight-fitting glass Dounce tissue grinder. The homogenate was centrifuged at 1,000×g for 10 min, and the resulting supernatant was centrifuged at 10,000×g for 15 min. Both the pellet (P2) and the supernatant (S2) were stored. The pellet P2 was homogenized in homogenization buffer containing 0.5 % Triton X-100 with ten strokes of a tight-fitting glass Dounce tissue grinder, incubated 40 min on ice, and centrifuged at 32,000×g for 20 min. The resulting pellet (TxP) containing PSDs was processed for protein extraction. The supernatant S2 was centrifuged at 100,000×g for 1 h, and the microsomal-enriched proteins were extracted from the resulting pellet (P3). Briefly, TxP and P3 were re-suspended in RIPA buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 % Triton X-100, 0.25 % sodium deoxycholate, 0.1 % SDS, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, 5 mM NaF, and protease inhibitor cocktail), sonicated, and incubated on ice for 20 min. The samples were centrifuged at 11,500×g for 10 min, and the protein concentration of resulting supernatant was determined by Bradford method.
Immunoblotting Analysis and Antibodies
Proteins were applied to SDS-PAGE and electroblotted on a PVDF membrane. Immunoblotting analysis was performed using a chemiluminescence detection kit. The relative levels of immunoreactivity were determined by densitometry using the software ImageJ. Data analysis was performed with a two tailed Student’s t test. p < 0.05 was considered to be statistically significant.
Primary antibodies: GluA1 (1:1,000; Millipore 04-855), GluA1pSer831 (1:1,000; Millipore 04-823), GluA1pSer845 (1:1,000; Millipore AB5849), PSD-95 (1:1,000; Millipore MAB1598), NR1 (1:1,000; Santa Cruz sc-1467), NR2A (1:250; Santa Cruz sc-1468), β-tubulin (1:100,000; Sigma T4026), actin (1:25,000; Sigma A5060), synaptophysin (1:10,000; Abcam ab32127), and Rab11 (1:1,000; BD Biosciences 610656).
Coomassie Staining
PSD (TxP) and microsome-enriched proteins (P3) were resolved by SDS-PAGE. After electrophoresis, the gels were fixed for 30 min in cold fixing solution (50 % methanol, 10 % acetic acid), stained with Coomassie solution (0.2 % Coomassie Blue, 20 % methanol, 10 % acetic acid) for 30 min and washed abundantly with cold destaining solution (45 % methanol, 10 % acetic acid).
References
Balducci, C., Mehdawy, B., Mare, L., Giuliani, A., Lorenzini, L., Sivilia, S., et al. (2011). The γ-secretase modulator CHF5074 restores memory and hippocampal synaptic plasticity in plaque-free Tg2576 mice. Journal of Alzheimers Disease, 24, 799–816.
Cavallucci, V., D’Amelio, M., & Cecconi, F. (2012). Aβ toxicity in Alzheimer’s disease. Molecular Neurobiology, 45, 366–378.
Cimini, A., Moreno, S., D’Amelio, M., Cristiano, L., D’Angelo, B., Falone, S., et al. (2009). Early biochemical and morphological modifications in the brain of a transgenic mouse model of Alzheimer’s disease: A role for peroxisomes. Journal of Alzheimers Disease, 18, 935–952.
D’Amelio, M., Cavallucci, V., Middei, S., Marchetti, C., Pacioni, S., Ferri, A., et al. (2011). Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nature Neuroscience, 14, 69–76.
D’Amelio, M., & Rossini, P. M. (2012). Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: From animal models to human findings. Progress in Neurobiology, 99, 42–60.
D’Amelio, M., Sheng, M., & Cecconi, F. (2012). Caspase-3 in the central nervous system: Beyond apoptosis. Trends in Neurosciences, 35, 700–709.
de Calignon, A., Fox, L. M., Pitstick, R., Carlson, G. A., Bacskai, B. J., Spires-Jones, T. L., et al. (2010). Caspase activation precedes and leads to tangles. Nature, 464, 1201–1204.
de Calignon, A., Polydoro, M., Suárez-Calvet, M., William, C., Adamowicz, D. H., Kopeikina, K. J., et al. (2012). Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron, 73, 685–697.
Dineley, K. T., Hogan, D., Zhang, W. R., & Taglialatela, G. (2007). Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiology of Learning and Memory, 88, 217–224.
Dineley, K. T., Kayed, R., Neugebauer, V., Fu, Y., Zhang, W., Reese, L. C., et al. (2010). Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. Journal of Neuroscience Research, 88, 2923–2932.
Esteban, J. A., Shi, S. H., Wilson, C., Nuriya, M., Huganir, R. L., & Malinow, R. (2003). PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neuroscience, 6, 136–143.
Gladding, C. M., Collett, V. J., Jia, Z., Bashir, Z. I., Collingridge, G. L., & Molnár, E. (2009). Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Molecular and Cellular Neuroscience, 40, 267–279.
He, K., Song, L., Cummings, L. W., Goldman, J., Huganir, R. L., & Lee, H. K. (2009). Stabilization of Ca2 + -permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America, 106, 20033–20038.
Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., et al. (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 274, 99–102.
Lee, S. H., Kim, B. C., Yang, D. H., Park, M. S., Choi, S. M., Kim, M. K., et al. (2008). Calcineurin inhibitor-mediated bilateral hippocampal injury after bone marrow transplantation. Journal of Neurology, 255, 929–931.
Li, Z., Jo, J., Jia, J. M., Lo, S. C., Whitcomb, D. J., Jiao, S., et al. (2010). Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell, 141, 859–871.
Middei, S., Houeland, G., Cavallucci, V., Ammassari-Teule, M., D’Amelio, M., & Marie, H. (2013). CREB is necessary for synaptic maintenance and learning-induced changes of the AMPA receptor GluA1 subunit. Hippocampus, 23, 488–499.
Moult, P. R., Gladding, C. M., Sanderson, T. M., Fitzjohn, S. M., Bashir, Z. I., Molnár, E., et al. (2006). Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. Journal of Neuroscience, 26, 2544–2554.
Mukerjee, N., McGinnis, K. M., Park, Y. H., Gnegy, M. E., & Wang, K. K. (2000). Caspase-mediated proteolytic activation of calcineurin in thapsigargin-mediated apoptosis in SH-SY5Y neuroblastoma cells. Archives of Biochemistry and Biophysics, 379, 337–343.
Nisticò, R., Cavallucci, V., Piccinin, S., Macrì, S., Pignatelli, M., Mehdawy, B., et al. (2012a). Insulin receptor β-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. NeuroMolecular Medicine, 14, 262–269.
Nisticò, R., Pignatelli, M., Piccinin, S., Mercuri, N. B., & Collingridge, G. (2012b). Targeting synaptic dysfunction in Alzheimer’s disease therapy. Molecular Neurobiology, 46, 572–587.
Rozkalne, A., Hyman, B. T., & Spires-Jones, T. L. (2011). Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiology of Disease, 41, 650–654.
Schreiber, S. L., & Crabtree, G. R. (1992). The mechanism of action of cyclosporin A and FK506. Immunology Today, 13, 136–142.
Wijdicks, E. F., Wiesner, R. H., Dahlke, L. J., & Krom, R. A. (1994). FK506-induced neurotoxicity in liver transplantation. Annals of Neurology, 35, 498–501.
Wu, H. Y., Hudry, E., Hashimoto, T., Kuchibhotla, K., Rozkalne, A., Fan, Z., et al. (2010). Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. Journal of Neuroscience, 30, 2636–2649.
Acknowledgments
MDA is financially supported by a grant from the Alzheimer’s Association (NIRG-11-204588). We thank Cecilia Giusti for technical support. Tg2576 mice used in the present study were kindly provided by Prof. Francesco Cecconi.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Virve Cavallucci and Nicola Berretta contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12017_2013_8241_MOESM1_ESM.eps
Supplementary Fig. S1 a Immunoblot analysis of GluA1, PSD-95, synaptophysin, and Rab11 demonstrating the purity of the synaptic fractionation. Representative immunoblots of the PSD-enriched fraction (TxP) and of the microsomal-enriched fraction (P3) are shown. b PSD (TxP) and microsome-enriched protein (P3) preparations (9 μg protein) from wild-type (WT) and Tg2576 (Tg) mice injected with saline (Ctrl) or calcineurin inhibitor FK506 were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. No major differences are seen at this level of resolution. (EPS 2896 kb)
Rights and permissions
About this article
Cite this article
Cavallucci, V., Berretta, N., Nobili, A. et al. Calcineurin Inhibition Rescues Early Synaptic Plasticity Deficits in a Mouse Model of Alzheimer’s Disease. Neuromol Med 15, 541–548 (2013). https://doi.org/10.1007/s12017-013-8241-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8241-2