Abstract
Induced pluripotent stem (iPS) cells can differentiate into nearly all types of cells. In contrast to embryonic stem cells, iPS cells are not subject to immune rejection because they are derived from a patient’s own cells without ethical concerns. These cells can be used in regenerative medical techniques, stem cell therapy, disease modelling and drug discovery investigations. However, this application faces many challenges, such as low efficiency, slow generation time, partially reprogrammed colonies and tumourigenicity. Numerous techniques have been formulated in the past decade to improve reprogramming efficiency and safety, including the use of different transcription factors, small molecule compounds and non-coding RNAs. Recently, microRNAs (miRNAs) were found to promote the generation and differentiation of iPS cells. The miRNAs can more effectively and safely generate iPS cells than transcription factors. This process ultimately leads to the development of iPSC-based therapeutics for future clinical applications. In this comprehensive review, we summarise advances in research and the application of iPS cells, as well as recent progress in the use of miRNAs for iPS cell generation and differentiation. We examine possible clinical applications, especially in cardiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spectrum of diseases worldwide is mainly composed of non-communicable diseases (NCDs), which account for 68% of global deaths in 2012. Cardiovascular diseases (CVD) are thought to be the most common cause of death worldwide in 2013, during which approximately 17.3 million (32%) of 54 million total deaths were related to CVDs. In 2013, an estimated 8.56 million people suffered from acute myocardial infarction (MI) [1]. The incidence of coronary heart disease (CHD) increased in recent years. In 2004, the World Health Organization (WHO) estimated 652,000 CHD cases in China, and almost 400,000 patients died from this disease [2]. Even though CHD can be treated by drugs and/or surgical operations, these treatments have limited curative effects. Induced pluripotent stem (iPS) cells have been extensively applied in regenerative medicine. These cells are advantageous because of their simplicity and reproducibility and have shown considerable potential for NCD therapy, especially heart disease therapy [3].
In general, cells can only differentiate unidirectionally. After the discovery of cellular reprogramming by nuclear transfer, cells have also been found to differentiate bilaterally [4]. However, this technique has many disadvantages, including ethical controversies and complex operations, which hamper its extensive application to clinical therapy and fundamental studies. In 2006, Yamanaka et al. reported that terminally differentiated cells could be reprogrammed by defined factors, including OSKM (OCT4, SOX2, KLF4 and c-Myc) [5]. This process avoided ethical controversy and has facilitated the development of cellular programming in regenerative medicine [6, 7].However, this method has drawbacks that result from low efficiency and tumourigenicity [8].
Numerous studies have developed various novel strategies to enhance the efficiency of reprogramming and/or reduce tumourigenicity. These strategies include the use of different transcription factors [9], chemical inhibitors [10], genetic factors [11], microRNAs (miRNAs) [12] and signalling molecules [13]. Small non-coding RNAs play an important role in regulating reprogramming by enhancing iPS cell generation and differentiation [14, 15].Additionally, miRNAs regulate the self-renewal, differentiation, proliferation, senescence, migration, pluripotency and survival of mesenchymal stem cells [16]. Considerable advances in the field of iPS cells have been achieved in the past decade. Numerous studies have shown the successful application of various combinations of miRNAs and delivery methods to reprogramme diverse cell lines [15, 17]. Safe and efficient reprogramming methods are currently being explored for clinical applications.
Overview of iPS Cells
IPS cell technology has been developed for more than ten years. In 2006, Takahashi et al. showed that iPS cells can be generated from mouse fibroblasts with specific factors (OSKM) (Fig. 1), and their characteristics are similar to those of embryonic stem cells (ESCs) [5]. In 2007, Thomson et al. generated human iPS cells by introducing different factors, including OSNL (OCT4, SOX2, NANOG and LIN28) [18]. In October 2012, Yamanaka and Gurdon were awarded the Nobel Prize in Physiology or Medicine for their outstanding contribution to the reprogramming of adult cells to an embryonic-like state [19].
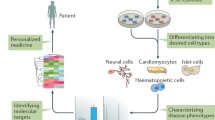
Somatic cells are reprogrammed with the help of OCT4, SOX2, KLF4 and c-Myc (OSKM) to generate iPS cells, which can differentiate into nearly all types of cells, including cardiomyocytes, blood cells, fat cells and neurons. The iPS cells can be used for regenerative medical techniques, disease modelling, cell therapy and drug discovery
In 2014, the first human trial of an iPS cell-based therapy was conducted, in which a retinal pigment epithelium (RPE) layer was implanted in the right eye of a 70-year-old female patient. The therapy stopped macular degeneration in the patient and significantly restored her vision. However, Yamanaka et al. found two small gene mutations in the patients with iPS and RPE cells in the laboratory during the preparation for the second clinical trial. Thus, for security reasons, Yamanaka advised Takahashi to defer the second trial. Consequently, numerous related studies have also been suspended [20, 21]. In 2016, RIKEN from Japan announced the resumption of the preclinical trial for retina iPS cells. In March 2017, Mandai et al. reported that the transplanted sheet remained intact after one year of autologous iPS cell-derived RPE cell sheet transplantation, and the best corrected visual acuity did not improve or worsen, although cystic macular oedema remained [22]. Another opposed report showed that three patients developed severe bilateral visual loss after receiving intravitreal injection of autologous adipose tissue-derived “stem cells” in a clinic in the USA [23]. Currently, iPS cell technology is safe and effective in promoting the specific differentiation of iPS cells, such as cardiomyocytes [24], pancreatic β-cells [25], functional hepatocyte-like cells [26] and chondrocytes [27]. The iPS cells can also differentiate into dopaminergic neurons [28]. Thus, these cells can be used for clinical treatments and basic research.
Challenges and the Development of iPS Cells
The traditional approach is to reprogramme somatic cells into iPS cells. However, this approach faces many challenges; efficiency and security are the two most prominent issues with iPS cells. Cells reprogrammed using the traditional method exhibit very low success rate. That is, only a small proportion of cells become iPS cells (less than 1%) [29]. Conventional methods for genomic insertion integrate transcription factors into the genome. This process limits the use of transcription factor methods because the target cell genomes inserted with foreign genes are at risk of mutations [30].The reprogramming factor c-Myc is an oncogene that has a potential carcinogenic risk. The other three transcription factors, OCT4, SOX2 and KLF4, are also highly expressed in various types of cancers [31, 33]. Additionally, epigenetic aberrations or mutations in donor cells will be carried to the iPS cells and its derived tissues, thereby leading to increased tumourigenicity, dysfunction and significant safety problems [34, 35].Moreover, the incomplete reprogramming and the selection of source cells also cannot be ignored.
Several methods have been proposed to improve the efficiency and quality of iPS cells. These techniques include the use of different transcription factors [36], small molecules [37] and non-coding RNAs [38] and improvements to delivery methods and culturing conditions (Fig. 2) [37]. Although many new methods can replace the transcription factor method, Oct4 is usually irreplaceable [39]. Currently, the efficiency of reprogramming has increased by more than 100-fold [40, 41], and this process can occur without c-Myc [42, 43]. For example, reprogramming may be performed using only miRNAs without transcription factors [44]. This process reduces the risk of tumourigenicity, which is beneficial for patients. However, the reprogramming efficiency is decreased in the absence of c-Myc. Recent studies have shown that neural stem cells only require one additional factor (Oct4) for successful reprogramming [45]. Numerous human somatic cell types have been successfully reprogrammed. However, reprogramming efficiencies and dynamics vary amongst somatic cell types, and excellent cell sources should be obtained easily, suitable for reprogramming and highly efficient [46].
Regulation of miRNAs in iPS Cells
MiRNAs in iPS Cell Generation
MiRNAs are endogenous, small non-coding RNAs. Their production is successive through a multi-step process (Fig. 3). The mature miRNA is approximately 22 nucleotides (nts) in length and can regulate the expression of target genes at the post-transcriptional level. MiRNAs participate in a number of biological events, such as cell proliferation, differentiation and death. In mammals, miRNAs are involved in the early maturation of embryos, stem cell differentiation and apoptosis [47,48,49]. Numerous studies have suggested that regulating miRNAs can remarkably improve the efficiency of iPS cell generation (Fig. 4). In 2009, Judson et al. reported that miRNA-291-3p, miRNA-294, miRNA-295 and miRNA-302d enhance mouse iPS cell generation with three transcription factors (Oct4, Sox2 and Klf4); miRNA-291-3p, miRNA-294 and miRNA-295 belong to the miRNA-290 cluster and are amongst the most highly expressed miRNAs in ESCs [29].
The miRNA genes are transcribed by RNA polymerase II (pol II) to generate the primary transcripts (pri-miRNAs). The initiation step is mediated by the Drosha–DGCR8 complex. The product of this nuclear processing step is an ~ 70-nucleotide (nt) pre-miRNA, which possesses a short stem and an ~ 2-nucleotide 3′-overhang. This structure might serve as a signature motif recognized by nuclear export factor exportin-5. Precursor miRNAs (pre-miRNA) are exported from the nucleus by exportin5 (EXPO5). Subsequently, cytoplasmic RNase III Dicer triggers the second processing step to produce miRNA duplexes. Then, the duplexes are separated. One strand is usually selected as the mature miRNA, and is loaded into the RNA-induced silencing complex. MiRNAs function through the degradation of protein-coding transcripts (perfect complementarity with the 3′-UTR of the target mRNAs) or translational repression (imperfect complementarity between the miRNAs and 3′ -UTR regions of the target mRNAs)
In contrast to miR-195, miR-138 and miR-302/367 promote iPS cell generation. MiR-21, miR-211, miR-155, miR-1, miR-199a, miR-199b and miR-449a promote differentiation, whereas miR-495 inhibits differentiation. The miR-302/367 cluster without any transcription factors can directly reprogramme somatic cells into pluripotent stem cells
A previous study reported that miR-138 dramatically enhances the efficiency of iPS cell generation using a combination of Sox2, Oct4 and Klf4 with or without c-Myc. MiR-138 directly targets the 3′-untranslated region of p53, thereby down-regulating the expression of p53 and its downstream genes [50].P53 plays a critical role in inhibiting iPS cell production [51]. Notably, p53 is also a tumour suppressor gene, and its inhibition leads to a significant increase in tumourigenicity. Although p53 directly regulates hundreds of target genes, p21 and miR-34 are major downstream targets that are important for the synergistic inhibition of iPS cell production [52].MiR-34 belongs to an evolutionarily conserved family, in which three mammalian homologues, namely, miR-34a, miR-34b and miR-34c, are localized in two different genomic loci, miR-34a and miR-34b/c [53]. As a p53 transcription target,miR-34 overexpression leads to cell cycle arrest or apoptosis [54]. MiR-34 can regulate p53 downstream activity by inhibiting specific target genes including cyclin D1, cyclin E2, Cdk4, Cdk6, Bcl2 and c-Met. MiR-34a hinders somatic cells reprogramming, at least partially, by inhibiting pluripotency genes, including Sox2, Nanog and Mycn. Moreover, miR-34b and miR-34c also inhibit reprogramming, and all three miR-34 s show synergy in this process. MiR-34a deficiency in mice significantly enhances reprogramming efficiency and kinetics. Compared with a P53 deficiency, the genetic ablation of miR-34a promotes iPS cell production without affecting self-renewal or differentiation [52]. ESCs and iPS cells can generate all embryonic cell lineages but rarely produce extra-embryonic cell types. A recent study showed that a miR-34a deficiency strongly induces MuERV-L (MERVL) endogenous retroviruses and significantly enhances the efficiency of iPS cell generation by targeting the transcription factor GATA binding protein 2 (Gata2). MiR-34a deficiency also expands the developmental potential of mouse pluripotent stem cells to yield both embryonic and extra-embryonic lineages [55].
Introducing miR-93 (or 106b) into the mouse embryonic fibroblasts can also promote the generation of iPS cells [56]. In comparison with scrambled miRNAs, a miR-195 blockade significantly increased the reprogramming efficiency (2.2-fold increase) of old skeletal myoblasts (SkMs). Intriguingly, anti-miR-195 transduction does not alter the pluripotency marker expression. IPS cells from old SkMs transduced with anti-miR-195 successfully form embryoid bodies that spontaneously differentiated into three germ layers. The potential target of miR-195 may be Sirtuin 1 (SIRT1). MiR-195 inhibition might up-regulate SIRT1 expression, thereby enhancing the reprogramming efficiency. A new strategy is introduced, in which highly efficient iPS cells may be produced from ageing donor subjects by blocking age-induced miR-195. Thus, this process has the potential for the autologous transplantation of iPS cells in elderly patients [57]. Ambasudhan et al. reported that miR-124 in combination with two transcription factors, POU class 3 homeobox 2(POU3F2, also known as BRN2)and myelin transcription factor 1 like (MYT1L), can directly reprogramme postnatal and adult primary skin fibroblasts into functional neurons [58].
MiRNA Alone-mediated Reprogramming
MiRNAs alone can more effectively and safely generate iPS cells than transcription factors. The sequences of miR-302/367 are highly conserved across species. The cluster contains five different miRNAs, including miR-302a/b/c/d and miR-367 [59]. Using this method, researchers found that mouse and human somatic cells can be reprogrammed to iPS cells without the participation of exogenous transcription factors such as OSKM. Additionally, the efficiency of this method is two orders of magnitude higher than that of the standard OSKM-mediated approach. MiR-367 is required for miR-302/367-mediated reprogramming and activation of Oct4 gene expression; it also inhibits histone deacetylase 2 (Hdac2). Histone deacetylase (Hdac) inhibition can enhance OSKM reprogramming, and low levels of Hdac2 or Hdac2 inhibition is required for efficient pluripotent stem cell reprogramming by miR-302/367 [17]. MiR-302 reportedly inhibits the transcription factor nuclear receptor subfamily 2 group F member 2 (NR2F2) and increases reprogramming efficiency through indirect positive regulation of OCT4 [60, 61].
A previous study found that reprogramming using miRNA-302 markedly enhanced drug sensitivity in hepatocarcinoma cells. MiR-302 induced histone 3 lysine 4 (H3K4) methylation by down-regulating amine oxidase flavin containing domain protein 2 (AOF2), thereby increasing c-Myc repression, apoptosis and sensitization to drugs [62]. Moreover, miRNA-302 combined with a chemically defined media could promote human adult hepatocytes to be reprogrammed into islet-like cells [63]. Miyoshi et al. reprogrammed mouse and human fibroblasts into iPS cells by direct transfection of miR-200c plus the miR-302 and miR-369 families [64].Thus, miRNA-based reprogramming does not require vector-mediated gene transfer, thereby indicating its potential application in regenerative medicine.
Role of miRNAs in iPS Cell Differentiation
The specific differentiation of iPS cells can be better achieved using miRNAs. In humans, miR-375 is important for pancreatic endocrine function, and its inactivation leads to impaired glucose balance, increased α-cell mass and reduced β-cell fraction. Both miR-375 and miR-186 play critical roles in the differentiation of iPS cells into insulin-like cell clusters. By overexpressing miR-186 and miR-375 via chemical transfection, iPS cells are differentiated into insulin-secreting β-like cells. These cells can express pancreatic endocrine-related genes, such as PDX1, PAX4, PAX6, KIR6.2, NKX6.1, NGN3 and GLUT2. Although the amount of secreted insulin is lower than that of adult human β-cells, transplantation of these islet β-cells into diabetic mice can normalize blood glucose levels [65].
Age-related macular degeneration is the main cause of blindness in developed countries and the third leading cause of blindness worldwide [61]. MiR-184, which is located at 15q25.1, is an evolutionarily conserved non-coding RNA oligonucleotide [66]. MiR-184 expression is up-regulated during differentiation from human induced pluripotent stem cells (hiPSCs) to RPE cells. MiR-184 overexpression promotes RPE differentiation by inhibiting the protein kinase B β (PKB β, also known as Akt2)/mammalian target of rapamycin (mTOR) signalling pathway and miR-184 dysfunction plays a key role in the pathogenesis of age-related macular degeneration [67]. AKT2 is homologue 2 of the v-akt oncogene, which is a main downstream effector of the phosphatidylinositol 3′ kinase pathway, that can activate the mTOR pathway [68]. Furthermore, miR-449a up-regulates Runx2 expression by binding to the 3′-UTR of HDAC1, thereby maintaining the histone acetylation status and stimulating the differentiation of human iPS cells into osteoblasts [69]. Runx2, a runt domain family protein, is essential for osteoblast differentiation and bone formation. Runx2 regulates the expression of osteoblast-specific genes, including alkaline phosphatase (ALP), type I collagen and osteocalcin [70]. Ozeki et al. showed that miR-211 up-regulated autophagy-related gene (Atg14) expression in osteoblast-like cells, thereby leading to the enhanced differentiation of iPS cells [71].
In addition to humans and mice, pigs are a good source of iPS cells. The organ size, immunology and physiology of pigs are similar to those of humans. Porcine cells can be used in studies on heredity and breeding, as animal models of human disease and xenotransplantation [72].The overexpression of miR-302a, miR-302b and miR-200c can enhance the reprogramming efficiency and reduce the induction time of porcine iPS cells (piPSCs) in OSKM or OSK induction systems. The reprogramming efficiency of piPSCs using miR-302a, miR-302b and miR-200c is equivalent to OSKM, which is more efficient than OSK and reduces the tumourigenicity of piPSCs caused by the lack of c-Myc [73]. Moreover, miR-720 can promote the differentiation of dental pulp stem/progenitor cells by repressing the stem cell marker NANOG [74].
Regulation of miRNAs in iPS Cells in Heart Disease
CVD is considered the leading cause of death across the world. CVD is associated with loss of myocardial cells, such as myocardial infarction. Cardiomyocytes are permanent cells with very poor division potential. Given that drug therapy has limited efficacy in the treatment of myocardial injury-associated heart diseases, such as myocardial infarction and cardiomyopathy, developing new strategies to treat these disorders is essential. IPS cells exhibit new prospects for CVD treatment. MiRNAs are closely involved in the maintenance, proliferation, differentiation and reprogramming of stem cells. One miRNA generally targets several genes, and a single gene may be regulated by several miRNAs [75].
As a highly conserved miRNA among different species, miR-199a inhibits the differentiation of iPS cells into smooth muscle cells by targeting SIRT1 [76]. Notably, that miR-199a expression is up-regulated during endothelial cell (EC) differentiation, especially in the later stages of this process. MiR-199a can induce the differentiation of iPS cells into ECs by targeting SIRT1 directly or indirectly. SIRT1 is a member of the NAD+-dependent class III group of histone deacetylases and the Silencing Information Regulatory Protein family. SIRT1 protein is highly expressed in ESCs, and its transient overexpression enhances the efficiency of producing iPS cells derived from mouse embryonic fibroblasts through the miR-34a-SIRT1-p53 pathway [77,78,79]. SIRT1 protein is a critical mediator in the regulation of various developmental genes during stem cell differentiation [80] and is important in the differentiation of various cells, including endothelial progenitor cells [81].
MiR-199b can direct iPS cell differentiation towards endothelial lineages by regulating critical signalling angiogenic responses. MiR-199b modulates vascular cell fate by targeting the Notch ligand JAG1, thereby resulting in vascular endothelial growth factor (VEGF) transcriptional activation and secretion through the transcription factor Signal transducer and activator of transcription 3 (STAT3) [82]. Notch signalling is essential for vascular development, homeostasis and angiogenesis; however, the molecular basis for its upstream regulation remains ambiguous [83]. During the differentiation of iPS cells into ECs, VEGF-induced miR-155 promotes endothelial angiogenesis via direct silencing of the E2F transcription factor 2 (E2F2) [84]. The transcription factor E2F2, an important member of the E2F family, participates in cell proliferation, apoptosis and death [85].
Anti-angiogenic miR-495 belongs to the Dlk1-Dio3 miRNA cluster. MiR-495 is highly expressed in the non-EC fraction, but it is down-regulated in the EC fraction. This miRNA mediates the expression of endothelial or angiogenic genes by directly targeting vascular endothelial zinc finger 1 (VEZF1). VEZF1 is an important transcription factor that can regulate EC differentiation and angiogenesis [86]. MiR-495 inhibition promotes EC generation from iPS cells and enhances angiogenesis and engraftment of hiPSCs due to increased VEZF1 expression. MiR-495 inhibition upregulates VEZF1 in hiPSCs and then activates downstream EC genes (such as insulin-like growth factor 1 (IGF1) and cluster of differentiation 31 (CD31)) by occupying their promoter regions. After transplantation in immunodeficient MI mice derived ECs significantly increase neovascularization in the infarcted heart, prevent functional deterioration and inhibit infarct size expansion [87].
Overexpression of miR-21 can activate the Akt /TGF-β2 signalling pathway by directly targeting phosphatase and tensin homologue deleted on chromosome ten (PTEN), thereby increasing the differentiation of iPS cells into ECs. MiR-21 overexpression increases the mRNA and protein levels of transforming growth factor-β2 (TGF-β2). TGF-β2 knockdown or neutralization by its antibody inhibits miR-21-induced EC marker expression of targets such as VE-cadherin (VE-cad) and CD31 [88]. TGF-β is a multifunctional cytokine that regulates the proliferation, differentiation, migration and survival of multiple cell types. Deficiency or mutations of the TGF-β gene causes vascular remodelling disorders and absence of mural cell formation, thereby leading to severe vascular diseases [89]. Upon binding to transforming growth factor-beta receptor type 1 (TGF-RI), TGF-β stimulates the phosphorylation of the SMAD family (SMAD2/3), thereby inhibiting lumen formation, proliferation and migration of ECs [90]. SMAD2/3 is a downstream effector of activin/Nodal signalling that plays a significant role in maintaining vascular integrity through regulation of VE-cadherin, N-cadherin and sphingosine-1-phosphate receptor-1 (S1PR1) expression [91, 92].
Resveratrol (RSV), a natural polyphenol, protects heart tissue from damage and exhibits anti-inflammatory, anti-oxidative, anti-aging and anti-cancer properties. A recent study has shown that RSV can promote the differentiation of human iPS cells into myocardial cells by inhibiting the classical Wnt signalling pathway and enhancing the SRF-miR-1 axis [93].
The miR-149, miR-125a-5p, miR- 27b, miR-296-5p, miR-181a, miR-100 and miR-137 are up-regulated in both human embryonic stem cell (hESC)-derived endothelial cells (hESC-ECs) and hiPSC-derived endothelial cells (hiPSC-ECs) during endothelial differentiation [94]. Jayawardena et al. described the use of miRNAs to reprogramme cardiac fibroblasts directly into cardiomyocytes both in vivo and in vitro. MiR-1 promotes cardiac differentiation of human iPS cells by suppressing the Wnt and fibroblast growth factor(FGF)pathways and directly targets frizzled class receptor 7 (FZD7) and fibroblast growth factor receptor substrate 2 (FRS2) [95]. FZD7 transduces extracellular signals into the cytoplasm to activate the canonical Wnt pathway [96]. FRS2 is a lipid-anchored Grb2-binding protein that is important in signal transduction from FGF receptors to the Ras/mitogen-activated protein kinase (MAPK) signalling pathway [97]. Furthermore, miR-1 alone can induce cardiac reprogramming, but its effects are significantly enhanced when combined with miR-133, miR-208 and miR-499 [98].
Conclusion and Perspectives
Chronic non-infectious diseases are a major cause of death worldwide. Ischaemic heart diseases, stroke, lower respiratory infections and chronic obstructive lung diseases are the top killers of the past decade. Although drugs and surgical treatments are available, their curative effects are unsatisfactory. Regenerative medicine can provide more effective treatments. The discovery of iPS cells has led to the development of a revolutionary strategy for disease research. Simulation of several human diseases in vitro has been performed to determine the molecular mechanisms underlying a particular pathology and discover new therapeutic avenues. Significant advances in disease mechanisms and treatment have been achieved by combining human iPS cells with other new technologies [83, 99, 100]. However, several important issues still need a resolution. The current regular strategy for iPS cell generation is the ectopic expression of OSKM. The reprogramming process has low overall efficiency; only ∼0.1–1% of cells are fully reprogrammed when viral integration vectors are used [101]. Since the first isolation of human ES cell lines from human blastocysts [4], limitations of this process have been identified, thereby resulting in the development of improved methods to enhance the efficiency of iPS cell generation [102].
MiRNAs are usually associated with a protein complex called the RNA-induced silencing complex; the play a major role in post-transcriptional gene regulation in higher eukaryotes [103]. The application of miRNAs can improve the efficiency of iPS cell generation, as has been shown by a significant increase in the number of iPS cell colonies. Moreover, miRNA-induced pluripotent stem cells (miR-iPSCs) can be derived at a faster rate than cells from traditional reprogramming experiments. Further study of iPS cells has elucidated the mechanism for cellular reprogramming, thereby increasing the efficiency and eliminating tumourigenicity. MiR-iPSCs have been used to study and treat various diseases (Table 1).
Numerous almost pure human cardiomyocytes can be formed, but they still cannot be used as therapeutic agents. The main limitation is the immaturity of cells produced manually in the laboratory [104]. Cardiomyocyte death is a major contributor to CVD. Thus, this type of cell is an important target for designing therapeutic strategies. Stem cell therapy, such as transplantation of iPS cell-derived cardiomyocytes, has emerged as a promising alternative therapeutic avenue for CVD [105]. MiRNAs promote iPSC-based disease therapy, pathology research and drug development by promoting the production and differentiation of iPS cells. A large body of evidence has suggested the involvement of miRNAs in iPS cell generation and differentiation. For example, miR-138 and miR-302/367 promote iPS cell generation, whereas miR-34a and miR-195 inhibit it. MiR-21, miR-211, miR-155, miR-1, miR-184, miR-199a, miR-199b and miR-449 promote differentiation, whereas miR-495 prevents this process. Using only the miR-302/367 cluster or direct transfection of miR-200c plus the miR-302 and miR-369 families without any transcription factors can reprogramme somatic cells into pluripotent stem cells. The promotion or inhibition of related microRNAs can be selected depending on the actual need. Moreover, the target cells of specific miRNAs can be obtained to improve efficiency and safety. MiR-iPSC generation may ultimately become a beneficial technology for biochemical research and clinical regenerative medicine.
References
Benjamin, E. J., Blaha, M. J., Chiuve, S. E., et al. (2017). Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 135, e146–e603.
Zhang, X. H., Lu, Z. L., & Liu, L. (2008). Coronary heart disease in China, Heart, 94, 1126–1131.
Zhang, J., Wilson, G. F., Soerens, A. G., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res, 104, e30–e41.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Maherali, N., & Hochedlinger, K. (2008). Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell, 3, 595–605.
Lo Sardo, V., Ferguson, W., Erikson, G. A., Topol, E. J., Baldwin, K. K., & Torkamani, A. (2017). Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol, 35, 69–74.
Feng, B., Ng, J. H., Heng, J. C., & Ng, H. H. (2009). Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell, 4, 301–312.
Melton, C., Judson, R. L., & Blelloch, R. (2010). Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature, 463, 621-U645.
Zhou, J. X., Su, P., Li, D., Tsang, S., Duan, E. K., & Wang, F. High-Efficiency Induction of Neural Conversion in Human ESCs and Human Induced Pluripotent Stem Cells with a Single Chemical Inhibitor of Transforming Growth Factor Beta Superfamily Receptors, Stem Cells. 28 (2010)1741–1750.
Gong, L., Pan, X., Chen, H., et al. (2016). p53 isoform Delta133p53 promotes efficiency of induced pluripotent stem cells and ensures genomic integrity during reprogramming. Sci. Rep, 6, 37281.
Di Stefano, B., Maffioletti, S. M., Gentner, B., et al. (2011). A microRNA-Based System for Selecting and Maintaining the Pluripotent State in Human Induced Pluripotent Stem Cells. Stem Cells, 29, 1684–1695.
Worringer, K. A., Rand, T. A., Hayashi, Y., et al. (2014). The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell, 14, 40–52.
Subramanyam, D., Lamouille, S., Judson, R. L., et al. (2011). Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol, 29, 443–448.
Li, Z., Yang, C. S., Nakashima, K., & Rana, T. M. (2011). Small RNA-mediated regulation of iPS cell generation. EMBO J, 30, 823–834.
Clark, E. A., Kalomoiris, S., Nolta, J. A., & Fierro, F. A. Concise Review: MicroRNA Function in Multipotent Mesenchymal Stromal Cells, Stem Cells. 32 (2014)1074–1082.
Anokye-Danso, F., Trivedi, C. M., Juhr, D., et al. (2011). Highly Efficient miRNA-Mediated Reprogramming of Mouse and Human Somatic Cells to Pluripotency. Cell Stem Cell, 8, 376–388.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920.
Chen, I. Y., Matsa, E., & Wu, J. C. (2016). Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nature Reviews Cardiology, 13, 333–349.
Reardon, S., & Cyranoski, D. (2014). Japan stem-cell trial stirs envy. Nature, 513, 287–288.
Scudellari, M. (2016). A DECADE OF iPS CELLS. Nature, 534, 310–312.
Mandai, M., Watanabe, A., Kurimoto, Y., et al. (2017). Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration, N. Engl. J. Med, 376, 1038–1046.
Kuriyan, A. E., Albini, T. A., Townsend, J. H., et al. (2017). Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD, N. Engl. J. Med, 376, 1047–1053.
Malan, D., Friedrichs, S., Fleischmann, B. K., & Sasse, P. (2011). Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circ. Res, 109, 841–847.
Micallef, S. J., Li, X., Schiesser, J. V., et al. (2012). INSGFP/w human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia, 55, 694–706.
Mizumoto, H., Matsushita, S., & Kajiwara, T. (2016). Generation Of Functional Hepatocyte-like Cells From Human Induced Pluripotent Stem Cells In A Three-dimensional Culture Using Hollow Fibers. Tissue Engineering Part A, 22, S76-S76.
Zhu, Y. X., Wu, X. M., Liang, Y. H., et al. (2016). Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol, 16, 11.
Soldner, F., Hockemeyer, D., Beard, C., et al. (2009). Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136, 964–977.
Judson, R. L., Babiarz, J. E., Venere, M., & Blelloch, R. (2009). Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat. Biotechnol, 27, 459–461.
Selvaraj, V., Plane, J. M., Williams, A. J., & Deng, W. (2010). Switching cell fate: the remarkable rise of induced pluripotent stem cells and lineage reprogramming technologies. Trends Biotechnol, 28, 214–223.
Tian, Y. Y., Luo, A. P., Cai, Y. R., et al. (2010). MicroRNA-10b Promotes Migration and Invasion through KLF4 in Human Esophageal Cancer Cell Lines. J. Biol. Chem, 285, 7986–7994.
Lambertini, C., Pantano, S., & Dotto, G. P. Differential Control of Notch1 Gene Transcription by Klf4 and Sp3 Transcription Factors in Normal versus Cancer-Derived Keratinocytes., PLoS One. 5 (2010).
Wang, Y. J., Meng, L., Hu, H. Y., et al. (2011). Oct-4B isoform is differentially expressed in breast cancer cells: hypermethylation of regulatory elements of Oct-4A suggests an alternative promoter and transcriptional start site for Oct-4B transcription. Biosci. Rep, 31, 109–115.
Ben-David, U., & Benvenisty, N. (2011). The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer, 11, 268–277.
Lee, A. S., Tang, C., Rao, M. S., Weissman, I. L., & Wu, J. C. (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med, 19, 998–1004.
Xiao, X., Li, N., Zhang, D., Yang, B., Guo, H., & Li, Y. (2016). Generation of Induced Pluripotent Stem Cells with Substitutes for Yamanaka’s Four Transcription Factors. Cell Reprogram, 18, 281–297.
Lian, X. J., Hsiao, C., Wilson, G., et al. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. U. S. A, 109, E1848–E1857.
Shafiee, M., Aleyasin, S. A., Vasei, M., Semnani, S., & Mowla, S. J. (2016). Down-Regulatory Effects of miR-211 on Long Non-Coding RNA SOX2OT and SOX2 Genes in Esophageal Squamous Cell Carcinoma. Cell Journal, 17, 593–600.
Mali, P., Chou, B. K., Yen, J., et al., Butyrate Greatly Enhances Derivation of Human Induced Pluripotent Stem Cells by Promoting Epigenetic Remodeling and the Expression of Pluripotency-Associated Genes, Stem Cells. 28 (2010)713–720.
Huangfu, D. W., Maehr, R., Guo, W. J., et al. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol, 26, 795–797.
Aasen, T., Raya, A., Barrero, M. J., et al. (2008). Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol, 26, 1276–1284.
Deng, W., Cao, X., Chen, J., et al. (2015). MicroRNA Replacing Oncogenic Klf4 and c-Myc for Generating iPS Cells via Cationized Pleurotus eryngii Polysaccharide-based Nanotransfection. ACS Appl Mater Interfaces, 7, 18957–18966.
Nakagawa, M., Koyanagi, M., Tanabe, K., et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol, 26, 101–106.
Bao, X., Zhu, X., Liao, B., et al. (2013). MicroRNAs in somatic cell reprogramming. Curr. Opin. Cell Biol, 25, 208–214.
Kim, J. B., Greber, B., Arauzo-Bravo, M. J., et al., Direct reprogramming of human neural stem cells by OCT4, Nature. 461 (2009)649-U693.
Brouwer, M., Zhou, H., & Nadif Kasri N. (2016). Choices for induction of pluripotency: Recent developments in human induced pluripotent stem cell reprogramming strategies. Stem Cell Rev, 12, 54–72.
Krol, J., Loedige, I., & Filipowicz, W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet, 11, 597–610.
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell, 136, 215–233.
Qu, K., Wang, Z., Lin, X. L., Zhang, K., He, X. L., & Zhang, H. MicroRNAs: Key regulators of endothelial progenitor cell functions., Clinica Chimica Acta. 448 (2015)65–73.
Ye, D., Wang, G. Y., Liu, Y., et al. (2012). miR-138 Promotes Induced Pluripotent Stem Cell Generation through the Regulation of the p53 Signaling (vol 30, pg 1645. Stem Cells, 31, (2013)2585–2586.
Kawamura, T., Suzuki, J., Wang, Y. V., et al. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460, 1140-U1107.
Choi, Y. J., Lin, C. P., Ho, J. J., et al. (2011). miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol, 13, 1353–1360.
He, L., He, X. Y., Lim, L. P., et al. (2007). A microRNA component of the p53 tumour suppressor network. Nature, 447, 1130-U1116.
Chang, T. C., Wentzel, E. A., Kent, O. A., et al. (2007). Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Molecular Cell, 26, 745–752.
Choi, Y. J., Lin, C.-P., Risso, D., et al., Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells., Science (New York). 355 (2017).
Li, Z., & Rana, T. M. Using microRNAs to enhance the generation of induced pluripotent stem cells., Curr. Protoc. Stem Cell Biol. Chapter 4 (2012)Unit 4A 4.
Kondo, H., Kim, H. W., Wang, L., et al. (2016). Blockade of senescence-associated microRNA-195 in aged skeletal muscle cells facilitates reprogramming to produce induced pluripotent stem cells. Aging Cell, 15, 56–66.
Ambasudhan, R., Talantova, M., Coleman, R., et al. (2011). Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell, 9, 113–118.
Card, D. A. G., Hebbar, P. B., Li, L. P., et al. (2008). Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol. Cell. Biol, 28, 6426–6438.
Hu, S. J., Wilson, K. D., Ghosh, Z., et al., MicroRNA-302 Increases Reprogramming Efficiency via Repression of NR2F2, Stem Cells. 31 (2013)259–268.
Kuo, C. H., Deng, J. H., Deng, Q., & Ying, S. Y. (2012). A novel role of miR-302/367 in reprogramming, Biochem. Biophys. Res. Commun, 417, 11–16.
Koga, C., Kobayashi, S., Nagano, H., et al. (2014). Reprogramming Using microRNA-302 Improves Drug Sensitivity in Hepatocellular Carcinoma Cells. Ann. Surg. Oncol, 21, S591-S600.
Lu, J., Dong, H. Y., Lin, L. J., Wang, Q. H., Huang, L. H., & Tan, J. M. (2014). miRNA-302 facilitates reprogramming of human adult hepatocytes into pancreatic islets-like cells in combination with a chemical defined media, Biochem. Biophys. Res. Commun, 453, 405–410.
Miyoshi, N., Ishii, H., Nagano, H., et al. (2011). Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell, 8, 633–638.
Shaer, A., Azarpira, N., & Karimi, M. H. (2014). Differentiation of Human Induced Pluripotent Stem Cells into Insulin-Like Cell Clusters with miR-186 and miR-375 by using chemical transfection. Appl. Biochem. Biotechnol, 174, 242–258.
Nomura, T., Kimura, M., Horii, T., et al. (2008). MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum. Mol. Genet, 17, 1192–1199.
Jiang, C., Qin, B., Liu, G. H., et al. (2016). MicroRNA-184 promotes differentiation of the retinal pigment epithelium by targeting the AKT2/mTOR signaling pathway. Oncotarget, 7, 52340–52353.
Liu, L. L., Lu, S. X., Li, M., et al., FoxD3-regulated microRNA-137 suppresses tumour growth and metastasis in human hepatocellular carcinoma by targeting AKT2, Oncotarget. 5 (2014)5113–5124.
Liu, T., Hou, L., Zhao, Y., & Huang, Y. (2015). Epigenetic silencing of HDAC1 by miR-449a upregulates Runx2 and promotes osteoblast differentiation. Int. J. Mol. Med, 35, 238–246.
Nishimura, R., Wakabayashi, M., Hata, K., et al. (2012). Osterix Regulates Calcification and Degradation of Chondrogenic Matrices through Matrix Metalloproteinase 13 (MMP13) Expression in Association with Transcription Factor Runx2 during Endochondral Ossification. J. Biol. Chem, 287, 33179–33190.
Ozeki, N., Hase, N., Hiyama, T., et al. (2017). MicroRNA-211 and autophagy-related gene 14 signaling regulate osteoblast-like cell differentiation of human induced pluripotent stem cells, Exp. Cell Res, 352, 63–74.
Hall, V. (2008). Porcine embryonic stem cells: a possible source for cell replacement therapy. Stem Cell Rev, 4, 275–282.
Ma, K., Song, G., An, X., et al. (2014). miRNAs promote generation of porcine-induced pluripotent stem cells. Mol. Cell. Biochem, 389, 209–218.
Hara, E. S., Ono, M., Eguchi, T., et al. (2013). miRNA-720 controls stem cell phenotype, proliferation and differentiation of human dental pulp cells. PLoS One, 8, e83545.
Takaya, T., Nishi, H., Horie, T., Ono, K., & Hasegawa, K. (2012). Roles of microRNAs and myocardial cell differentiation. Prog. Mol. Biol. Transl. Sci, 111, 139–152.
Gu, S., & Chan, W. Y. (2012). Flexible and Versatile as a Chameleon-Sophisticated Functions of microRNA-199a. Int. J. Mol. Sci, 13, 8449–8466.
Lee, Y. L., Peng, Q., Fong, S. W., et al. (2012). Sirtuin 1 facilitates generation of induced pluripotent stem cells from mouse embryonic fibroblasts through the miR-34a and p53 pathways. PLoS One, 7, e45633.
Yang, Y., Duan, W. X., Li, Y., et al. (2013). Novel Role of Silent Information Regulator 1 in Myocardial Ischemia. Circulation, 128, 2232–2240.
Li, Z. B., Margariti, A., Wu, Y. T., et al. (2015). MicroRNA-199a induces differentiation of induced pluripotent stem cells into endothelial cells by targeting sirtuin 1. Mol. Med. Report, 12, 3711–3717.
Calvanese, V., Lara, E., Suarez-Alvarez, B., et al. (2010). Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc. Natl. Acad. Sci. U. S. A, 107, 13736–13741.
Cheng, B. B., Yan, Z. Q., Yao, Q. P., et al. (2012). Association of SIRT1 expression with shear stress induced endothelial progenitor cell differentiation. J. Cell. Biochem, 113, 3663–3671.
Chen, T., Margariti, A., Kelaini, S., et al., MicroRNA-199b Modulates Vascular Cell Fate During iPS Cell Differentiation by Targeting the Notch Ligand Jagged1 and Enhancing VEGF Signaling, Stem Cells. 33 (2015)1405–1418.
Suchting, S., Freitas, C., le Noble, F., et al. (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. U. S. A, 104, 3225–3230.
Yang, D., Wang, J., Xiao, M., Zhou, T., & Shi, X. (2016). Role of Mir-155 in Controlling HIF-1alpha Level and Promoting Endothelial Cell Maturation. Sci. Rep, 6, 35316.
Dimova, D. K., & Dyson, N. J. (2005). The E2F transcriptional network: old acquaintances with new faces. Oncogene, 24, 2810–2826.
Zou, Z., Ocaya, P. A., Sun, H., Kuhnert, F., & Stuhlmann, H. (2010). Targeted Vezf1-null mutation impairs vascular structure formation during embryonic stem cell differentiation. Arterioscler. Thromb. Vasc. Biol, 30, 1378–1388.
Liang, J., Huang, W., Cai, W., et al., Inhibition of microRNA-495 Enhances Therapeutic Angiogenesis of Human Induced Pluripotent Stem Cells, Stem Cells. 35 (2017)337–350.
Di Bernardini, E., Campagnolo, P., Margariti, A., et al. (2014). Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor beta2 (TGF-beta2) pathways. J. Biol. Chem, 289, 3383–3393.
Verhamme, F. M., Bracke, K. R., Joos, G. F., & Brusselle, G. G. (2015). Transforming Growth Factor-beta Superfamily in Obstructive Lung Diseases. Am. J. Respir. Cell Mol. Biol, 52, 653–662.
Vargel, O., Zhang, Y., Kosim, K., et al., Activation of the TGF beta pathway impairs endothelial to haematopoietic transition., Sci. Rep. 6 (2016).
Massague, J., Blain, S. W., & Lo, R. S. (2000). TGF beta signaling in growth control, cancer, and heritable disorders. Cell, 103, 295–309.
Itoh, F., Itoh, S., Adachi, T., et al. (2012). Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood, 119, 5320–5328.
Liu, H., Zhang, S., Zhao, L., et al., Resveratrol Enhances Cardiomyocyte Differentiation of Human Induced Pluripotent Stem Cells through Inhibiting Canonical WNT Signal Pathway and Enhancing Serum Response Factor-miR-1 Axis., Stem Cells Int. 2016 (2016)2524092.
Wang, L. N., Su, W. J., Du, W., et al. (2015). Gene and MicroRNA Profiling of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells. Stem Cell Reviews and Reports, 11, 219–227.
Lu, T. Y., Lin, B., Li, Y., et al. (2013). Overexpression of microRNA-1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J. Mol. Cell. Cardiol, 63, 146–154.
Pinson, K. I., Brennan, J., Monkley, S., Avery, B. J., & Skarnes, W. C. (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature, 407, 535–538.
Kouhara, H., Hadari, Y. R., SpivakKroizman, T., et al. (1997). A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell, 89, 693–702.
Jayawardena, T. M., Egemnazarov, B., Finch, E. A., et al. (2012). MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res, 110, 1465–1473.
Itzhaki, I., Maizels, L., Huber, I., et al. (2011). Modelling the long QT syndrome with induced pluripotent stem cells. Nature, 471, 225-U113.
Yazawa, M., Hsueh, B., Jia, X. L., et al., Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome., Nature. 471 (2011)230-U120.
Aoi, T., Yae, K., Nakagawa, M., et al. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 321, 699–702.
Shi, Y., Zhao, Y., & Deng, H. K. (2010). Powering Reprogramming with Vitamin C. Cell Stem Cell, 6, 1–2.
Ameres, S. L., & Zamore, P. D. (2013). Diversifying microRNA sequence and function. Nature Reviews Molecular Cell Biology, 14, 475–488.
Barbuti, A., Benzoni, P., Campostrini, G., & Dell’Era, P. (2016). Human derived cardiomyocytes: A decade of knowledge after the discovery of induced pluripotent stem cells. Dev. Dyn, 245, 1145–1158.
Guo, C., Deng, Y., Liu, J., & Qian, L. (2015). Cardiomyocyte-specific role of miR-24 in promoting cell survival. J. Cell. Mol. Med, 19, 103–112.
Acknowledgements
This study was supported by the Natural Science Foundation of China (No. 81070221; 81600342) and the Innovative Research Team for Science and Technology in Higher Educational Institutions of Hunan Province and the Construct Program of the Key Discipline in Hunan Province (No. 15C1201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zeng, ZL., Lin, Xl., Tan, LL. et al. MicroRNAs: Important Regulators of Induced Pluripotent Stem Cell Generation and Differentiation. Stem Cell Rev and Rep 14, 71–81 (2018). https://doi.org/10.1007/s12015-017-9785-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9785-6