Abstract
Three experiments were conducted to investigate the effects of inorganic and organic Mn sources on MnSOD mRNA, protein and enzymatic activity and the possible signal pathways. The primary broiler myocardial cells were treated with MnCl2 (I) or one of organic chelates of Mn and amino acids with weak, moderate (M) or strong (S) chelation strength for 12 and 48 h. Cells were preincubated with superoxide radical anions scavenger N-acetylcysteine (NAC) or specific inhibitors for MAPKs and protein tyrosine kinase (PTK) or protein kinase C (PKC) for 30 min before treatments of I and M. The MnSOD mRNA, protein and enzymatic activity, phosphorylated MAPKs or protein kinases activations were examined. The results showed that additions of Mn increased (P < 0.05) MnSOD mRNA levels and M was more effective than I. Additions of Mn elevated (P < 0.05) MnSOD protein levels and enzymatic activities, and no differences were found among I and M. Addition of NAC did not decrease (P > 0.05) Mn-induced MnSOD mRNA and protein levels. None of the three MAPKs was phosphorylated (P > 0.05) by Mn. Additions of Mn decreased (P < 0.05) the PTK activities and increased (P < 0.05) the membrane PKC contents. Inhibitors for PTK or PKC decreased (P < 0.05) Mn-induced MnSOD protein levels. The results suggested that Mn-induced MnSOD mRNA and protein expressions be not related with NAC, and MAPK pathways might not involve in Mn-induced MnSOD mRNA expression. PKC and PTK mediated the Mn-induced MnSOD protein expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese superoxide dismutase (MnSOD), a vital antioxidant enzyme localized in mitochondrial matrix, catalyzes the dismutation of superoxide radical anions (ROS) into hydrogen peroxide and oxygen. The physiological role of MnSOD as a cytoprotective enzyme has been clearly confirmed by the extremely short life-span of MnSOD knockout mice, which died shortly after birth with dilated cardiomyopathy and neurodegeneration (Li et al. 1995; Lebovitz et al. 1996). Manganese (Mn) as a crucial component of MnSOD has been proved to be necessary for fast-growing broilers. Previous in vivo studies from our laboratory have demonstrated that Mn elevated MnSOD mRNA and protein levels, as well as the enzymatic activity in broiler heart (Li et al. 2004, 2005, 2008, 2011; Luo et al. 2007). Moreover, the organic Mn source chelated with amino acids with moderate chelation strength was more effective than inorganic Mn sulfate or the organic Mn sources with weak or strong chelation strength in activating heart MnSOD gene expression of broilers at both mRNA and protein levels (Li et al. 2004, 2005, 2008, 2011; Luo et al. 2007). Previous in vitro study from our laboratory also indicated that Mn elevated MnSOD mRNA and protein levels, and its enzymatic activity in the primary broiler myocardial cells in dose- and time-dependent manners (Gao et al. 2011). However, the signaling pathways responsible for Mn-induced MnSOD mRNA and protein expressions in broiler heart are still unclear. Whether the organic Mn source with moderate chelation strength mediated the heart MnSOD gene expression through the same signaling pathways as inorganic Mn did remains unknown.
One of the central means of regulating gene expressions is through signal pathways involving kinase/phosphatase cascades (Sun and Tonks 1994) The mitogen-activated protein kinases (MAPKs) are a group of protein serine/threonine kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus. Two of the MAPKs pathways p38 mitogen-activated protein kinase (p38 MAPK) and/or c-Jun N-terminal kinase (JNK) have been implicated to be involved in the signal transduction of MnSOD gene expression induced by arachidonic acid (Bianchi et al. 2002) and hepatitis C virus (Qadri et al. 2004). Meanwhile, protein kinase C (PKC) activation mediates the induction of MnSOD gene expression by tumor necrosis factor-α (TNF-α) (Karube-Harada et al. 2001), phorbol esters (Fujii and Taniguchi 1991) and anticancer drugs such as vinblastine and vincristine (Das et al. 1998). Reactive oxygen intermediates cause an increase in protein tyrosine kinase (PTK) activity and a decreased synthesis of MnSOD protein (Brumell et al. 1996). However, whether the known signal pathways related to MnSOD induction were involved in the Mn-induced MnSOD gene expression of broiler heart is unpredictable.
The aim of the present study was to investigate the effects of inorganic and organic Mn sources on MnSOD mRNA and protein levels and the possible signal pathways involved in MnSOD regulation in the primary broiler myocardial cells.
Materials and methods
Cell culture and treatments
All experimental procedures were approved by the Office of the Beijing Veterinarians and performed in accordance with the guidelines. The primary myocardial cells were isolated from the heart tissue of 7-day-old male Arbor Acres broilers (Huadu Broiler Breeding Corp., Beijing, China) fed the Mn-deficient diet for 7 days as described previously (Gao et al. 2011). The cardiac muscles were digested and the isolated cells were pooled for all tested parameters in each experiment. For treatments with inhibitors of protein kinases, the primary myocardial cells were seeded into 100-mm tissue culture dishes (Cat no. 430167, BD Biosciences), otherwise were seeded into 6-well plates (Cat no. 353046, BD Biosciences). All cells were cultured in DMEM/F-12 medium (Cat no. 1344212, Invitrogen) supplemented with 10 % fetal bovine serum (Cat no. SH30084.03, Hyclone), 200 µmol/L l-glutamine (Cat no. 25030081, Invitrogen), 100 units/mL penicillin and 100 µg/mL streptomycin (Cat no. 15140-122, Invitrogen) at 37 °C until 80–90 % confluence. There were 6 replicate cell culture wells or dishes for each treatment.
The aim of experiment 1 was to determine whether the effects of inorganic and organic Mn sources on MnSOD mRNA and protein levels in the primary myocardial cells in vitro were consistent with our previous in vivo studies. Our preliminary experiments showed that cells treated with 0.5 mmol/L inorganic MnCl2 as long as 48 h had similar viability compared with those not treated with Mn (Gao et al. 2011). The MnSOD protein level increased from 12 h, while MnSOD mRNA response occurred after stimulation with inorganic Mn until up to 24 h and kept on increasing by 48 h (Gao et al. 2011). Therefore, in Expt 1, the primary myocardial cells were cultured with serum-free medium for 12 h, and then treated with no Mn supplementation (C, medium) or 0.5 mmol/L of Mn as the inorganic MnCl2 (I, reagent grade, Beijing Biochemical Reagent Company, Beijing, China) or one of three organic Mn sources for 12 or 48 h. The three organic Mn sources (chelates of Mn and amino acids) with weak (W, formation quotient (Qf) = 2.35 lower than 10, containing Mn 7.49 % and total amino acids 30.42 %, Zinpro Corp.), moderate (M, Qf = 16.85 between 10 and 100, containing Mn 9.06 % and total amino acids 29.09 %, Sanbao Additive Company, Beijing, China), or strong (S, Qf = 147.00 between 100 and 1000, containing Mn 10.18 % and total amino acids 45.27 %, Alltech) chelation strengths were the same as those described by Li et al. (2011). The Qf is a quantitative measurement of chelation strength between Mn and amino acids according to the shift in half-wave potential (E1/2) in polarography, as described by Holwerda et al. (1995) and Li et al. (2004). The MnSOD mRNA and protein levels and the enzymatic activities were determined after Mn source treatments for 12 and 48 h.
The aim of experiment 2 was to investigate possible signal pathways involved in MnSOD mRNA regulation by Mn sources. To determine whether MAPKs or ROS was involved in the transcriptional activation of MnSOD gene by Mn, the primary myocardial cells were preincubated in the serum-free medium containing one of specific inhibitors of p38 MAPK (10 µmol/L, SB203580, Cat no. S8307, Sigma) (Kujime et al. 2000; Tanaka et al. 2000), JNK (50 nmol/L, JNKI1, Cat no. J2580, Sigma) (Qadri et al. 2004), and ERK (30 µmol/L, PD98059, Cat no. P215, Sigma) (Kujime et al. 2000; Tanaka et al. 2000), or a ROS scavenger N-acetylcysteine (NAC, 500 µmol/L, Cat no. A9165, Sigma) (Kujime et al. 2000) for 30 min prior to Mn treatments. The results in Expt 1 showed that MnSOD mRNA levels among C and Mn source treatments differed at 48 h and the organic Mn source M showed the highest value compared with I and the other two organic Mn sources, therefore, in Expt 2 cells were only treated with C or addition of 0.5 mmol/L of Mn as I or M for 48 h after inhibitors or NAC treatment. The mRNA levels and the phosphorylations of MAPKs were determined.
The aim of experiment 3 was to investigate possible signal pathways involved in MnSOD protein regulation by Mn sources. The results in Expt 1 showed that MnSOD mRNA levels were not increased while the protein levels were elevated by Mn source treatments at 12 h, which indicated that the induction of MnSOD protein expression by Mn was independent of transcription at 12 h. Therefore, the primary myocardial cells were preincubated with the serum-free medium containing PTK inhibitor (50 µmol/L, genistein, Cat no. G6649, Sigma) (Tanaka et al. 2000; Knirsch and Clerch 2001), PKC inhibitor (1 µmol/L, calphostin C, Cat no. C6303, Sigma) (Tanaka et al. 2000; Knirsch and Clerch 2001) or NAC (500 µmol/L) (Kujime et al. 2000) for 30 min before 12 h incubations of 0.5 mmol/L of Mn as I or M. The MnSOD protein levels and the activation of PTK and PKC were determined in C or additions of Mn sources.
Determinations of MnSOD mRNA, protein and activity
At the end of each experiment, the medium was removed, and the cell monolayers were washed three times with ice-cold PBS. Total cellular RNA was isolated using Trizol reagent (Cat No. 15596-026, Invotrogen) according to the manufacturer’s instructions, and the total RNA concentration of each specimen was estimated by measuring UV light absorbance at 260 nm with spectrophotometer (ND-100, NanoDrop Technologies). Briefly, cDNA was synthesized using the SuperScript® III First-Strand Synthesis for RT-PCR kit (Cat No. 18080-051, Invitrogen) with DNA Engine PCR instrument (Bio-RAD). The MnSOD mRNA level was determined by relative quantitative real-time PCR using the power SYBR® Green PCR mater mix kit (Cat no. 4367659, Applied Biosystems) with ABI Prism 7500 (Applied Biosystems) as described by Gao et al. (2011). β-actin was used as an internal control in all reactions. The primers for MnSOD (accession number: AF299388; forward, 5′-AGGAGGGGAGCCTAAAGGAGA-3′, reverse, 5′-CCAGCAATGGAATGAGACCTG-3′; product length, 215 bp) and β-actin (accession number: L08165; forward, 5′-GAGAAATTGTGCGTGACATCA-3′, reverse, 5′-CCTGAACCTCTCATTGCCA-3′; product length, 152 bp) were used for the amplification reactions, respectively.
For MnSOD protein analysis, cells were lysed in ice-cold lysis buffer (50 mmol/L Tris, pH 7.4, containing 150 mmol/L NaCl, 1 mmol/L PMSF, 1 mmol/L EDTA, 1 % Triton x-100, 1 % sodium deoxycholate, and 0.1 % SDS), and then sonicated at 4 °C for 2 min (2 s with 10 s intervals), and incubated on ice for 30 min. Lysates were centrifuged at 12,000×g for 5 min at 4 °C. The supernatant was subjected to western blotting analysis as described by Gao et al. (2011). Briefly, protein concentrations were determined by BCA protein assay kit (Cat no. 233225, Thermo) and 30 µg of protein was electrophoresed in a SDS polyacrylamide gel (15 % running gels and 5 % stacking gels). After being transferred to nitrocellulose membrane (Cat no. 88018, Invotrogen), the proteins were blotted with primary antibodies for MnSOD (Cat no. ab13534, Abcam, 1:3000) and β-actin (Cat no. A2103, Sigma, 1:5000). The secondary antibody was horseradish peroxidase-goat anti-rabbit IgG (Cat no. CW0103, CWBIO, Beijing, China, 1:5000). After exposition to the Chemiluminescence Western Blotting Kit (Cat no. 32106, Pierce), the bands were quantitated by using an image analysis system (FR-980, Shanghai FURI Science & Technology, Shanghai, China). β-actin protein was used for calibrating protein loading, and fold change was calculated relative to no Mn supplementation C.
The MnSOD activity was measured by the nitrite method as described by Li et al. (2004). Cells were scraped in ice-cold saline after being washed three times with ice-cold PBS, and then sonicated at 4 °C for 2 min (2 s with 10 s intervals). Lysates were centrifuged at 1000×g for 10 min at 4 °C. The protein concentrations in supernatants were determined by BCA protein assay kit (Cat no. 233225, Thermo), and the MnSOD activities were determined with superoxide dismutase typed assay kit by hydroxylamine method (Cat no. A001-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The MnSOD activity was expressed as nitrite units (NU) per milligram of protein (NU/mg protein), and one NU was defined as the amount of enzyme needed to obtain 50 % inhibition of nitrite formation.
Western blot analyses of total and phosphorylated p38 MAPK, JNK and ERK
Cells were lysed in 500 μL buffer A (10 mmol/L HEPES, pH 7.9, containing 10 mmol/L KCl, 2 mmol/L MgCl2, 0.1 mmol/L EDTA, 1 mmol/L DTT, 0.4 mmol/L PMSF, 0.2 mmol/L NaF, 0.2 mmol/L NaOrthovanadate, 100 μg/mL leupeptin 10 μg/mL aprotinin, and 10 μg/mL pepstatin). After 15 min of incubation on ice, 30 μL of buffer B consisting of 10 % NP-40 was added to the cell lysate and mixed vigorously. Following centrifugation at 14,000 rpm for 30 s, the supernatant was collected and saved at −80 °C for Western blot analysis of the MAPK.
Fifteen microgram of cytoplasmic protein was loaded onto a 10 % SDS-PAGE and then electroblotted onto a nitrocellulose membrane. The membrane was firstly incubated in the primary antibody for the phosphorylated form (p-p38MAPK, Cat no. PA1-84807, Thermo, 1:100; p-ERK1/2, Cat no. ab76165, Abcam, 1:200; p-JNK, Cat no. 54390, AnaSpec, 1:200), and then incubated with secondary antibody conjugated with HRP (Cat no. CW0103, CWBIO, Beijing, China, 1:5000) and visualized by the Chemiluminescence Western Blotting Kit (Cat no. 32106, Pierce). The membranes were then stripped at 50 °C for 30 min, and exposed to the antibody of the nonphosphorylated form of the MAPK (p38MAPK, Cat no. ab19329, Abcam, 1:200; ERK1/2, Cat no. ab79853, Abcam, 1:200; JNK, Cat no. 54389, AnaSpec, 1:200). The data were expressed as relative ratios of the densitometry units of phosphorylated MAPKs to total MAPKs in each experimental group compared with those of the C group.
Determination of intracellular activation of PKC
The monolayers were resuspended in lysis buffer (25 mmol/L Tris, pH 7.4, containing 0.5 mol/L sucrose, 2 mmol/L PMSF, 20 mmol/L 2-mercaptoethanol, 4 mmol/L EDTA, and 1 mmol/L EGTA, and 0.02 % leupeptin), sonicated, centrifuged at 100,000×g for 60 min at 4 °C, and the cytosolic fraction was collected. The pellet was sonicated in the buffer with 1 % Triton X-100 and centrifuged for 10 min at 14,000×g at 4 °C to obtain the supernatant (membrane fraction). Equal amounts of cytosolic or membrane protein (30 µg) were subjected to SDS/PAGE and Western blot analysis with PKC antibody (Cat no. ab69531, Abcam, 1:2000), and detected by chemiluminescence as described in MnSOD protein determination.
PTK activity assay
The monolayers in dishes were lysed in 1 mL freshly prepared lysis buffer (50 mmol/L HEPES, pH 7.4, containing 1 % Triton X-100, 10 % glycerol, 1 mmol/L dithiothreitol, and 1 mmol/L activated sodium vanadate, 1 mmol/L benzamidine, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 2 μg/mL pepstatin) for 15 min at 4 °C. The cell lysate was centrifuged at 10,000×g for 15 min at 4 °C. PTK activity in the supernatant was measured using Protein Tyrosine Kinase Assay Kit (Cat no. PTK101, Sigma) in a 96-well microtiter plate according to the manufacturer’s instructions. The PTK activity was expressed as the activity units per milligram of protein (U/mg protein).
Statistical analysis
Values were expressed as mean ± SEMs of the 6 replicate wells or dishes (n = 6). Data were analyzed by 1-way ANOVA using the GLM procedure of SAS. When ANOVA was significant, a Fisher PLSD test was applied to compare the differences between means. The replicate well or dish served as the experimental unit. Statistical significance was detected at P < 0.05.
Results
Effect of Mn source on MnSOD mRNA, protein and enzymatic activity
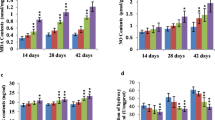
In Expt 1, the additions of Mn sources did not increase (P > 0.05) MnSOD mRNA levels when cells were treated with each of Mn sources for 12 h (Fig. 1a). The MnSOD mRNA levels were increased (P < 0.05) to 1.74, 2.06, 2.23 and 1.66 compared to C (0.91) when cells were treated with I, W, M or S for 48 h, respectively. The MnSOD mRNA level in M group was higher (P < 0.05) than those in I and S groups. No differences were observed (P > 0.05) between M and W or among I, W and S. The MnSOD protein levels (Fig. 1b) and MnSOD enzymatic activities (Fig. 1c) were increased (P < 0.05) by the Mn source additions at 12 and 48 h. No differences (P > 0.05) were observed in MnSOD protein levels or enzymatic activities among the four Mn sources at either 12 or 48 h. These results indicated that MnSOD mRNA level was more sensitive than MnSOD protein level or enzymatic activity in discriminating the differences among the four Mn sources, and M was more effective than I and S in inducing MnSOD mRNA expression. In addition, the MnSOD protein level was independent of transcriptional regulation when cells were treated with Mn for 12 h.
Effect of Mn source on MnSOD mRNA (a), protein (b), and enzymatic activity (c) in Expt 1. The primary myocardial cells were treated with Mn un-supplementation (C, filled square) or 0.5 mmol/L of Mn as the inorganic MnCl2 (I,  ) or one of three organic Mn sources with weak (W,
) or one of three organic Mn sources with weak (W,  ), moderate (M,
), moderate (M,  ) or Strong (S,
) or Strong (S,  ) chelation strengths for 12 or 48 h. All data were expressed as mean ± SE. a, b
P < 0.05 compared with the corresponding C and among Mn sources. *P < 0.05 between C in the two time-points
) chelation strengths for 12 or 48 h. All data were expressed as mean ± SE. a, b
P < 0.05 compared with the corresponding C and among Mn sources. *P < 0.05 between C in the two time-points
Possible signal pathways involved in the Mn-induced MnSOD transcription
In Expt 2, the MnSOD mRNA levels were increased (P < 0.05) by I and M compared with the Mn-unsupplemented C of medium as shown in Expt 1 (Fig. 2a). Additions of the three MAPKs inhibitors or NAC in the Mn-unsupplemented C did not affect (P > 0.05) MnSOD mRNA levels. The mRNA levels were higher in Mn-treated cells compared with C, and the M-treated cells had higher (P < 0.05) MnSOD mRNA level than I-treated cells when NAC was added to the cultures. The MnSOD mRNA levels in I were not decreased (P < 0.05) by the additions of each MAPKs inhibitor, however, no differences in MnSOD mRNA level were observed (P > 0.05) between I and M. Compared with C, the phosphorylations of the three numbers of MAPKs were not affected (P > 0.05) by each of Mn sources, as well as MAPKs inhibitors (Fig. 2b). These results suggested that the MnSOD mRNA expression induced by I or M might not be through activation of p38 MAPK, ERK or JNK pathway, as well as superoxide anions.
Effects of MAPK inhibitors or ROS scavenger on Mn-induced MnSOD mRNA levels (a) and MAPKs phosphorylation (b) in Expt 2. The primary myocardial cells were preincubated with inhibitors for p-38 mitogen-activated protein kinases (MAPK) (SB203580, 10 µmol/L), c-Jun N-terminal kinase (JNK) (JNKI1, 50 nmol/L), extracellular-signal-regulated kinase (ERK) (PD98059, 30 µmol/L), or N-acetylcysteine (NAC, 500 µmol/L) for 30 min before 48-h treatment of Mn un-supplementation (C, filled square) or 0.5 mmol/L of Mn as inorganic MnCl2 (I,  ) or organic Mn source with moderate chelation strength (M,
) or organic Mn source with moderate chelation strength (M,  ). All data were presented as the mean ± SE. a, b
P < 0.05 compared with the corresponding C and between I and M. The figures in a, b, and c of B indicate the relative abundances of phosphorylated MAPKs to MAPKs compared with the Mn-unsupplemented C
). All data were presented as the mean ± SE. a, b
P < 0.05 compared with the corresponding C and between I and M. The figures in a, b, and c of B indicate the relative abundances of phosphorylated MAPKs to MAPKs compared with the Mn-unsupplemented C
Protein kinases and the Mn-induced MnSOD translation
In Expt 3, the MnSOD protein levels were increased (P < 0.05) by I and M compared with the Mn-unsupplemented C of medium as shown in Expt 1 (Fig. 3a). The addition of calphostin C did not affect (P > 0.05) the MnSOD protein level, but genistein increased (P < 0.05) and NAC decreased (P < 0.05) the MnSOD protein levels in the Mn-unsupplemented C. The MnSOD protein levels were higher (P < 0.05) in Mn treatments when the cells were cultured with addition of NAC compared with the corresponding C. There were no differences (P > 0.05) in MnSOD protein level among C and the two Mn sources when genistein or calphostin C was added into media. The PTK activities were decreased (P < 0.05) when cells were treated with Mn sources compared with the Mn-unsupplemented C medium, respectively (Fig. 3b). Compared with the Mn-unsupplemented C of medium, addition of calphostin C or NAC did not affect (P > 0.05) PTK activity, but addition of genistein decreased (P < 0.05) PTK activity in Mn-unsupplemented C. No decreases in PTK activity were observed (P > 0.05) in Mn-treated cells when either genistein or calphostin C was added to the media, however, the PTK activities were lower (P < 0.05) in Mn treatments than in C when NAC was added to the media. The membrane PKC proteins in Mn-treated cells were increased (P < 0.05) compared with the Mn-unsupplemented C medium, in which the membrane PKC protein in M was higher (P < 0.05) than that in I (Fig. 3c). Additions of genistein, calphostin C or NAC did not affect membrane PKC proteins in their corresponding C. The membrane PKC proteins were higher (P < 0.05) in Mn-treated cells than in C cells when genistein or NAC was added into the media, however, there were no differences (P > 0.05) in membrane PKC proteins among C and the two Mn sources when calphostin C was added. These results indicated that Mn might elevate MnSOD protein level through the activations of PKC and PTK pathways, which was not correlated with ROS.
Effects of PTK and PKC inhibitors or ROS scavenger on Mn-induced MnSOD protein levels (a), PTK activities (b) and PKC activations (c) in Expt 3. The primary myocardial cells were preincubated with inhibitors for PTK (genistein, 50 µmol/L), PKC (calphostin C, 1 µmol/L) or N-acetylcysteine (NAC, 500 µmol/L) for 30 min before 12-h incubation of Mn un-supplementation (C, filled square) or 0.5 mmol/L of Mn as inorganic MnCl2 (I,  ) or organic Mn source with moderate chelation strength (M,
) or organic Mn source with moderate chelation strength (M,  ). All data were presented as the means ± SE. a, b
P < 0.05 compared with the corresponding C and between I and M. *P < 0.05) compared with Mn un-supplementation (C) without inhibitor
). All data were presented as the means ± SE. a, b
P < 0.05 compared with the corresponding C and between I and M. *P < 0.05) compared with Mn un-supplementation (C) without inhibitor
Discussion
Our results in Expt 1 revealed a close relationship between Mn sources and MnSOD mRNA and protein levels and enzymatic activities, which was consistent with our previous in vivo studies (Li et al. 2004, 2005, 2008, 2011; Luo et al. 2007). These results further confirmed that the organic Mn source with moderate chelation strength is more effective than other Mn sources in regulation of MnSOD gene expression. As found in our preliminary myocardial cell cultural experiment (Gao et al. 2011), the disparity between MnSOD mRNA and MnSOD protein levels at 12 h of Mn treatments further indicated that the Mn-induced MnSOD gene expression in the primary broiler myocardial cells could be modulated at transcriptional and translational levels, or the stabilities of synthesized mRNA and protein.
Although MnSOD gene is highly inducible by various stimuli triggering oxidative stress, such as TNF-α (Fujii and Taniguchi 1991), 12-O-tetradecanoylphorbol-3-acetate (TPA) (Whitsett et al. 1992), and irradiation (Akashi et al. 1995), the induction of MnSOD protein expression by MnCl2 in human breast cancer Hs578T cells was not inhibited by superoxide scavenger 5, 5-dimethyl-1-pyrroline-1-oxide or ROS scavenger NAC (Thongphasuk et al. 1999). Our results that the MnSOD mRNA levels of Mn-treated cells in Expt 2 were not decreased by NAC suggested that Mn-induced MnSOD mRNA expression or stability might not be related with ROS in heart cells. The results that MnSOD protein level of Mn-unsupplemented C in Expt 3 was decreased by NAC suggested that cellular MnSOD protein level depend on ROS. However, the results that MnSOD protein levels of Mn-treated cells were not affected by NAC compared to the corresponding C suggested that Mn-induced MnSOD protein expression or stability might be independent of ROS.
The signaling pathways involved in MnSOD induction may be stimulant-dependent and cell type-specific. Both JNK and p38 MAPK contributed to the regulation of MnSOD expression by hepatitis C virus (Qadri et al. 2004), but it did not occur in the induction of MnSOD expression by TNF-α (Rogers et al. 2001) or LPS (White et al. 2000). PKC mediated the TNF-α-induced MnSOD transcription in human endometrial stromal cells (Karube-Harada et al. 2001) but not in human kidney cells (Daniel et al. 1995). Signaling transductions responsible for Mn have almost been focused on the toxic effect of Mn on catecholaminergic rich pheochromocytoma (PC12). A low-level Mn exposure led to activation of MAPK kinase and the subsequencial activation of JNK (Hirata and Kazutoshi 1998; Ramesh et al. 2002; Wise et al. 2004), resulting in a cytotoxic effect on neuronal function. However, induction of MnSOD gene expression by MnCl2 may not be through the reducing property of Mn(II) and the Mn-induced cell killing was not caused by induction of MnSOD gene expression in human breast cancer Hs578T cells (Thongphasuk et al. 1999). Our results in Expt 2 that the phosphorylations of the three numbers of MAPKs were not activated by the two Mn sources suggested that p38MAPK, JNK or ERK signaling pathway might be not involved in the Mn-induced MnSOD transcriptional modulation in the primary broiler myocardial cells. The additions of MAPKs inhibitors resulting in the decreases in MnSOD mRNA levels in M-treated cells but not in I-treated cells indicated that organic Mn source M might have an interaction with MAPKs on the MnSOD gene transcriptional regulation. The exact mechanism need to be further investigated.
Kinase/phosphatase signal transduction cascades are also critical in the regulation of the activity of initiation and elongation factors in the control of protein synthesis (Rhoads 1999). Inhibition of PTK increases MnSOD RNA-binding protein activity and MnSOD protein expression without change in MnSOD mRNA level (Knirsch and Clerch 2001). Hyperoxia (Sheth et al. 1997) or reactive oxygen intermediates (Brumell et al. 1996) causes an increase in PTK activity and PTK inhibitor genistein attenuated the H2O2-mediated increase in protein tyrosine phosphorylation (Natarajan et al. 1996). Our results in Expt 3 that Mn increased the MnSOD protein level along with the lower PTK activities in Mn-treated cells suggested that Mn might induce MnSOD protein expression in heart by activation of tyrosine phosphatase pathway.
Because PKC inhibitor also reduced the elevation of Mn-induced MnSOD protein level, we detected the PKC activation in Mn-treated cells. In general, PKC is located in the cytosol of unstimulated cells. After stimulation, PKC is translocated to the membrane region (Martelly and Castagna 1989). Hence, translocation of PKC to the membrane fraction provides one indicator of its activation. In the present study, we found that the inductions of MnSOD protein by the Mn sources were in according with the elevations of membrane PKC levels. This observation implied that activation of PKC might mediate the Mn-induced MnSOD protein expression. Meanwhile, PKC inhibitor calphostin C also inhibited the decrease of PTK activities in Mn-treated cells, suggesting that PTK might be a downstream mediator in the Mn-induced MnSOD protein expression by PKC in broiler heart. Organic Mn source M was more effective in the PKC activation than I, implied that PKC was also a sensitive indicator in distinguishing the effectiveness of M in MnSOD gene expression regulation. These findings could provide a new strategy for controlling cellular MnSOD protein level with nutrients.
In conclusion, the Mn-induced MnSOD mRNA and protein expressions were not correlated with ROS. Mn might exert its control on MnSOD protein expression regulation through PTK/phosphatase signal transduction pathway, in which PKC might be the upstream mediator. No significant differences were found in signal pathways between I and M. The present study reveals a novel role of Mn in Mn-induced MnSOD gene expression modulation through protein kinases.
Abbreviations
- MnSOD:
-
Manganese-containing superoxide dismutase
- ROS:
-
Reactive oxygen species
- Mn:
-
Manganese
- MAPKs:
-
Mitogen-activated protein kinases
- p38 MAPK:
-
p38 Mitogen-activated protein kinase
- JNK:
-
C-Jun N-terminal kinase
- PKC:
-
Protein kinase C
- PTK:
-
Protein tyrosine kinase
- I:
-
Inorganic MnCl2
- Qf :
-
Formation quotient
- W:
-
Organic chelates of Mn and amino acids with weak chelation strength
- M:
-
Organic chelates of Mn and amino acids with moderate chelation strength
- S:
-
Organic chelates of Mn and amino acids with strong chelation strength
- ERK:
-
Extracellular-signal-regulated kinase
- NAC:
-
N-acetylcysteine
References
Akashi M, Hachiya M, Paquette RL, Osawa Y, Shimizu S, Suzuki G (1995) Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. Possible mechanisms for its accumulation. J Biol Chem 270:15864
Bianchi A, Becuwe P, Franck P, Dauca M (2002) Induction of MnSOD gene by arachidonic acid is mediated by reactive oxygen species and p38 MAPK signaling pathway in human HepG2 hepatoma cells. Free Rad Biol Med 32:1132–1142
Brumell JH, Burkhardt AL, Bolen JB, Grinstein S (1996) Endogenous reactive oxygen intermediates activate tyrosine kinases in human neutrophils. J Biol Chem 271:1455–1461
Daniel LW, Civoli F, Rogers MA, Smitherman PK, Raju PA, Roederer M (1995) ET-18-OCH3 inhibits nuclear factor-κb activation by 12-0-tetradecanoylphorbol-13-acetate but not by tumor necrosis factor-α or interleukin lα. Cancer Res 55:4844–4849
Das KC, Guo XL, White CW (1998) Protein kinase C & #x03B4;-dependent induction of manganese superoxide dismutase gene expression by microtubule-active anticancer drugs. J Biol Chem 273:34639–34645
Fujii J, Taniguchi N (1991) Phorbol ester induces manganese-superoxide dismutase in tumor necrosis factor-resistant cells. J Biol Chem 266:23142–23146
Gao TQ, Wang FN, Li SF, Luo XG, Zhang KY (2011) Manganese regulates manganese-containing superoxide dismutase (MnSOD) expression in the primary broiler myocardial cells. Biol Trace Elem Res 144:695–704
HirataY Kayo A, Kazutoshi K (1998) Activation of JNK pathway and induction of apoptosis by manganese in PC12 cells. J Neurochem 71:1607–1615
Holwerda RA, Albin RC, Madsen FC (1995) Chelation effectiveness of zinc proteinates demonstrated. Feedstuffs 67(12–13):23
Karube-Harada A, Sugino N, Kashida S, Takiguchi S, Takayama H, Yamagata Y et al (2001) Induction of manganese superoxide dismutase by tumour necrosis factor-alpha in human endometrial stromal cells. Mol Hum Reprod 7:1065–1072
Knirsch L, Clerch LB (2001) Tyrosine phosphorylation regulates manganese superoxide dismutase (MnSOD) RNA-binding protein activity and MnSOD protein expression. Biochem 40:7890–7895
Kujime K, Hashimoto S, Gon Y, Shimizu K, Horie T (2000) p38 Mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol 164:3222–3228
Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr, Dionnel L, Lu N et al (1996) Neurodegeneration, myocardial injury and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci 93:9782–9787
Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL et al (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11:376–381
Li SF, Luo XG, Liu B, Crenshaw TD, Kuang X, Shao GZ et al (2004) Use of chemical characteristics to predict relative bioavailability of supplemental organic manganese sources for broilers. J Anim Sci 82:2352–2363
Li SF, Luo XG, Lu L, Crenshaw TD, Bu YQ, Liu B et al (2005) Bioavailability of organic manganese sources in broilers fed high dietary calcium. Anim Feed Sci Technol 123–124:703–715
Li SF, Luo XG, Lu L, Liu B, Kuang X, Shao GZ et al (2008) Effect of intravenously injected manganese on the gene expression of manganese-containing superoxide dismutase in broilers. Poult Sci 87:2259–2265
Li SF, Lu L, Hao SF, Wang YP, Zhang LY, Li SB et al (2011) Dietary manganese modul ates the expression of manganese-containing superoxide dismutase gene in chickens. J Nutr 141:189–194
Luo XG, Li SF, Lu L, Liu B, Kuang X, Shao GZ et al (2007) Gene expression of manganese-containing superoxide dismutase as a biomarker of manganese bioavailability for manganese sources in broilers. Poult Sci 86:888–894
Martelly I, Castagna M (1989) Protein kinase C and tumor promoters. Curr Opin Cell Biol 1:206–210
Natarajan DV, Vepa S, Verma RS, Scribner WM (1996) Role of protein tyrosine phosphorylation in H2O2-induced activation of endothelial cell phospholipase D. Am J Physiol 271:L400–L408
Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J et al (2004) Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J 378:919–928
Ramesh GT, Ghosh D, Gunasekar PG (2002) Activation of early signaling transcription factor, NF-kB following low-level manganese exposure. Toxicol Lett 136:151–158
Rhoads RE (1999) Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem 274:30337–30340
Rogers RJ, Monnier JM, Nick HS (2001) Tumor necrosis factor-alpha selectively induces MnSOD expression via mitochondria-to-nucleus signaling, whereas interleukin-1 beta utilizes an alternative pathway. J Biol Chem 276:20419–20427
Sheth MV, Goodman BE, Friese JL, Eyster KM (1997) Protein phosphatase activity in the lungs of normoxic versus hyperoxic rats. Exp Lung Res 23:475–494
Sun H, Tonks NK (1994) The coordinated action of protein tyrosine phosphatases and kinases in cell signaling. Trends Biochem Sci 19:480–485
Tanaka T, Kurabayashi M, Aihara Y, Ohyama Y, Nagai R (2000) Inducible expression of manganese superoxide dismutase by phorbol 12-myristate 13-acetate is mediated by Sp1 in endothelial cells. Arterioscler Thromb Vasc Biol 20:392–401
Thongphasuk J, Oberley LW, Oberley TD (1999) Induction of superoxide dismutase and cytotoxicity by manganese in human breast cancer cells. Arch Biochem Biophys 365:317–327
White JE, Lin HY, Davis FB, Davis PJ, Tsan MF (2000) Differential induction of tumor necrosis factor alpha and manganese superoxide dismutase by endotoxin in human monocytes: role of protein tyrosine kinase, mitogen-activated protein kinase, and nuclear factor kappaB. J Cell Physiol 182:381–389
Whitsett JA, Clark JC, Wispe JR, Pryhuber GS (1992) Effects of TNF-alpha and phorbol ester on human surfactant protein and MnSOD gene transcription in vitro. Am J Physiol 262:L688–L693
Wise K, Manna S, Barr J, Gunasekar P, Ramesh G (2004) Activation of activator protein-1 DNA binding activity due to low level manganese exposure in pheochromocytoma cells. Toxicol Letters 147:237–244
Acknowledgments
This work was supported by the Key International Cooperation Program of the National Natural Science Foundation of China (31110103916), the Agricultural Science and Technology Innovation Program (ASTIP-IAS08), the National Natural Science Foundation of China (31272465), and China Agriculture Research System (CARS-42). We thank Yongwen Zhu and Xiaoming Sun for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Lu, L., Liao, X. et al. Manganese elevates manganese superoxide dismutase protein level through protein kinase C and protein tyrosine kinase. Biometals 29, 265–274 (2016). https://doi.org/10.1007/s10534-016-9913-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9913-9