Abstract
Heat stress threatens severely cardiac function by caused myocardial injury in poultry. Our previous study has showed that manganese (Mn) has a beneficial effect on heat-stress resistance of broiler. Therefore, we tried to confirm the alleviation mechanism through proteomic analysis after heat stress exposure to primary broiler myocardial cells pretreated with Mn. The experiment was divided into four groups: CON group (37 °C, cells without any treatment), HS group (43 °C, cells treatment with heat stress for 4 h), HS+MnCl2 group (cells treated with 20 μM MnCl2 before heat stress), and HS+Mn-AA group (cells treated with 20 μM Mn compound amino acid complex before heat stress). Proteome analysis using DIA identified 300 differentially expressed proteins (DEPs) between CON group and HS group; 93 and 121 DEPs were identified in inorganic manganese treatment group and organic manganese treatment group, respectively; in addition, there were 53 DEPs identified between inorganic and organic manganese group. Gene Ontology (GO) analysis showed that DEPs were mainly involved in binding, catalytic activity, response to stimulus, and metabolic process. DEPs of manganese pretreatment involved in a variety of biological regulatory pathways, and significantly influenced protein processing and repair in endoplasmic reticulum, apoptosis, and DNA replication and repair. These all seem to imply that manganese may help to resist cell damage induced by heat stress by regulating key node proteins. These findings contribute to a better understanding of the effects of manganese on overall protein changes during heat-stress and the possible mechanisms, as well as how to better use manganese to protect heart function in high temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High temperature in summer is a difficult problem that is often encountered in the concentrated feeding of poultry, which brings great financial losses. Unlike other livestock, poultry are susceptible to heat stress because they are covered with feathers, have no sweat glands, and have a high basal body temperature. These characteristics make it difficult for poultry to dissipate heat [1]. The continued heat stress causes an increase of body temperature, respiratory rate, heart rate, and body temperature (rectal temperature) of animals, which in turn affects feed intake and production performance of animals [2]. Heat stress decreased feed efficiency and metabolic rate of poultry [3], especially in high-density poultry in captivity, and this not only affects the health and welfare of poultry, but also maybe increase production costs and reduce the profitability of the farming industry [4]. The heart is a highly thermal sensitivity organ. Under heat stress, the respiratory metabolism, energy demand, and blood circulation of poultry were increased rapidly, which aggravate the burden of the heart [5]. Studies have shown that myocardial cell is the early targets of heat stress and myocardial cell damage is a prominent feature of severe heat stroke [6]. Studies have shown that broilers have high mortality rates exposed to heat stress resulted from pathological changes in the heart characterized by the acute degeneration of myocardial cells and the necrosis and rupture of myocardial fibers, which induced heart failure [7]. Cardiomyocytes have limited proliferation capacity as they are terminally differentiated cells. Therefore, inhibiting cardiomyocyte apoptosis is of great significance in maintaining the normal structure and morphology of the heart.
Naturally, a defense system was initiated in poultry to response to the challenge of heat stress. Heat stress induces lipid peroxidation via the accumulation of reactive oxygen species (ROS) [8], which leads to disruptions in the antioxidant mechanism and oxidative damage [9, 10]. Thus, scavenging excess free radicals and reduction of ROS using the antioxidant function of enzymes such as superoxide dismutase (SOD) and catalase (CAT) is one of the main regulatory strategies in response to heat stress [11, 12]. Manganese (Mn) showed a promising anti-heat stress via promoting the activity and gene expression of Mn superoxide dismutase (MnSOD) [13], which maybe achieved through the protein tyrosine kinase (PTK) signal transduction pathway [14]. Our previous research revealed that Mn decreased the content of ROS and the expression levels of heat shock protein 70 (HSP70, a key marker of heat stress) in heat-stressed chick embryonic myocardial cells [15]. Study has shown that exogenous Mn significantly increases the activity and gene expression of MnSOD in heat-stress chicken and even protects embryonic development from heat stress through epigenetic activation of antioxidant and anti-apoptotic abilities [16]. However, protection mechanisms of Mn for poultry under heat stress is complex and perhaps involve numerous regulatory genes and proteins; thus, it is important to understand the molecular mechanisms that operate in response to interaction mechanism of Mn and heat stress at the protein level.
In recent years, the rapid development of proteomics technology, especially quantitative proteomics technology based on mass spectrometry (MS) with high sensitivity and resolution, has been helpful for large-scale studies of gene and cell function at the protein level [17]. Data-independent acquisition (DIA) is a two-stage MS acquisition strategy with continuous and cyclic window acquisition, which collected all fragment ion signals in full coverage [18]. Compared with data-dependent acquisition (DDA) and selected reaction monitoring (SRM), DIA has the advantages of high repeatability and high throughput, then which greatly meets the needs of the analysis of complex protein samples [19], and was widely used in biological and clinical studies [20].
Based on the above, it has been confirmed that Mn could increase the resistibility to heat stress of poultry. However, to our knowledge, it is not well known about the effect of Mn on overall protein changes in heat-stress poultry. Mn might be caused some interesting changes and those changed proteins through Mn may lead to increasing their resistance to heat stress. Thus, in the present study, we analyzed proteome changes in heat-stress primary chick embryonic myocardial cells with and without Mn pretreatment using DIA technology, to explore interaction mechanism of Mn and heat stress at the protein level.
Materials and Methods
Isolate, Culture, and Treatment of the Primary Chick Myocardial Cells
All procedures were approved by the Animal Welfare Committee of the Institute of Animal Science, Gansu Agricultural University. As we previously described [15], chicken embryo myocardial cells were prepared from 10-day-old embryonated eggs. First, the embryonic heart was removed and minced under sterile conditions and then was digested with 0.12% collagenase II digestion solution (Solarbio, China) in a shaking water bath at 37 °C for 8 min (repeat digestion 5 times). The digested solution were collected and centrifuged at 1000 rpm for 5 min. These cells was resuspended and cultured in the DMEM/F-12 medium (BasalMedia, China) supplemented with 20% fetal bovine serum (FBS, Gibco, USA) and 1% Penicillin-Streptomycin Liquid (Solarbio, China) at 37 °C under 5% CO2, and then the medium was changed every day. When the cells reached approximately 80% confluence, the myocardial cells were treated with 20 μM Mn (MnCl2, Sigma-Aldrich, USA; Mn compound amino acid complex, Trouw Nutrition, Netherlands) for 24 h. The culture medium was replaced with fresh medium, and the cells were exposed to 42 °C for 4 h (heat-stress treatment).
Sample Collection
Cell samples were collected from CON group (37 °C, cells without any treatment), HS group (43 °C, cells treatment with heat stress for 4 h), HS+MnCl2 group (cells treated with 20 μM MnCl2 before heat stress), and HS+Mn-AA group (cells treated with 20 μM Mn compound amino acid complex before heat stress). Cells were digested with 0.25% trypsin, collected in the retained culture medium, and centrifuged at 1000 rpm for 5 min. The collected cell pellets were flash-frozen in liquid nitrogen and stored at −80 °C for proteome quantification.
Sample Preparation
Total proteins were extracted using the cold acetone method: cell samples were disrupted in lysis buffer (including 2% sodium dodecyl sulfate (SDS), 7 M urea, and 1 mg/ml protease inhibitor cocktail) and homogenized for 3 min in ice using an ultrasonic homogenizer. The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C, and the supernatant was collected.
BCA Protein Assay Kit (Promega, Madison, WI) was used to detect the protein concentration of the supernatant. Next, a total of 50 μg proteins were suspended in 50 μL solution, then 1 μL 1 M dithiothreitol (DTT) was added to the solution. The solution was maintained at 55 °C for 1 h to reduce the disulfide bonds of peptides and alkylated by adding 5 μL 20 mM iodoacetamide (IAA) in a darkroom at 37 °C for 1 h. Then, the sample was precipitated using 300 μL prechilled acetone at −20 °C overnight. The precipitate was washed twice with cold acetone and resuspended in 50 mM ammonium bicarbonate. Finally, the proteins were digested with sequence-grade modified trypsin (Promega, Madison, WI) at a substrate/enzyme ratio of 50:1 (w/w) for 16 h at 37 °C.
The sample was redissolved in the buffer A (20 mM ammonium formate in water, pH 10.0, adjusted with ammonium hydroxide), and then fractionated by high pH separation using Ultimate 3000 system (Thermo Fisher Scientific, MA, USA) connected to a reverse phase column (XBridge C18 column, 4.6 mm × 250 mm, 5 μm; Waters Corporation, MA, USA). Peptides were separated using a linear gradient from 5 to 45% B (20 mM ammonium formate in 80% acetonitrile (ACN), pH 10.0, adjusted with ammonium hydroxide) in 40 min. The column was re-equilibrated at the initial condition for 15 min. The column flow rate was maintained at 1 mL/min and the column temperature was maintained at 30 °C. Twelve fractions were collected; each fraction was dried in a vacuum concentrator for the next step.
DDA: Nano-HPLC-MS/MS Analysis
The peptides were dissolved in 30 μL solvent A (0.1% formic acid in water) and analyzed by online nanospray LC-MS/MS on an Orbitrap Fusion Lumos coupled to EASY-nLC 1200 system (Thermo Fisher Scientific, MA, USA). Sample (3 μL) was loaded onto Acclaim PepMap C18 analytical column (75 μm × 25 cm) and separated with a 120-min gradient from 5 to 35% B (0.1% formic acid in ACN). The column flow rate was maintained at 200 nL/min with the column temperature of 40 °C and the electrospray voltage of 2 kV versus the inlet of the mass spectrometer. The mass spectrometer was run under data-dependent acquisition mode, and automatically switched between MS and MS/MS mode. The parameters were as follows: (1) MS: scan range (m/z) = 350–1200, resolution = 120,000, AGC target = 400,000, maximum injection time = 50 ms, filter dynamic exclusion duration = 30 s; (2) HCD-MS/MS resolution = 15,000, AGC target = 50,000, maximum injection time = 35 ms, collision energy = 32.
Database Search and Quantification
The MS/MS data was processed using Spectronaut X (Biognosys AG, Switzerland) with default settings to generate an initial target list. Spectronaut was set up to search the Gallus database and contaminant database assuming trypsin as the digestion enzyme. Carbamidomethyl (C) was specified as the fixed modification and oxidation (M) was specified as variable modifications. q value (FDR) thresholds were specified at 1%.
DIA Data Collection
The peptides were redissolved in 30 μL solvent A (0.1% formic acid in water) and analyzed by online nanospray LC-MS/MS on an Orbitrap Fusion Lumos coupled to EASY-nLC 1200 system (Thermo Fisher Scientific, MA, USA). Sample (3 μL) was loaded onto Acclaim PepMap C18 analytical column (75 μm × 25 cm) with a 120-min gradient from 5 to 35% B (0.1% formic acid in ACN). The column flow rate was maintained at 200 nL/min with the column temperature of 40 °C and the electrospray voltage of 2 kV versus the inlet of the mass spectrometer. The mass spectrometer was run under data independent acquisition mode, and automatically switched between MS and MS/MS mode. The parameters were as follows: (1) MS: scan range (m/z) = 350–1200, resolution = 120,000, AGC target = 1e6, maximum injection time = 50 ms; (2) HCD-MS/MS resolution = 30,000, AGC target = 1e6, collision energy = 32, stepped CE = 5%; (3) DIA was performed with variable isolation window, and each window overlapped 1 m/z, and the window number was 60.
DIA Data Analysis
Raw data of DIA were processed using Spectronaut X (Biognosys AG, Switzerland) with default parameters. Retention time prediction type was set to dynamic iRT. Data extraction was determined by Spectronaut X based on the extensive mass calibration. Spectronaut Pulsar X determined the ideal extraction window dynamically depending on iRT calibration and gradient stability. q value (FDR) thresholds were specified at 1%. Decoy generation was set to mutated which similar to scrambled but will only apply a random number of AA position swamps (min = 2, max = length/2). All selected precursors passing the filters were used for quantification. The average top 3 filtered peptides which passed the 1% q value cutoff were used to calculate the major group quantities. Proteins with a P value < 0.05 and a fold change ≥ 1.2 or fold change ≤ 1/1.2 were considered differentially expressed proteins (DEPs).
Protein Functional Annotation and Enrichment Analysis
Proteins were annotated against Gene Ontology (GO, http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/) database to obtain their functions. GO terms were assigned by using Blast2GO (version 2.8), GO classification was performed using the OmicShare tools online platform for data analysis (http://www.omicshare.com/tools), and KEGG pathway annotation was performed using Blastp (version 2.6.0) software against the KEGG database (version 3.0). Significant GO functions and pathways were examined within differentially expressed proteins with P value < 0.05. Venn diagrams were also performed using the OmicStudio tools online platform. A PPI network was constructed using the STRING database (http://string-db.org/).
Validation of Proteins by Western Blot
Western blot was performed as previously described (Qin et al.2021) using the following antibodies: anti-HSP70 (1:400; Bioss, Beijing, China), anti-Opa1 (1:1000; Proteintech, Wuhan, China), anti-β-actin (1:5000; Bioss). An HRP-conjugated anti-rabbit IgG (1:6000; Proteintech) antibody was used as the secondary antibody. Signal was visualized using ECL Reagent (Servicebio, servicebio, Wuhan, China) and the chemiluminescence was detected by Amersham Imager 600 (GE, USA) and analyzed with the ImagerJ software (National Institutes of Health, USA).
Statistical Analysis
The experimental data for protein expression was analyzed with GraphPad Prism (version 8.01). The significance of differences in protein expression was determined using one-way ANOVA with Tukey’s multiple comparisons test, significance was set at P < 0.05, and all data are expressed as the mean ± SD. Additionally, Adobe Photoshop (version CS5) was used to draw pictures.
Results
Protein Identification and Quantitative Analysis
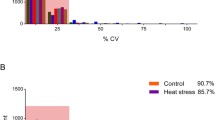
All protein and peptide identifications were obtained by database searching using raw data from DDA (Supplementary file 1) and stringent data filtering (FDR ≤ 0.01). In total, precursors (58,674), peptides (39,056), proteins (6488), and protein groups (4227) were identified and the screening rates of peptides, proteins, and protein groups were 51.81%, 58.51%, and 59.41%, respectively (Fig. 1A, Supplementary file 2). The most identified proteins were constituted by 1–10 peptides, especially, more than half (54.98%) were in the range of 1–6 peptides. In addition, about 27% of the identified proteins had eleven or more peptides (Fig. 1B). By comparing the strength of peptides signal in samples, the relative quantity of proteins was carried out. After protein quantification normalization, the strength of signal in most samples achieved basically the same response strength (Fig. 1C), which shown the overwhelming majority of the proteins were accurately quantified (Fig. 1D, Supplementary file 3).
Differential Expression Analysis of Proteins
Using strict thresholds (P value < 0.05, fold change ≥ 1.2 or ≤1/1.2), as shown in Fig. 2, a total of 437 proteins (161 upregulated and 276 downregulated) were differentially expressed between CON group and HS group. However, the heat affect was changed by Mn. In MnCl2 pretreatment group, 93 (30 upregulated and 63 downregulated) of the 437 proteins were changed. In Mn-AA pretreatment group, 121 proteins (51 upregulated and 70 downregulated) were changed. In addition, there were 53 DEPs identified between organic Mn (HS+Mn-AA group) and inorganic Mn (HS+MnCl2 group), of which 20 were upregulated and 33 were downregulated.
Differential expression analysis of proteins. A Numbers of DEPs. B Volcano plot of DEPs. The abscissa is log2 (FC), the ordinate is −log (P value), the auxiliary line is the threshold line, green represents downregulated proteins, red represents upregulated proteins, and other colors represent proteins with no difference
Bioinformatic Analysis of the DEPs in Primary Chick Embryonic Myocardial Cells Pretreated with Mn Under Heat Stress
Functional classification of 93 DEPs in HS vs HS+MnCl2 group showed that these proteins are mainly involved in processes such as cellular metabolism, biological regulation, response to stimulus, localization, and biosynthesis (Fig. 3A). GO enrichment showed that these DEPs were enriched in 44 GO terms (Supplementary file 4), and above 30% DEPs were enriched in binding (GO:0005488), metabolic process (GO:0008152), biological regulation (GO:0065007), response to stimulus (GO:0050896), catalytic activity (GO:0003824), cellular process (GO:0009987), and cellular component organization or biogenesis (GO:0071840). KEGG enrichment showed that DEPs were enriched in 81 pathways, and fifteen pathways were significantly enriched (P < 0.05) and three of which have more than three DEPs. These are DNA replication (ko03030), nucleotide excision repair (ko03420), and oxidative phosphorylation (ko00190), respectively (Fig. 3A, Table 1, and Supplementary file 5).
Similarly, GO functional annotation showed that the screened DEPs in HS vs HS+Mn-AA group were also mainly involved in cellular metabolism processes, biological regulation, response to stimulus, localization, and biosynthesis processes (Fig. 3B). A total of 46 typical terms were identified by GO enrichment of 121 DEPs in HS vs HS+Mn-AA group (Supplementary file 4), and above 30% DEPs were enriched in cellular process (GO:0009987), binding (GO:0005488), metabolic process (GO:0008152), biological regulation (GO:0065007), response to stimulus (GO:0050896), and cellular component organization or biogenesis (GO:0071840). KEGG enrichment showed that DEPs were enriched in 84 pathways, but only three pathways were significantly enriched (P < 0.05) and two of which have more than three DEPs: lysosome (ko04142) and VEGF signaling pathway (ko04370) (Fig. 3B, Table 1, and Supplementary file 5).
Furthermore, the DEPs between organic Mn and inorganic Mn were involved primarily in endoplasmic reticulum unfolded protein response (GO:0030968), cellular response to unfolded protein (GO:0034620), response to unfolded protein (GO:0006986), and participated in misfolded protein binding, ubiquitin-related enzymes activity (ubiquitin protein ligase, ubiquitin-like protein ligase, ubiquitin-protein transferase, ubiquitin-like protein transferase), and hydrolase activity. KEGG enrichment showed the DEPs were mainly enriched in oxidative phosphorylation (ko00190) and protein processing in endoplasmic reticulum (ko04141) (Fig. 4).
Proteins Related to Response to Heat Stimulus
In HS vs HS+MnCl2 group, a total of 38 DEPs related to response to heat stimulus were identified (Table 2), among which 13 were upregulated, and 25 were downregulated. In HS vs HS+Mn-AA group, a total of 37 DEPs related to response to heat stimulus were identified (Table 2), among which 21 were upregulated under heat stress, and 16 were downregulated. There were 12 overlap DEPs between the two groups of the organic and inorganic Mn pretreatments, of which five DEPs (RPA3, BAG3, TMCO1, HSPB8, DNAJB1) were upregulated under heat stress, with fold changes above 1.5. And the change of small heat shock proteins (HSPB8, DNAJB1), molecular chaperone BAG3 (an anti-apoptosis protein), and TMCO1 (a transmembrane protein in endoplasmic reticulum) were dramatic (P values < 0.01). In addition, seven DEPs were downregulated, including apoptosis-inducing factor (AIFM1), tumor related gene (GLTSCR2, NOV), metallothionein (MT4), and the Borg (binder of Rho GTPases) family proteins (Cdc42EP1).
These overlap DEPs were mainly divided into four categories based on functions: protein binding, catalytic activity of various enzymes, DNA damage, and energy metabolism. KEGG enrichment analysis showed that these overlap DEPs were mainly enriched in metabolic pathways (ko01100), protein processing in endoplasmic reticulum (ko04141), PPAR signaling pathway (ko03320), apoptosis (ko04210), MAPK signaling pathway (ko04010), p53 signaling pathway (ko04115), DNA replication (ko03030), and nucleotide excision repair (ko03420).
Nucleic acid metabolism related proteins
GO molecular functional enrichment showed that above 20% of significantly enriched GO term in both HS vs HS+MnCl2 group and HS vs HS+Mn-AA group are involved in nucleic acid metabolism process (Supplementary file 6). And comparatively, the major difference is that the former were more involved in DNA binding and helicase activity, while the latter tends more to methylation-associated binding. In HS vs HS+MnCl2 group, a total of 27 DEPs related to nucleic acid metabolism were identified (Table 3), among which 8 were upregulated, and 19 were downregulated. In HS vs HS+Mn-AA group, a total of 28 DEPs related to nucleic acid metabolism were identified (Table 3), among which 11 were upregulated under heat stress, and 17 were downregulated. There were 9 overlap DEPs between the two groups of the organic and inorganic Mn pretreatments, of which three DEPs (RPA3, BAG3, DNAJB1) were upregulated under heat stress, with fold changes above 1.5. In addition, six DEPs were downregulated (AIFM1, GTPBP4, TAF15, MRPL12, NOP53, NOP14).
As shown Fig. 5, with further analysis of DEPs related to heat stress response and nucleic acid metabolism, five cross-proteins were screened out, namely, RPA3 (replication protein A3), BAG3 (BCL2 associated athanogene 3), DNAJB1 (dnaJ homolog subfamily B member 1-like), AIFM1 (apoptosis inducing factor, mitochondria associated 1), and NOP53 (glioma tumor suppressor candidate region gene 2). These genes were mainly associated with DNA replication (ko03030), nucleotide excision repair (ko03420), mismatch repair (ko03430), protein processing in endoplasmic reticulum (ko04141), and apoptosis (ko04210). Therefore, these genes should be the key nodes of Mn in regulating the metabolism of genetic material at high temperature, and through these key nodes, Mn regulates DNA replication, DNA repair, cell apoptosis, and protein synthesis in endoplasmic reticulum, and then ultimately influences the cell response to thermal stimulation.
Validation of protein identification and quantification
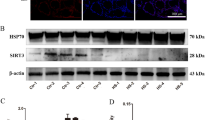
To verify the reliability of DEPs identified by DIA, the protein expression of heat shock protein 70 (Hsp70) and optic atrophy 1 (Opa1) were detected by western blot analysis. As shown in Fig. 6, the expression of Hsp70 was significantly upregulated and Opa1 was significantly downregulated in HS groups as compared with CON group (P < 0.0001). The Hsp70 expression levels were significantly decreased in HS+MnCl2 group and HS+Mn-AA group than in HS group (P < 0.05 and <0.01, respectively), and there was significant difference between HS vs HS+MnCl2 group and HS vs HS+Mn-AA group (P < 0.01). Conversely, the Opa1 expression levels were significantly increased in HS vs HS+MnCl2 group and HS vs HS+Mn-AA group than in HS group (P < 0.001 and <0.01, respectively), and there was significant difference between HS vs HS+MnCl2 group and HS vs HS+Mn-AA group (P < 0.05). The change in the protein expression of western blot analysis presented a similar trend to that of the DIA analysis.
Validation part of differentially expressed proteins. A Western blot was used to analyze the protein expression levels of Hsp70 (B) and Opa1 (C); β-actin was used as an internal control. D–E Comparison of the expression patterns of DEPs acquired by DIA and by western blot validation. The data shown represent the mean ± SD and *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared between groups
Discussion
Mn is an essential trace mineral that plays an important role in intracellular metabolic reactions of lipids, proteins, carbohydrates, and various enzymes. As a cofactor, Mn is participates in a series of biochemical reactions, especially in various metabolic and antioxidant stress processes [21]. Mn is required for both Mn catalase (Mn-CAT) and Mn superoxide dismutase (Mn-SOD), so Mn is involved in detoxification of oxygen free radicals and reduction of oxidative stress [22]. High temperature stimulation is one of the main stressors of oxidative stress. Previous studies have confirmed that protein metabolism in animals can be altered by heat stress [23]. Especially, heat stress leads to protein denaturation, more commonly various enzymes, and the disruption of normal physiological processes [24, 25].
In this study, the effects of Mn on the whole protein changes were studied for the first time in chicken embryo cardiomyocytes under heat stress using DIA technology. In total, 6488 proteins and 39,056 peptides were identified; these high-throughput data contribute to a comprehensive and in-depth analysis of the effects of Mn on the proteome of heat-stressed cardiomyocytes.
Respectively, 93 and 121 differentially expressed proteins were identified in inorganic and organic Mn treatments. Interestingly, although GO annotation analysis showed that these DEPs had similar functional classifications, specific molecular functions disclosed differences between the two Mn sources. The DEPs in inorganic Mn pretreatment cells were more prominently distributed in respiratory chain, organelle lumen, mitochondrion, and membrane-bounded organelle, and effected on catalytic activity on RNA, protein dimerization activity, DNA helicase activity, and hydrolase activity. By contrast, the DEPs in organic Mn pretreatment cells were more significantly located in nucleolus, nucleoplasm, nuclear lumen, membrane-enclosed lumen, and organelle lumen, and play a role in methyl-CpG binding, heat shock protein binding, transcription coregulator activity, chaperone binding, methylated histone binding, and methylation-dependent protein binding. Therefore, these results indicate that inorganic Mn maybe engage in DNA replication and repair, and protein synthesis process, meanwhile, organic Mn tend more to contribution consist mostly in regulating gene methylation under heat stress. In addition, KEGG analysis showed that some important biological processes have been found, specifically, DNA replication, nucleotide excision repair, and oxidative phosphorylation were significantly enriched in inorganic Mn group, and lysosome and VEGF signaling pathway were significantly enriched in organic Mn group. We hypothesized that the different effects of inorganic Mn and organic Mn on heat stress cells might be related to the properties of amino acids chelated by organic Mn.
Further analysis found that the DEPs between inorganic Mn and organic Mn were strongly associated with protein processing and repair in the ER and protein ubiquitination modification, which might suggest that there were differences in the regulation of ubiquitination modification of protein by different Mn sources. And beyond that, these DEPs were mainly associated with cardiac muscle contraction, vascular smooth muscle contraction, and adrenergic signaling in cardiomyocytes. Evidence has shown that Mn obviously affects the vascular system, which is manifested in leading to reduce of vascular diastolic pressure by dilating blood vessel and diminishing cardiac systolic strength [26]. Based on the above analysis, we believe that under heat stress, both inorganic and organic Mn all have affected the activity of various enzymes and protein modifications, especially DNA methylation and histone ubiquitination. At the same time, the protective effect of inorganic Mn and organic Mn on myocardial contraction was different with the difference of bioavailability. The underlying mechanisms need to be further explored.
Exposed in thermal environment, cells have been stimulated an adaptive self-protection response by activating stress response of multiple organelles. Mild heat stress would enhance heat tolerance and cell survival rate via upregulating the expression of various genes, especially heat shock proteins [27]. However, when the synthesis of intracellular self-protective proteins is not enough to compensate for the loss of proteins, heat stress will cause a series of damage to cells, and apoptosis is one of them [28]. In the present study, small heat shock proteins (HSPB8, DNAJB1) and molecular chaperone BAG3 were significantly upregulated under heat stress, and then Mn further enhanced the upregulation. Small heat shock protein (sHSP) is a highly conserved chaperone family that contains the α-crystallin domain (ACD), characterized by their small molecular weight (ranging from 15 to 40 kDa) [29]. The function of sHSPs is to bind and stabilize denatured or non-native proteins, and then defense against aggregation to maintain protein homeostasis [30, 31]. In addition, sHSPs can activate the ATPase activity of high-molecular-weight chaperones, such as HSP70, prevent clumping of misfolded proteins, and assist protein renaturation [29]. As a small heat shock protein, HSPB8 is mainly expressed in the heart and skeletal muscle and acts as a molecular chaperone to regulate protein quality control [32,33,34]. BAG3, a member of the co-chaperone family of Bag domain-containing proteins, is the only protein known to bridge ATP-independent sHSPs and the ATP-dependent HSP70 family to form ternary complexes [32, 34]. HspB8 and Bag3 formed a chaperone complex that stimulates degradation of protein substrates by macroautophagy [35] and plays a role in maintaining normal cardiac function and regulating cardiac stress [36]. Correspondingly, KEGG analysis showed that some DEPs were significantly enriched in lysosome and protein processing in endoplasmic reticulum pathways. These all seems to imply that Mn may help to promote protein repair by upregulating these genes and resist cell damage induced by heat stress. Specifically, the cooperation of HSPB8 and BAG3 have promoted spatial sequestration of ubiquitinated proteins and coordinate the cellular adaptive response to proteasome insufficiency [37], which indicated that Mn was involved in protein ubiquitination modification under heat stress.
Heat stress can cause excessive accumulation of intracellular reactive oxygen species (ROS), activate cell apoptosis signals, and ultimately induce cell apoptosis [14]. Studies have confirmed that small heat shock proteins serve as a negative regulator of apoptosis [38], contributing to improved survival rate of cells under heat stress. BAG3, belongs to antiapoptotic protein (BAG) family, the silence or lack of BAG3 results in enhancing cell apoptosis, and oppositely, BAG3 overexpression can inhibit cell apoptosis [39, 40]. One of the reasons for the anti-apoptotic function of BAG3 is to increase the stability of the pro-survival bcl-2 family proteins (including Bcl-2, Bcl-XL and McL-1), and then support the apoptotic antagonistic function of these proteins [41]. Apoptosis-inducing factor (AIF) is an inducer of apoptosis located in intermembrane space [42]. Studies suggest that the apoptotic effect of AIF is independent of caspase, after being cleaved in mitochondria, AIF has the activity of inducing apoptosis and released from mitochondria into the nucleus to shear DNA, which directly cause nuclear condensation and promoted cell apoptosis [43, 44]. Additionally, AIFM1 not only mediates large-scale DNA degradation, but is also essential for the stability and function of respiratory complex I [45]. In this study, the upregulation of BAG3 and downregulation of AIFM1 suggest that Mn has an anti-apoptotic effect. Especially, KEGG analysis showed that some DEPs were significantly enriched in apoptosis and p53 signaling pathway. Based on the above analysis, we believe that Mn can reduce cell apoptosis induced by heat stress via regulating the expression of these apoptosis-related genes, which is consistent with our previous research results [46, 47].
Heat stress inhibits DNA synthesis and mRNA transcription in cells and inhibits cell proliferation at a certain stage of the cell proliferation cycle, thereby preventing cell division [27, 48]. In this study, some DEPs were related to nucleic acid metabolism, particularly DNA metabolism, and important pathways associated with that were significantly enriched, such as DNA replication, nucleotide excision repair, and mismatch repair. Replication protein A3 (RPA3) is a single stranded DNA binding protein in eukaryotic cells and is a component of replication origin recognition complex, which plays an important role in DNA elongation and replication initiation, is essential for the stability of single stranded DNA [49]. Recent studies [50, 51] have shown that RPA is not only involved in DNA replication, but also in DNA damage repair. The overexpression of RPA3 enhanced the capacity for DNA repair, whereas inhibiting RPA3 expression to decreased the DNA repair capacity. In this present study, RPA3 was significantly upregulated under the intervention of Mn, which suggested that Mn regulates cellular DNA replication and promotes DNA repair under heat stress by acting on key node proteins of nucleic acid metabolism.
Conclusion
In this study, we analyzed proteome changes in heat-stress primary chick embryonic myocardial cells with inorganic and organic Mn pretreatment using DIA technology. In total, 93 and 121 DEPs were identified in inorganic and organic Mn pretreatment group, respectively; in addition, there were 53 DEPs identified between inorganic Mn pretreatment group and organic Mn pretreatment group. Many significant DEPs were identified, including sHSP, apoptosis-related proteins, and nucleic acid metabolism related proteins. These DEPs involved in a variety of biological regulatory pathways, and significantly influenced protein processing and repair in endoplasmic reticulum, apoptosis, and DNA replication and repair. These all seem to imply that Mn may help to resist cell damage induced by heat stress by regulating key node proteins. The findings are helpful to better understand the effect of Mn on overall protein changes in heat-stress and possible action mechanism.
References
Jahejo AR, Rajput N, Rajput NM, Leghari IH, Kaleri RR, Mangi RA, Sheikh MK, Pirzado MZ (2016) Effects of heat stress on the performance of Hubbard broiler chicken. Cells 2:1–5
Zaboli GR, Rahimi S, Shariatmadari F, Torshizi MAK, Baghbanzadeh A, Mehri M (2017) Thermal manipulation during pre and post-hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult Sci 96(2):478–485. https://doi.org/10.3382/ps/pew344
Kamel NN, Ahmed AMH, Mehaisen GMK, Mashaly MM, Abass AO (2017) Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int J Biometeorol 61(9):1637–1645. https://doi.org/10.1007/s00484-017-1342-0
St-Pierre NR, Cobanov B, Schnitkey G (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86:E52–E77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5
Chang CP, Hsu YC, Lin MT (2003) Magnolol protects against cerebral ischaemic injury of rat heatstroke. Clin Exp Pharmacol P 30(5-6):387–392. https://doi.org/10.1046/j.1440-1681.2003.03847.x
Wu D, Xu J, Song E, Tang S, Zhang XH, Kemper N, Hartung J, Bao E (2015) Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperon 20(4):687–696. https://doi.org/10.1007/s12192-015-0596-x
Xu Y, Jie Z, Lin L, Bo Y, Chen BX, Bao ED, Zhang XH (2023) Hsp90 protected chicken primary myocardial cells from heat-stress injury by inhibiting oxidative stress and calcium overload in mitochondria. Biochem Pharmacol 209:115434
Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH (2010) Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Phys C 151(2):204–208. https://doi.org/10.1016/j.cbpc.2009.10.010
Slimen IB, Najar T, Ghram A, Dabbebi H, Mrad MB, Abdrabbah M (2014) Reactive oxygen species, heart stress and oxidative-induced mitoehondrial damage. A review Int J Hyperther 30(7):513–523. https://doi.org/10.3109/02656736.2014.971446
Gu XH, Hao Y, Wang XL (2012) Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2 intestinal oxidative stress. Poultry Sci 91(4):790–799. https://doi.org/10.3382/ps.2011-01628
Víctor MV, Milagros R, Mónica DLF (2003) Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol 3(1):97–106. https://doi.org/10.1016/s1567-5769(02)00232-1
Zeng T, Li JJ, Wang DQ, Li GQ, Wang GL, Lu LZ (2014) Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperon 19(6):895–901. https://doi.org/10.1007/s12192-014-0514-7
Zhu YE, Lu L, Liao XD, Li WX, Zhang LY, Ji C, Lin X, Liu HC, Jack O, Luo XG (2017) Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and antiapoptotic abilities. Oncotarget 8(52):89665–89680. https://doi.org/10.18632/oncotarget.20804
Li SF, Lu L, Liao XD, Gao TQ, Wang F, Zhang LY, Xi L, Liu SB, Luo XG (2016) Manganese elevates manganese superoxide dismutase protein level through protein kinase C and protein tyrosine kinase. Biometals 29(2):265–274. https://doi.org/10.1007/s10534-016-9913-9
Qin SZ, Huang L, Lu L, Zhang LY, Guo YL, Xi L, Liao XD, Luo XG (2023) Manganese alleviates heat stress of primary cultured chick embryonic myocardial cells via enhancing manganese superoxide dismutase expression and attenuating heat shock response. J Therm Biol 112:103440
Liao XD, Zhu YW, Lu L, Li WX, Zhang LY, Ji C, Lin X, Luo XG (2019) Maternal manganese activates anti-apoptotic-related gene expressions via miR-1551 and miR-34c in embryonic hearts from maternal heat stress (Gallus gallus). J Therm Biol 84:190–199. https://doi.org/10.1016/j.jtherbio.2019.07.014
Monti C, Zilocchi M, Colugnat I, Alberio T (2019) Proteomics turns functional. J Proteomics 198:36–44. https://doi.org/10.1016/j.jprot.2018.12.012
Allison D (2015) DIA mass spectrometry. Nat Methods 12(1):35–35. https://doi.org/10.1038/nmeth.3234
Vidova V, Spacil Z (2017) A review on mass spectrometry-based quantitative proteomics: targeted and data independent acquisition. Anal Chim Acta 964:7–23. https://doi.org/10.1016/j.aca.2017.01.059
Floriou-Servou A, Lukas VZ, Luzia S, Oliver S, Mattia P, Anahita R, Alessio C, Beat T, Johannes B (2018) Distinct proteomic, transcriptomic and epigenetic stress-responses in dorsal and ventral hippocampus. Biol Psychiat 84(7):531–541. https://doi.org/10.1016/j.biopsych.2018.02.003
Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M (2015) Manganese is essential for neuronal health. Annu Rev Nutr 35:71–108. https://doi.org/10.1146/annurev-nutr-071714-034419
Gunter EJM Gavin CE, Aschner M Thomas EG (2013) Manganese neurotoxicity and the role of reactive oxygen species. Free Radical Bio Med 62:65-75. https://doi.org/10.1016/j.freeradbiomed.2013.01.032.
Lopez V, Heijden EVD, Villar M, Michel A, Alberdi P, Gortázar C, Rutten V, Fuente JDL (2018) Comparative proteomics identified immune response proteins involved in response to vaccination with heat-inactivated Mycobacterium bovis and mycobacterial challenge in cattle. Vet Immunol Immunop 206:54–64. https://doi.org/10.1016/j.vetimm.2018.10.013
Matsuki SG, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J (2003) Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Bioph Res Co 312(3):843–849. https://doi.org/10.1016/j.bbrc.2003.10.191
Saelao P, Wang Y, Chanthavixay G, Yu V, Gallardo RA, Dekkers JM (2018) Integrated proteomic and transcriptomic analysis of differential expression of chicken lung tissue in response to NDV infection during heat stress. Genes-Basel 9(12):579. https://doi.org/10.3390/genes9120579
Ari M,and Hrusti O (1975) Exposure to airborne manganese and arterial blood pressure. Environ Res 10(2):314-318. https://doi.org/10.1016/0013-9351(75)90092-4.
Meares GP, Zmijewska AA, Jope RS (2004) Heat shock protein-90 dampens and directs signaling stimulated by insulin-like growth factor-1 and insulin. FEBS Lett 574(1-3):181–186. https://doi.org/10.1016/j.febslet.2004.08.026
Langer T, Neupert W (1991) Heat shock proteins hsp60 and hsp70: their roles in folding, assembly and membrane translocation of proteins. Curr Top Microbiol 167:3–30. https://doi.org/10.1007/978-3-642-75875-1_1
Fang X, Bogomolovas J, Trexler C, Chen J (2019) The BAG3-dependent and -independent roles of cardiac small heat shock proteins. JCI Insight 4(4):e126464. https://doi.org/10.1172/jci.insight.126464
Carra S, Simon A, Arrigo PA, Benesch JL, Benjamin IJ (2017) The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperon 22(4):601–611. https://doi.org/10.1007/s12192-017-0787-8
Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J (2017) The chaperone activity and substrate spectrum of human small heat shock proteins. J Biol Chem 292(2):672–684. https://doi.org/10.1074/jbc.M116.760413
Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu XZ, Kudej RK, Wagner T, Sadoshima J, Vatner SF (2002) H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res 91(11):1007–1014. https://doi.org/10.1161/01.RES.0000044380.54893.4B
Rauch JN, Tse E, Freilich R, Mok SA, Makley LN, Southworth DR, Gestwicki JE (2017) BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Cell Biol 429(1):128–141. https://doi.org/10.1016/j.jmb.2016.11.013
Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC (2003) Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol 82(10):523–530. https://doi.org/10.1078/0171-9335-00337
Carra S, Seguin SJ, Landry J (2008) HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 4(2):237–239. https://doi.org/10.4161/auto.5407
Li FZ, Xiao H, Hu ZP, Zhou FF, Yang BB (2018) Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur J Cell Biol 97(3):216–229. https://doi.org/10.1016/j.ejcb.2018.03.003
Guilbert SM, Lambert H, Rodrigue MA, Fuchs M, Landry J, Lavoie JN (2018) HSPB8 and BAG3 cooperate to promote spatial sequestration of ubiquitinated proteins and coordinate the cellular adaptive response to proteasome insufficiency. Faseb J 32(7):3518–3535. https://doi.org/10.1096/fj.201700558RR
Mehlen P, Mehlen A, Godet J, Arrigo AP (1997) Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 272(50):31657–31665. https://doi.org/10.1074/jbc.272.50.31657
Doong H, Vrailas A, Kohn EC (2002) What’s in the ‘BAG’?–a functional domain analysis of the BAG-family proteins. Cancer Lett 188(1-2):25–32. https://doi.org/10.1016/s0304-3835(02)00456-1
Festa M, Valle LD, Khalili K, Franco R, Scognamiglio G, Graziano V, Laurenzi VD, Turco MC, Rosati A (2011) BAG3 protein is overexpressed in human glioblastoma and is a potential target for its therapy. Am J Surg Pathol 178(6):2504–2512. https://doi.org/10.1016/j.ajpath.2011.02.002
Rosati A, Graziano V, Laurenzi VD, Pascale M, Turco MC (2011) BAG3: a multifaceted protein that regulates major cell pathway. Cell Death Dis 2(4):e141. https://doi.org/10.1038/cddis.2011.24
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397(6718):441–446. https://doi.org/10.1038/17135
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410(6828):549–554. https://doi.org/10.1038/35069004
Norberg E, Orrenius S, Zhivotovsky B (2010) Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF). Biochem Bioph Res Co 396(1):95–100. https://doi.org/10.1016/j.bbrc.2010.02.163
Bonora M, Pedro JMB, Kroemer G, Galluzzi L, Pinton P (2014) Novel insights into the mitochondrial permeability transition. Cell Cycle. 13(17):2666–2670. https://doi.org/10.4161/15384101.2014.949082
Qin SZ, Wang R, Tang DF, Qin SJ, Guo YL, Shi ZG (2022) Manganese mitigates heat stress-induced apoptosis by alleviating endoplasmic reticulum stress and activating the NRF2/SOD2 pathway in primary chick embryonic myocardial cells. Biol Trace Elem Res 200:2312–2320. https://doi.org/10.1007/s12011-021-02810-2
Wang R, Shi ZG, Li JL, Tang DF, Qin SZ, Guo YL (2022) Protective effect of manganese on apoptosis and mitochondrial function of heat-stressed primary chick embryonic myocardial cells. Biol Trace Elem Res 200:4419–4429. https://doi.org/10.1007/s12011-021-03016-2
Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C (2003) Characterization of the signal that directs Bcl-x[sub L], but not Bcl-2, to the mitochondrial outer membrane. J Mol Cell Biol 160(1):53–64. https://doi.org/10.1083/jcb.200210084
Iftode C, Daniely Y, Borowiec JA (1999) Replication protein A (RPA): the eukaryotic SSB. Crit Rev Biochem Mol 34(3):141–180. https://doi.org/10.1080/10409239991209255
Burns JL, Guzder SN, Sung P, Prakash S, Prakash L (1996) An affinity of human replication protein A for ultraviolet-damaged DNA. J Biol Inorg Chem 271(20):11607–11610. https://doi.org/10.1074/jbc.271.20.11607
Costa RMA, Chiganças V, Galhardo RDS, Carvalho H, Menck CFM (2003) The eukaryotic nucleotide excision repair pathway. Biochimie 85(11):1083–1099. https://doi.org/10.1016/j.biochi.2003.10.017
Funding
This research was funded by the National Natural Science Foundation of China (No. 31960668) and Discipline Team Project of Gansu Agricultural University (NO: GAU-XKTD-2022-24).
Author information
Authors and Affiliations
Contributions
Shizhen Qin: conceptualization, methodology, writing—original draft preparation; Rui Wang: conceptualization, methodology, software; Jinlu Li: contributed materials, data curation, methodology; Defu Tang: methodology, software; Zhaoguo Shi: project administration, methodology.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, S., Wang, R., Li, J. et al. Quantitative Proteomics Reveals Manganese Alleviates Heat Stress of Broiler Myocardial Cells via Regulating Nucleic Acid Metabolism. Biol Trace Elem Res 202, 1187–1202 (2024). https://doi.org/10.1007/s12011-023-03731-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03731-y