Abstract
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by impairments in synaptic plasticity and cognitive performance. Current treatments are unable to achieve satisfactory therapeutic effects or reverse the progression of the disease. Calcineurin has been implicated as part of a critical signaling pathway for learning and memory, and neuronal calcineurin may be hyperactivated in AD. To investigate the effects and underlying mechanisms of FK506, a calcineurin inhibitor, on Alzheimer-like behavior and synaptic dysfunction in the 3 × Tg-AD transgenic mouse model of Alzheimer’s disease, we investigated the effect of FK506 on cognitive function and synaptic plasticity in the 3 × Tg-AD transgenic mouse model of Alzheimer’s disease. The results showed that FK506 treatment ameliorated cognitive deficits, as indicated by the decreased latency in the water maze, and attenuated tau hyperphosphorylation in 3 × Tg-AD mice. Treatment with FK506 also reduced the levels of certain markers of postsynaptic deficits, including PSD-95 and NR2B, and reversed the long-term potentiation deficiency and dendritic spine impairments in 3 × Tg-AD mice. These findings suggest that treatment with calcineurin inhibitors such as FK506 can be an effective therapeutic strategy to rescue synaptic deficit and cognitive impairment in familial Alzheimer’s disease and related tauopathies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the most common cause of dementia in the elderly. Its clinical characteristics include gradual memory loss accompanied by language problems. Eventually, neurons in parts of the brain that enable a person to carry out basic bodily functions, such as walking and swallowing, are affected. Individuals become bed-bound and require around-the-clock care (Gaugler et al. 2022). Currently, there are no effective interventions that can cure, halt or prevent the progression of AD. Neuronal dysfunction and degeneration in brain regions such as the hippocampus, entorhinal cortex, basal forebrain, and frontal and parietal lobes, lead to behavioral abnormalities in AD (Umar et al. 2022). Synaptic and neuronal degeneration in these brain regions is closely associated with the aggregation of extracellular senile plaques composed of amyloid-β (Aβ) peptides and intracellular neurofibrillary tangles (NFTs) composed of abnormally hyperphosphorylated tau proteins (Scheltens et al. 2021). Thus, an ideal drug would be one that can effectively target the pathological changes as well as prevent and rescue cognitive impairment.
Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN) is the most abundant phosphatase in the central nervous system and is essential for synaptic plasticity and normal memory function. Modulated calcineurin signaling is associated with neural dysfunction in Alzheimer’s disease (Saraf et al. 2018). The activation of CaN leads to dephosphorylation of various proteins, which in turn induce various hallmarks of Alzheimer’s disease, such as inflammation, cell death, and hyperphosphorylation of tau (Tucker et al. 2021). For instance, in APP/PS1 transgenic mice, CaN is upregulated in astrocytes and triggers a typical AD inflammatory response (Norris et al. 2005). CaN over activation in AD patients triggers a cascade of NFAT signaling, leading to Aβ-induced cell death (Wu et al. 2010). Conversely, the dysregulation of Ca2+ plays a crucial role in oligomer-mediated toxicity in many amyloidogenic diseases due to their strong trans-membrane concentration gradients and involvement in cell dysfunction and death (Demuro et al. 2005), such as inhibition of CaN protects neuronal cells from Aβ-induced cell death (Rajput et al. 2020) and eliminates perturbations of LTP induced by Aβ (Chen et al. 2001). Therefore, inhibition of CaN may be a potential therapeutic strategy for patients with AD.
The 3 × Tg-AD model harbors two mutations associated with familial AD (APP Swedish and PSEN1 M146V), one Tau mutation, and P301L frontotemporal dementia; replicates some key histopathological and behavioral characteristics of AD; and is one of the most widely used animal models of AD. However, no study has used this AD model mouse to investigate the impact of CaN inhibitor on AD. So, in this study, we aimed to investigate the effect of FK506, one of the calcineurin inhibitors, on cognitive function and molecular markers of neuroplasticity in 3 × Tg-AD mice, intended to provide a more comprehensive research foundation for whether inhibition of CaN can become a therapeutic strategy for AD.
Materials and methods
Experimental groups and treatment with FK506
This study included homozygous 3 × Tg-AD mice (purchased from the Jackson Laboratory, Bar Harbor, Maine, USA) with a C57BL6/129S4 background. The 3 × Tg-AD mice and the male non-transgenic WT control mice (a hybrid of 129S4 and C57BL/6 J mice) were group-housed (four animals per cage) at 24 ± 2 °C with daily 12 h light–dark cycles and ad libitum access to food and water. There were three study groups, as follows: 3 × Tg-AD mice with FK506 treatment (3 × Tg + FK506, n = 9), 3 × Tg-AD mice without FK506 treatment (3 × Tg, n = 8), WT mice without FK506 treatment (wt, n = 9). The 3Tg + FK506 group mice were treated with FK506 (purchased from Sigma, St. Louis, MO) via intraperitoneal injection (10 mg FK506/kg body weight at a concentration of 10 mg/mL in 0.9% saline) once they reached 10 months of age, for 14 days. As a control, the other two groups received the same dose of 0.9% sterile saline via intraperitoneal injection. The Morris water maze (MWM) task was performed on the 8th day. The animals were euthanized on the 14th day for electrophysiological and spine analysis as well as western blotting. The flow chart of the experiment is shown in Fig. 1A. All efforts were made to minimize animal suffering in this study. All animal experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, and the Guidelines for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China. The study protocol was approved by the Institutional Animal Care and Use Committee in Tongji Medical College, Huazhong University of Science and Technology.
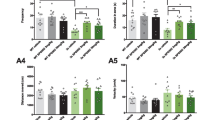
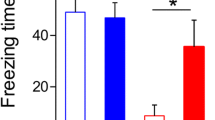
FK506 treatment ameliorated cognitive deficits in 3 × Tg-AD mice. A The experiments were designed as shown in panel A. During the 2nd week after FK506 treatment, the mice were trained in the Morris water maze (MWM) for 6 consecutive days to measure their learning capacity, and memory was assessed on the 7th day via removal of the hidden platform. B FK506 improved learning ability as indicated by the decreased escape latency during the MWM training test. C The representative swimming trace of the mice during the learning test on the 6th day in the water maze. D FK506 improved the memory capacity as demonstrated by the decreased escape latency, increased number oftimes crossing the site where the platform was placed before, and longer retention time in the target quadrant on the 7th day. E There was no significant difference in swimming speed among the three groups (n = 9 mice each for the wt and 3 × Tg + FK506 groups, n = 8 mice for the 3 × Tg group). *, p < 0.05, **, p < 0.01 (3 × Tg vs wt); #, p < 0.05 (3 × Tg + FK506 vs 3 × Tg). The data are expressed as the mean ± standard error of the mean
MWM
The MWM was used to assess spatial learning and memory. The test was performed in a circular pool (with a diameter of 180 cm and a height of 60 cm) filled with nontoxic black dye-tinted water and maintained at 23 ± 2 °C. Two virtual principal axes, with each line bisecting the maze perpendicular to the other, divided the maze into quadrants. A platform was positioned in the middle of one of the quadrants and submerged 1 cm below the water surface. The mice were trained to find the hidden platform from semi-random start positions in each quadrant facing the pool wall and ended as soon as the mice climbed onto the hidden platform for 6 consecutive days (four training trials per day, once per quadrant) from 14:00 to 20:00 pm. During training, the swimming path and the time used to find the platform (latency) were recorded each day. If a mouse failed to find the platform within 60 s, it was gently guided to the platform and the escape latency was recorded as 60 s. At the end of each trial, the mouse was left on the platform for 30 s, then removed, dried, and returned to its home cage. A 90 s probe test assessing spatial memory without the platform was performed 24 h after the final trial. The time spent passing through the previous platform-located quadrant and the first time to cross the platform (latency) were recorded. All trials were recorded using a video camera, which was fixed to the ceiling 1.5 m from the water surface and connected to a digital-tracking device attached to an IBM computer (Armonk, NY).
Electrophysiological recordings
Animals were anesthetized using ketamine and xylazine (Sigma-Aldrich, X1126). The mouse brains were cut into horizontal sections of 350 μm thickness using a Leica VT1000S vibratome (Leica, Germany) after continuously perfused with ice-cold oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 1, Na2PO4 1.25, NaHCO3 26, glucose 10, saturated with 95% O2 and 5% CO2 (pH 7.4). The hippocampal slices were incubated at 32 °C for 30 min, and allowed to equilibrate to 22 ± 2 °C for ≥ 30 min. For the induction of long-term potentiation (LTP), the slice was laid over an 8 × 8 microelectrode array and kept flat in a CSF. Field excitatory postsynaptic potentials (fEPSPs) were evoked with 0.2 ms pulses delivered via MED64 on mossy fibers, recorded extracellularly in the CA3 stratum radiatum and quantified as the initial slope of the field potential. Baseline responses were recorded by using a stimulation intensity that evoked a half-maximal response, defined as the maximal response without a population spike (pop-spike). High-frequency tetanus consisting of three epochs (100 Hz, 1 s duration) was delivered. The magnitude of LTP was calculated as the average (normalized to baseline) of the responses recorded 50–60 min after conditioning stimulation. All signals were recorded using a multi-electrophysiological recording setup (MED64 System, Alpha MED Sciences, Panasonic, Japan).
Golgi staining and spine analyses
Golgi staining was performed by using a FD Rapid Golgi Stain Kit (FD Neurotechnology, PK401). The mice were deeply anaesthetized and perfused for 5 min with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS for 15 min. The brains were dissected and immersed in impregnation solution (equal volumes of Solutions A and B, containing HgCl2, K2Cr2O7, and K2CrO4). All procedures were performed in the dark. The brain samples were soaked in the solution in the dark for 4 h at 24 ± 2 °C and placed in fresh impregnation solution. After 2 weeks, the brain tissues were transferred to Solution C and stored at 4 °C for 48 h, and placed in fresh Solution C after 4 h. Sagittal brain Sections (100 μm thick) were taken using a vibrate microtome (VT 1000 s, Leica, Nussloch, Germany) and mounted on gelatin-coated slides in Solution C. After drying, the slides were rinsed twice in distilled water (2 min each) and then placed in a mixture of Solution D: E: distilled water (1:1:2) for 10 min. The sections were rinsed in distilled water and dehydrated in 50, 75, 95, and 100% ethanol four times (4 min each), cleaned in xylene (Sinopharm Chemical Reagent, 10,023,418) three times (4 min each), and then cover-slipped with Permount solution (Daigger Scientific, EF15969A).
A Zeiss Axio imager microscope with a 63 × oil immersion objective was used to image the dendritic segments under bright-field illumination, and the spine morphology was analyzed. The segments of dendrites at a distance of 90–110 μm (proximal) and 190–210 μm (distal) from the soma were used to determine the spine density. Image-Pro Plus 6.0 was used to calculate the spine numbers from 10 μm of dendrite length per neuron. Data from five to seven neurons were averaged per animal (three mice per group) and used in further statistical analysis. All imaging data were analyzed using the double-blind method.
Western blotting
Mice were decapitated after the spatial memory retention test. The tissues were rapidly removed and homogenized at 4 °C using a Teflon glass homogenizer (Glas-Col, 099cs71) in 50 mM Tris–HCl, pH7.4, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 2 mM benzamidine (Bjoka-vip, XW020077), and 1mphenylmethylsulphonyl fluoride (Sigma-Aldrich, P7626). After mixing with sample buffer (3:1, v/v, containing 200 mM Tris–HCl [pH 7.6], 8% SDS, 40% glycerol, 40 mM dithiothreitol), the extract was boiled for 10 min and then centrifuged at 12, 000 × g for 10 min at 25 °C. The supernatant was used for western blotting. The proteins were transferred to PVDF membranes after separation by 10% SDS–polyacrylamide gel electrophoresis. The membranes were blocked for 1 h with 5% nonfat milk, dissolved in TBS-Tween-20 (50 mM Tris HCl, pH 7.6, 150 mM NaCl, 0.2% Tween-20 [Sinopharm Chemical Reagent, 31,089,328]), and incubated with primary antibodies (Table 1) at 4 °C overnight. Finally, the blots were incubated with anti-mouse IgG (LI-COR, 926-32210) or anti-rabbit IgG (LI-COR, 926-32211) conjugated to IRDyeTM (800CW) for 1 h at 24 ± 2 °C and visualized using the Odyssey Infrared Imaging System (Licor biosciences, Lincoln, NE, USA). The protein concentration was estimated using a bicinchoninic acid kit (Sigma-Aldrich, BCA-1) according to manufacturer’s instructions. Protein bands were quantitatively analyzed using Image-pro plus 6.0 software (Media Cybernetics, Washington, USA).
Statistical analysis
All data were collected and analyzed in a blinded manner. Data are expressed as the mean ± standard error of the mean and analyzed using SPSS 20.0 (SPSS Inc. Chicago, IL, USA). Statistical analysis was performed using a one-way analysis of variance followed by Bonferroni’s post hoc analysis. The level of significance was set at p < 0.05.
Results
FK506 treatment ameliorates memory deficits in 3 × Tg-AD mice
To determine the role of FK506 in AD, we first investigated whether FK506 could rescue spatial memory impairment in 3 × Tg-AD mice. On the MWM test, the 3 × Tg-AD mice exhibited learning and memory deficits compared to their age-matched littermates (Fig. 1B–D), while FK506 improved learning ability (days 3–5), as indicted by the decreased latency to find the submerged platform over 6 consecutive days of training (Fig. 1B, C). The improved memory of 3 × Tg-AD mice was demonstrated by this decreased latency as well as the increased time crossing the platform site and the increased time spent in the platform quadrant on day 7 after removing the platform (Fig. 1D). There was no significant difference in swimming speed among the three groups (Fig. 1E).
FK506 improved the synaptic plasticity of neurons in the hippocampal CA3 region of 3 × Tg-AD mice
LTP contributes to synaptic plasticity and synaptic strength, which underlies the formation of learning and memory (McLeod et al. 2020). Therefore, to investigate the underlying mechanisms of FK506-induced amelioration of synaptic deficits, we first measured the effect of FK506 on LTP in acute hippocampal slices. By stimulating mossy fibers, the slope of fEPSPs was recorded in CA3. We found that the slope value was much lower after high-frequency stimulation in 3 × Tg-AD mice than in wt mice. However, FK506 rescued the LTP deficit in 3 × Tg-AD mice (Fig. 2A, B).
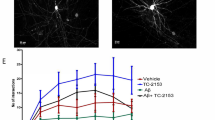
FK506 reverses long-term potentiation impairment and improves dendritic spine density in 3 × Tg-AD mice. A, B fEPSPs were recorded in hippocampal region CA3 by stimulating mossy fibers in brain slices from 3 × Tg-AD mice and wild-type littermates. The long-term potentiation (LTP) magnitude was calculated as the average (normalized to baseline) of the responses recorded 50–60 min after conditioning stimulation (n = 7–8 slices from three mice for each group). C, D Spine density was detected by Golgi staining (at least 18 neurons were analyzed from three mice per group). Scale bar: 5 μm. **, p < 0.01, ***, p < 0.001(3 × Tg vs wt); #, p < 0.05 (3 × Tg + FK506 vs 3 × Tg). Data are expressed as the mean ± standard error of the mean
Dendrite complexity and the morphologies of post-synaptic spines are critical components for learning and memory (Guedes-Dias and Holzbaur 2019). Therefore, we examined alterations in dendritic spines using Golgi staining. We found that the spine density in hippocampal CA3 was markedly reduced in 3 × Tg-AD mice and FK506 almost fully recovered the spine density (Fig. 2C, D).
Normal synaptic transmission is dependent on the stable expression of synaptic proteins. Several independent studies have demonstrated that N-methyl-D-aspartate receptor type 1 (NR1), NR2B, PSD95, AMPA receptor subunits GluR1 and GluR2, synaptophysin, and synapsin 1 are related to synaptic plasticity (Yan et al. 2023; Wang et al. 2023; Liu et al. 2020). To further explore the molecular mechanisms underlying the protective effects of FK506, we detected synapse-associated proteins using Western blotting. The expression of postsynaptic proteins NR2B and PSD95 were significantly reduced in the hippocampi of 3 × Tg-AD mice compared to matched wt mice, however, FK506 restored the NR2B and PSD95 levels (Fig. 3A, C). In addition, the levels of presynaptic proteins synaptophysin and synapsin 1, and postsynaptic proteins GluR1 and GluR2 did not significantly change (Fig. 3A–C).
FK506 increases synaptic protein levels in the hippocampi of 3 × Tg-AD mice. A The levels of presynaptic and postsynaptic proteins in whole hippocampal extracts were measured using Western blotting and quantitative analysis (n = 3 for each group). B The levels of GluR1 and GluR2 did not significantly differ among the three groups. C The expression levels of NR2B and PSD95 were significantly reduced in the hippocampi of 3 × Tg-AD mice, while FK506 restored the levels. D The levels of synapsin1 and synaptophysin were not significantly altered. *, p < 0.05, **, p < 0.01 (3 × Tg vs wt); #, p < 0.05 (3 × Tg + FK506 vs 3 × Tg). Data are expressed as the mean ± standard error of the mean
FK506 attenuates tau hyperphosphorylation in 3 × Tg-AD mice
We further detected the level of phosphorylated tau in 3 × Tg-AD mice. In the hippocampi of 3 × Tg-AD mice, the levels of total tau (Tau5), Tau1 (not phosphorylated at Ser198/199/202), and tau phosphorylated at Thr231, Ser214, Ser396, and Ser404 were increased significantly, while FK506 attenuated tau hyperphosphorylation at Ser396 and Ser404. The Tau5 level was also reduced by FK506 (Fig. 4A and B).
FK506 attenuated tau hyperphosphorylation in 3 × Tg-AD mice. A, B The levels of tau in whole hippocampal extracts were detected by western blotting and quantitative analysis (n = 3 for each group). The levels of Tau5, Tau1, and phosphorylated tau at Thr231, Ser214, Ser396, and Ser404 sites were significantly increased, while FK506 attenuated tau hyperphosphorylation at Ser396 and Ser404 sites. The level of the total tau (Tau5) was also decreased by FK506. **, p < 0.01 (3 × Tg vs wt); #, p < 0.05 (3 × Tg + FK506 vs 3 × Tg). Data are expressed as the mean ± standard error of the mean
These data suggest that tau hyperphosphorylation is attenuated by FK506, which may have played a major role in the improved cognitive function of the 3 × Tg-AD mice.
Discussion
In the present study, we found that treatment with CaN inhibition can improve the AD-like memory deficits and synaptic dysfunction, as well as attenuates tau hyperphosphorylation in adult 3 × Tg-AD mice. These data suggest that CaN inhibition may be a potential therapeutic strategy for the prevention of diseases associated with AD.
FK506, a product of the bacterium Streptomyces tsukubaensis and also known as tacrolimus, is a CaN inhibitor. FK506 inhibits CaN-mediated dephosphorylation by forming a complex with the FK506-binding protein FKBP12 (Juvvadi et al. 2020). As a clinically important immunosuppressant, FK506 is primarily used in liver, kidney, heart, and lung transplants (Parlakpinar and Gunata 2021). FK506 has a good therapeutic potential due to its antifungal, neuroprotective, and neuroregenerative activities (Jung et al. 2020). Whether FK506 can become one of the candidate drugs for AD treatment and bring hope to AD patients remains unknown. In clinical researches, a large single-center retrospective study showed that the long-term use of FK506 was associated with a significantly lower incidence of AD than that in age-matched control populations (Taglialatela et al. 2015). An open-label phase II study of FK506 is underway to investigate the efficacy in mild cognitive impairment (MCI) and AD (Yu et al. 2021). These clinical studies suggest that FK506 may become a candidate drug for AD treatment, therefore, extensive animal experimental research and cytological study have become necessary. The current researches includes FK506 reversed deficits in cognition and memory in Tg2576 APP transgenic mice as well as AD model mice created by the injection of Aβ oligomers into the lateral ventricles (Taglialatela et al. 2009; Dineley et al. 2007, 2010). Further studies have documented that FK506 treatment alleviated memory deficits in a lithium-pilocarpine-induced cognitive deficit SE rat model as well as STZ i.c.v. treated mice and aged mice (Liu et al. 2018, Amit et al. 2017), and our study also confirmed that FK506 can ameliorates memory deficits in 3 × Tg-AD mice.
The impairment of synaptic plasticity is a common change in the brain of AD patients. Synaptic plasticity refers to the property of synapses to undergo long-term changes in synaptic strength and is considered to be a cellular substrate of learning and memory (Stroebel et al. 2021). The impairment of synaptic plasticity often manifests as abnormalities in LTP, a decrease in synaptic spines and the abnormal expression of synaptic proteins. Cognitive deficits in AD are strongly associated with LTP failure and the aberrant expression of synaptic proteins in the hippocampus (Wong et al. 2022; Al-Onaizi et al. 2022). In the present study, we found that the LTP deficit was rescued and the levels of postsynaptic proteins NR2B and PSD95 were increased in 3 × Tg-AD mice following treatment with FK506, this indicates that the mechanism by which FK506 reverses cognitive deficits can occur at the synaptic protein level. As well as, we observed that FK506 reversed the decline in dendritic spine density in 3 × Tg-AD mice. There are also studies with similar results, such as Miller found that in cultured hippocampal neurons from APPSwe AD-transgenic mice and cultured rat hippocampal neurons, treatment with soluble Aβ oligomers impaired synaptic function by reducing the amplitude of miniature excitatory postsynaptic currents, and FK506 blocked this effect (Miller et al. 2014). FK506 prevented Aβ42-mediated loss of dendritic spines in wild type cells and in primary hippocampal neurons (Stallings et al. 2018; Zhao et al. 2010). Zhang et al. showed that the use of FK506 can rescue the maintenance defect of mushroom spines in the PS1-M146V KI mouse model (Zhang et al. 2015). Rozkalne also reported a 14% reduction in spine density that was effectively rescued with FK506 treatment in APP/PS1 mice (Rozkalne et al. 2011). These data and our research provide strong evidence that FK506 has a long-lasting effect on synaptic plasticity and can improve cognitive performance.
The aggregation of hyperphosphorylated tau proteins leads to the formation of NFTs, which is one of the characteristic changes in the brain of AD patients. In our study, FK506 attenuated hyperphosphorylation of tau at Ser396 and Ser404 sites in 3 × Tg-AD mice. Some studies are similar to our results, such as CaN inhibitors reduce CaN activity in SH-SY5Y cells, resulting in a corresponding increase in extracellular p-tau181 (Karch et al. 2013). But there is also study that contradict our results, for example, Luo et al. found that the injection of different doses of FK506 into the left ventricle of mice resulted in abnormal hyperphosphorylation of tau at multiple phosphorylation sites but did not affect the total amount of tau protein (Luo et al. 2008). The different effects of FK506 on tau protein may be caused by different experimental methods, such as variations in the drug dose, site of administration, and animal model. This difference highlights the need for further research.
Currently, five drugs approved by FDA for the treatment of AD—donepezil, rivastigmine, galantamine, memantine and memantine combined with donepezil—temporarily treat Alzheimer’s symptoms but do not change the underlying brain changes of Alzheimer’s or alter the course of the disease (Gaugler et al. 2022). The sixth drug, aducanumab, addressing the underlying biology of Alzheimer’s disease rather than the symptoms, is not appropriate for all individuals living with Alzheimer’s disease (Cummings et al. 2021). Thus, it is a matter of urgency to develop or identify treatments that can effectively target the pathological changes in AD and suitable for more AD patients and safe. The present study showed FK506 via intraperitoneal injection for 14 days rescue spatial memory impairment in 3 × Tg-AD mice which not accompanied by any negative effects. A study also has shown that the administration of FK506, over 1 year prevents age- and AD-associated microstructural changes in the hippocampus, parahippocampal cortex, and prefrontal cortex of the middle-aged beagle brain, with no noticeable adverse effects (Radhakrishnan et al. 2021). This suggest that FK506 would be a potential route for therapeutic intervention in AD. However, a double-blind placebo control human clinical trial remains to be carried out to learn the actual therapeutic potential of FK506. The further mechanism of FK506, its impact on Aβ and its efficacy in combination with other drugs are all directions that can be further studied in the future.
Conclusion
In summary, we found that intraperitoneal injections of FK506 can prevent learning and memory impairments and reduce synaptic plasticity dysfunction in 3 × Tg-AD mice, reduce tau hyperphosphorylation. Our findings encourage further consideration of calcineurin inhibition as a pharmacological strategy to ameliorate cognitive deficits in AD.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its additional files, or from the authors upon request.
References
Al-Onaizi M, Al-Sarraf A, Braysh K, Kazem F, Hetal A-H (2022) Impaired spatial navigation and age-dependent hippocampal synaptic dysfunction are associated with chronic inflammatory response in db/db mice. Eur J Neurosci 56(11):6003–6021. https://doi.org/10.1111/ejn.15835
Amit K, Nirmal S (2017) Calcineurin inhibitors improve memory loss and neuropathological changes in mouse model of dementia. Pharmacol Biochem Behav 153:147–159. https://doi.org/10.1016/j.pbb.2016.12.018
Chen QS, Wei WZ, Shimahara T, Xie CW (2002) Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem 77(3):354–371. https://doi.org/10.1006/nlme.2001.4034
Cummings J, Aisen P, Apostolova LG, Atri A, Setal S (2021) Aducanumab: appropriate use recommendations. The Journal of Prevention of Alzheimer’s Disease 8(4):398–410. https://doi.org/10.14283/jpad.2021.41
Demuro A, Mina E, Kayed R, Milton SC, Ietal P (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280(17):17294–17300. https://doi.org/10.1074/jbc.M500997200
Dineley KT, Hogan D, Zhang WR, Taglialatela G (2007) Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem 88(2):217–224. https://doi.org/10.1016/j.nlm.2007.03.010
Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W et al (2010) Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res 88(13):2923–2932. https://doi.org/10.1002/jnr.22445
Gaugler J, James BT, Reimer J, Solis M, Jetal W (2022) Alzheimer’s disease facts and figures. Alzheimers Dementia 18(4):700–789. https://doi.org/10.1002/alz.12638
Guedes-Dias P, Holzbaur ELF (2019) Axonal transport: driving synaptic function. Science 366(6462):eaaw 9997. https://doi.org/10.1126/science.aaw9997
Jung JA, Yoon YJ (2020) Development of non-immunosuppressive FK506 derivatives as antifungal and neurotrophic agents. J Microbiol Biotechnol 30(1):1–10. https://doi.org/10.4014/jmb.1911.11008
Juvvadi PR, Bobay BG, Gobeil SMC, Cole DC, Venters RA et al (2020) FKBP12 dimerization mutations effect FK506 binding and differentially alter calcineurin inhibition in the human pathogen aspergillus fumigatus. Biochem Biophys Res Commun 526(1):48–54. https://doi.org/10.1016/j.bbrc.2020.03.062
Karch CM, Jeng AT, Goate AM (2013) Calcium phosphatase calcineurin influences tau metabolism. Neurobiol Aging 34(2):374–386. https://doi.org/10.1016/j.neurobiolaging.2012.05.003
Liu B, Kou J, Li F, Huo D, Xu J et al (2020) Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging (albany NY) 12(9):8622–8639. https://doi.org/10.18632/aging.103179
Liu J, Si Z, Li S, Huang Z, He Y et al (2018) The calcineurin inhibitor FK506 prevents cognitive impairment by inhibiting reactive astrogliosis in pilocarpine-induced status epilepticus rats. Front Cell Neurosci 11:428. https://doi.org/10.3389/fncel.2017.00428
Luo J, Ma J, Da-Yu Y, Fan B, Zhang W et al (2008) Infusion of FK506, a specific inhibitor of calcineurin, induces potent tau hyperphosphorylation in mouse brain. Brain Res Bull 76(5):464–468. https://doi.org/10.1016/j.brainresbull.2007.12.00
McLeod F, Boyle K, Marzo A, Martin-Flores N, Moe TZ et al (2020) Wnt signaling through nitric oxide synthase promotes the formation of multi-innervated Spines. Front Synaptic Neurosci 12:575863. https://doi.org/10.3389/fnsyn.2020.575863
Miller EC, Teravskis PJ, Dummer BW, Zhao X, Huganir RL et al (2014) Tau phosphorylation and tau mislocalization mediate soluble Aβ oligomer-induced AMPA glutamate receptor signaling deficits. Eur J Neurosci 39(7):1214–1224. https://doi.org/10.1111/ejn.12507
Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V et al (2005) Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and alzheimer’s models. J Neurosci 25(8):4649–4658. https://doi.org/10.1523/JNEUROSCI.0365-05.2005
Parlakpinar H, Gunata M (2021) Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol 43(6):651–665. https://doi.org/10.1080/08923973.2021.1966033
Radhakrishnan H, Ubele MF, Krumholz SM, Boaz K, Mefford JL et al (2021) Tacrolimus protects against age-associated microstructural changes in the beagle brain. J Neurosci 41(23):5124–5133. https://doi.org/10.1523/JNEUROSCI.0361-21.2021
Rajput MS, Nirmal NP, Rathore D, Dahima R (2020) Dimethyl fumarate exerts neuroprotection by modulating calcineurin/NFAT1 and NFκB dependent BACE1 activity in Aβ1-42 treated neuroblastoma SH-SY5Y cells. Brain Res Bull 165:97–107. https://doi.org/10.1016/j.brainresbull.2020.08.024
Rozkalne A, Hyman BT, Spires-Jones TL (2011) Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing alzheimer model mice. Neurobiol Dis 41(3):650–654. https://doi.org/10.1016/j.nbd.2010.11.014
Saraf J, Bhattacharya P, Kalia K, Borah A, Sarmah D et al (2018) A friend or foe: calcineurin across the gamut of neurological disorders. ACS Cent Sci 4(7):805–819. https://doi.org/10.1021/acscentsci.8b00230
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G et al (2021) Alzheimer’s disease. Lancet 397(10284):1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ et al (2007) Natural oligomers of the alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27(11):2866–2875. https://doi.org/10.1523/JNEUROSCI.4970-06.2007
Stallings NR, O’Neal MA, Hu J, Kavalali ET, Bezprozvanny I et al (2018) Pin1 mediates A beta (42)-induced dendritic spine loss. Sci Signal 11(522):eaap8734. https://doi.org/10.1126/scisignal.aap8734
Stroebel D, Mony L, Paoletti P (2021) Glycine agonism in ionotropic glutamate receptors. Neuropharmacology 193:108631. https://doi.org/10.1016/j.neuropharm.2021.108631
Taglialatela G, Hogan D, Zhang WR, Dineley KT (2009) Intermediate and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res 200(1):95–99. https://doi.org/10.1016/j.bbr.2008.12.034
Taglialatela G, Rastellini C, Cicalese L (2015) Reduced incidence of dementia in solid organ transplant patients treated with calcineurin inhibitors. J Alzheimers Dis 47(2):329–333. https://doi.org/10.3233/JAD-150065
Tucker ES, Ibrahim R, Kakodkar R, Kreiling JA, Creton R (2021) A zebrafish model for calcineurin-dependent brain function. Behav Brain Res 416:113544. https://doi.org/10.1016/j.bbr.2021.113544
Umar T, Meena R, Mustehasan KP, Khan AA (2022) Recent updates in the development of small molecules as potential clinical candidates for alzheimer’s disease: a review. Chem Biol Drug Des 100(5):674–681. https://doi.org/10.1111/cbdd.14133
Wang T, Bai Y, Zheng X, Liu X, Xing S et al (2023) Sapap4 deficiency leads to postsynaptic defects and abnormal behaviors relevant to hyperkinetic neuropsychiatric disorder in mice. Cereb Cortex 33(4):1104–1118. https://doi.org/10.1093/cercor/bhac123
Wong LW, Wang Z, Ang SRX, Sajikumar S (2022) Fading memories in aging and neurodegeneration: is p75 neurotrophin receptor a culprit? Ageing Res Rev 75:101567. https://doi.org/10.1016/j.arr.2022.101567
Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A et al (2010) Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci 30(7):2636–2649. https://doi.org/10.1523/JNEUROSCI.4456-09.2010
Yan W, Guo T, Liu N, Cui X, Wei X et al (2023) Erythropoietin ameliorates cognitive deficits by improving hippocampal and synaptic damage in streptozotocin-induced diabetic mice. Cell Signal 106:110614. https://doi.org/10.1016/j.cellsig.2023.110614
Yu TW, Lane HY, Lin CH (2021) Novel therapeutic approaches for alzheimer’s disease: an updated review. Int J Mol Sci 22(15):8208. https://doi.org/10.3390/ijms22158208
Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E et al (2015) Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of alzheimer’s disease. J Alzheimers Dis 45(2):561–580. https://doi.org/10.3233/JAD-142427
Zhao WQ, Santini F, Breese R, Ross D, Zhang XD et al (2010) Inhibition of calcineurin-mediated endocytosis and alphaamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 285(10):7619–7632. https://doi.org/10.1074/jbc.M109.057182
Acknowledgements
This work was supported by The Foundation of Hubei Science & Technology Department (2022CFB974) to Dong-Sheng Sun.
Author information
Authors and Affiliations
Contributions
This study was initiated and designed by QF; QF directed and coordinated the study; JZ and XFH performed major animal behavior studies, Western blotting, and immunohistochemistry; DSS and JZM performed brain slice electrophysiology recordings, brain Golgi staining, dendritic morphology analysis, and collected and analyzed the data; XYH helped to collect and analyze the data; JZM performed immunohistochemistry, helped to interpret the results, and commented on the manuscript; and QF and JZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no financial or non-financial confict of interest.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, J., Hu, XF., Sun, DS. et al. Alzheimer-like behavior and synaptic dysfunction in 3 × Tg-AD mice are reversed with calcineurin inhibition. Exp Brain Res 242, 1507–1515 (2024). https://doi.org/10.1007/s00221-024-06841-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-024-06841-8