Abstract
Heat stress leads to oxidative stress and induces apoptosis in various cells. Endoplasmic reticulum (ER) stress is an important apoptosis pathway. Manganese (Mn) has been shown to enhance the activity of manganese superoxide dismutase (MnSOD). To explore the potential effect of Mn on ER stress and apoptosis induced by heat stress, we examined crucial factors associated with heat stress, ER stress, and apoptosis in cultured primary chick embryonic myocardial cells that had been pretreated with 20 μM Mn for 24 h and then subjected to 4 h of heat stress. The results showed that Mn decreased (P < 0.05) heat stress–induced reactive oxygen species (ROS) production and exerted antiapoptotic effects by increasing MnSOD enzymatic activity. The heat stress–induced accumulation of intracellular calcium was dramatically reduced (P < 0.05). Mn treatment significantly decreased (P < 0.05) the expression levels of the apoptosis-related gene Bax and ER stress markers glucose-regulated protein 78 (GRP78) and CCAAT/enhancer binding protein homologous protein (CHOP) in primary chick embryonic myocardial cells. Additionally, Mn reduced oxidative stress by activating the nuclear factor E2-related factor 2 (NRF2)/SOD2 signaling pathway. Taken together, our findings indicate that Mn attenuates heat stress–induced apoptosis by inhibiting ROS generation, intracellular calcium accumulation, and the ER stress pathway and activating the NRF2/SOD2 signaling pathway to protect myocardial cells from oxidative stress during chick embryonic development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial damage caused by excessive ambient temperature has been considered a risk factor that threatens the health of poultry and is characterized by rapid increases in respiratory rate, blood flow, and heart rate [1]. Heat stress may profoundly affect the heart and lead to sudden death in high-density poultry in captivity, especially in the summer. It is generally accepted that heat stress can change the activity of metabolic enzymes, thereby breaking the balance of the antioxidant system in the body, leading to oxidative stress and mediating apoptosis in multiple ways [2,3,4]. Heat stress causes intracellular reactive oxygen species (ROS) accumulation, and excessive ROS levels activate downstream regulatory factor, and induce apoptosis, which manifests as mitochondrial p53 translocation and dynamic calcium (Ca2+) balance disorder [5,6,7].
Oxidative stress and endoplasmic reticulum (ER) stress are closely linked. Recent studies have shown that prolonged heat stress can trigger ER stress in cardiac myocytes, and the ER stress pathway is associated with apoptosis [8,9,10]. By inhibiting the ER stress pathway, selenium can reduce chronic heat stress–induced apoptosis [11]. Under heat stress conditions, alpha lipoic acid significantly alleviates the adverse effects of heat stress by affecting the activity of antioxidant enzymes and the occurrence of ER stress–related apoptosis in chicken testes [12].
Manganese (Mn) is an essential micronutrient in animals and a component of manganese superoxide dismutase (MnSOD). MnSOD attenuates oxidative injury by enhancing ROS scavenging abilities and regulating the cellular redox status. Mn promotes the activity and gene expression of MnSOD, thus protecting myocardial cells from oxidative stress during chick embryonic development [13]. Studies by Li et al. showed that Mn could increase MnSOD gene expression and enzymatic activity via the protein tyrosine kinase (PTK) signal transduction pathway in the myocardium of broiler chickens [14]. Evidence has shown that a maternal diet supplemented with Mn significantly increases the activity and gene expression of MnSOD and increases the mRNA and protein expression of Bcl-2 in the myocardia of offspring chicken embryos, thus inhibiting heat stress–induced apoptosis [15, 16]. This finding suggests that maintaining or enhancing the function of MnSOD under heat stress may be beneficial in reducing heat stress–induced apoptosis.
Our previous study indicated that Mn could improve MnSOD activity [13] and reduce heat stress–induced apoptosis (the relevant data has been submitted, but yet published) in primary chick embryonic myocardial cells. However, it is still not fully understood whether Mn protects against heat stress–induced apoptosis in primary chick embryonic myocardial cells by inhibiting ER stress and ROS generation. We hypothesized that Mn pretreatment would ameliorate ROS production and ER stress, and thus attenuate heat stress–induced apoptosis in cultured primary chick embryonic myocardial cells.
Materials and Methods
Isolation, Culture, and Treatment of Primary Chick Embryonic Myocardial Cells
All procedures were approved by the Animal Welfare Committee of the Institute of Animal Science, Gansu Agricultural University. Chick embryonic myocardial cells were prepared from 10-day-old embryonated eggs as described by Qin et al. [13]. Briefly, the embryonic heart was removed and minced under sterile conditions. The myocardial tissue was digested with 0.12% collagenase II (Solarbio, China) in a shaking water bath at 37 °C for 8 min, and this process was repeated 4–5 times. The digested solution was centrifuged at 1000 g for 5 min. The cell pellet was resuspended and maintained in DMEM/F-12 medium (BasalMedia, China) supplemented with 20% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin–streptomycin solution (Solarbio, China) at 37 °C and 5% CO2. When the cells reached approximately 80% confluence, the myocardial cells were treated with 20 μM Mn (MnCl2, Sigma-Aldrich, USA; Mn compound amino acid complex, Trouw Nutrition, The Netherlands) for 24 h. The culture medium was replaced with fresh medium, and the cells were placed in a 42 °C incubator in 5% CO2 for heat stress for 4 h. Then, the cells were digested with 0.25% trypsin, collected in the retained culture medium, and centrifuged at 1000 rpm for 5 min. The cell pellet was harvested for subsequent examinations.
Measurement of ROS
The levels of intracellular ROS were assessed using a ROS assay kit (Solarbio, China). Briefly, chick embryonic myocardial cells were exposed to 42 °C for 4 h and then harvested. Then, the cell pellet was resuspended in serum-free DMEM containing 10 mM DCFH-DA and incubated at 37 °C for 20 min in the dark. DCFH-DA is a probe that enters cells and reacts with ROS to form the fluorescent product DCF. The fluorescence intensity of the ROS probe was measured (excitation 488 nm/emission 525 nm) using a multifunctional microplate reader (Thermo Fisher Scientific, Varioskan LUX, USA).
MnSOD Activity Assay
MnSOD activity was measured using a Cu/Zn-SOD and Mn-SOD assay kit with WST-8 (Beyotime, China) according to the manufacturer’s protocol. The protein concentrations were measured using a BCA protein assay kit (Solarbio, China). MnSOD activity is expressed as units per milligram of protein (U mg−1 protein), and one unit was defined as the SOD enzyme activity required to obtain 50% inhibition in the xanthine oxidase coupling reaction system.
Analysis of Apoptosis Rates
Apoptosis rates were analyzed using an Annexin V-FITC/PI apoptosis detection kit (Beyotime, China) according to the manufacturer’s protocol. The cells were washed with phosphate-buffered saline (PBS) and centrifuged (1500 rpm, 5 min), and then a 10 µL mixture of Annexin V-FITC and PI staining solutions (1:1) was added to the cells and mixed. After 10 min of incubation in the dark at 4 °C, apoptosis was analyzed immediately using flow cytometry (BD, FACSCanto, USA).
Ca2+ Assay
Cytosolic Ca2+ was measured by using the cell-permeable Ca2+ fluorophore Fluo-4 (Solarbio, China). After 4 h of heat stress at 42 °C, chick embryonic myocardial cells were harvested and incubated with 5 µM Fluo-4 AM for 30 min at 37 °C. Changes in cytoplasmic Ca2+ were evaluated by flow cytometry (BD, FACSCanto, USA).
Western Blot Analysis
Total cellular proteins were extracted with RIPA lysis buffer containing 1 mM PMSF (Solarbio, China), and protein concentrations were measured using a BCA protein assay kit (Solarbio, China). A total of 20 µg of protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Germany). The membranes were blocked in 5% skimmed milk for 2 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: HSP70 (1:300; Bioss, Beijing, China), Bax (1:1000; Proteintech, Wuhan, China), glucose-regulated protein 78 (GRP78) (1:2000; Proteintech, Wuhan, China), CCAAT/enhancer binding protein homologous protein (CHOP) (1:500; Proteintech, Wuhan, China), nuclear factor E2-related factor 2 (NRF2) (1:500; Proteintech, Wuhan, China), calmodulin (1:200; Bioss, Beijing, China), MnSOD (1:3 000; Abcam, England), and GAPDH (1:5000; Proteintech, Wuhan, China). After being incubated, the PVDF membranes were washed four times with Tris-buffered saline containing 0.1% Tween 20 (TBST) and then incubated with HRP-conjugated secondary antibodies (1:6000; Proteintech, Wuhan, China) for 1 h at room temperature. After being washed with TBST, the membranes were exposed to ECL reagent (Servicebio, China), and chemiluminescence was detected by an Amersham Imager 600 (GE, USA) and analyzed with ImageJ Software (National Institutes of Health, USA). GAPDH was used as a loading control.
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted from primary chick embryonic myocardial cells using TRIzol reagent (TransGen, China). RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara, Japan) according to the manufacturer’s instructions. Real-time PCR was performed on a LightCycler 480 System (Roche, USA) using a SYBR-Green Premix Pro Taq HS qPCR kit (AG, China). The primer sequences used to amplify the target genes are listed in Table 1. Target gene expression was normalized to the geometric mean of β-actin and GAPDH. Differential gene expression was calculated using the 2−△△Ct method.
Statistical Analysis
All data were analyzed by SPSS 19.0 (IBM SPSS Software, Armonk, NY, USA), and statistical analysis was performed using one-way ANOVA with Turkey’s multiple comparisons test. The data are shown as the mean ± SD, and P < 0.05 was considered statistically significant.
Results
The Effect of Manganese on ROS Production and Apoptosis Induced by Heat Stress in Primary Chick Embryonic Myocardial Cells
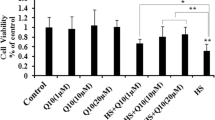
ROS generation plays an important role in heat stress and is known to be associated with apoptosis. To determine whether Mn, as a component of MnSOD, reduced ROS generation, intracellular ROS concentrations were measured using the DCFH-DA assay. As shown in Fig. 1A, we observed that Mn decreased (P < 0.05) heat stress–induced ROS production, and the reduction level of ROS by organic Mn was significantly higher than by inorganic Mn. To investigate whether Mn-mediated inhibition of ROS generation could alleviate apoptosis, we assessed MnSOD enzymatic activity, the apoptosis rate, and heat stress–related and apoptosis-related proteins in primary chick embryonic myocardial cells. Cells were pretreated with the antioxidant MnTMPyP (10 µmol/l) for 1 h and then treated with heat stress (42 °C) as a positive control. The data show that Mn exhibits antiapoptotic activity in primary chick embryonic myocardial cells by increasing the enzymatic activity of MnSOD (P < 0.05) (Fig. 1B–C). Mn significantly decreased the apoptosis rate (Fig. 1D) and the protein levels of HSP70 (a key marker of heat stress) and Bax (an apoptosis-related gene) in chick embryonic myocardial cells (P < 0.05) (Fig. 1E–G).
The effect of manganese on ROS production and apoptosis induced by heat stress. Primary chick embryonic myocardial cells were treated with Mn for 24 h and heat stressed at 42 °C for 4 h. (A) The levels of ROS were assayed by DCFH staining. (B–C) MnSOD activity was measured by WST-8. (D) Apoptosis was analyzed by flow cytometry. (E–G) Western blotting was used to analyze the protein expression levels of the heat stress–related marker protein HSP70 (F) and the apoptosis-related protein Bax (G). GAPDH was used as an internal control. The data shown represent the mean ± SD of at least three independent experiments, and different lowercase letters indicate significant differences (P < 0.05)
Manganese Mitigates Heat Stress–Induced Apoptosis and ER Stress in Primary Chick Embryonic Myocardial Cells
To investigate the effect of Mn on heat stress–induced apoptosis and ER stress, primary chick embryonic myocardial cells were treated with 20 μM Mn for 24 h and heat stressed at 42 °C for 4 h. Heat-treated primary chick embryonic myocardial cells were subjected to q-PCR and Western blotting as shown in Fig. 2. The results showed that the mRNA expression levels of HSP70, Caspase3, Bax, GRP78, and CHOP (ER stress–related proteins in heat-treated myocardial cells) were significantly increased (P < 0.05) compared with the those of the control (Fig. 2A–B). Mn decreased (P < 0.05) heat stress–induced mRNA expression of HSP70, Caspase3, Bax, GRP78, and CHOP, but there was no significant difference in the effects of Mn sources (P > 0.05) except regarding Bax expression. Similarly, after heat treatment, the protein levels of HSP70, Caspase3, Bax, GRP78, and CHOP increased prominently (P < 0.05) (Fig. 2C–H). Mn significantly decreased (P < 0.05) the protein levels of HSP70, Bax, GRP78, and CHOP in heat-stressed chick embryonic myocardial cells. However, the effect of the Mn source was not consistent.
Manganese mitigates heat stress–induced apoptosis and ER stress. Primary chick embryonic myocardial cells were treated with Mn for 24 h and heat stressed at 42 °C for 4 h. (A–B) qRT-PCR was used to analyze the mRNA levels of target genes. (C–H) Western blotting was used to analyze the protein expression levels of the heat stress–related marker protein HSP70 (E), cell apoptosis-related protein Bax (F), and ER stress activation markers GRP78 (G) and CHOP (H). GAPDH was used as an internal control. The data shown represent the mean ± SD of at least three independent experiments, and different lowercase letters indicate significant differences (P < 0.05)
Manganese Reduces Heat Stress–Induced Intracellular Calcium Elevation
Ca2+ is a critical second messenger involved in apoptosis, and in the early and late stages of apoptosis, increased intracellular Ca2+ has been observed [17, 18]. To investigate whether heat stress–induced apoptosis is associated with increased intracellular Ca2+ and to determine whether Mn reduces the accumulation of intracellular Ca2+, we examined the effect of Mn on changes in Ca2+ during heat stress–induced apoptosis using flow cytometric analysis of primary chick embryonic myocardial cells stained with the fluorescent probe Fluo-4. The results showed that heat stress caused a significant increase in the intracellular Ca2+ concentration, while similar to the changes in ROS levels, Mn dramatically reduced the heat stress–induced accumulation of intracellular Ca2+ (Fig. 3).
Manganese reduces heat stress–induced intracellular calcium elevation. Primary chick embryonic myocardial cells were treated with Mn for 24 h and heat stressed at 42 °C for 4 h. The effect of heat stress and Mn on Ca2+ overload was determined using flow cytometric analysis with the fluorescent probe Fluo-4. The data shown represent the mean ± SD of at least three independent experiments, and different lowercase letters indicate significant differences (P < 0.05)
Manganese Inhibits Oxidative Stress by Activating the NRF2/SOD2 Signaling Pathway
NRF2 is a key transcription factor that regulates the antioxidant stress response, can promote the expression of the antioxidant protein SOD2 (MnSOD) and activate the endogenous antioxidant response [19, 20]. To explore whether the NRF2/SOD2 pathway is involved in the effect of Mn on heat stress–induced apoptosis, we measured the mRNA and protein expression of NRF2 and SOD2. As shown in Fig. 4A, the mRNA expression levels of NRF2, Keap1 (a negative regulatory factor of NRF2) and SOD2 of the heat stress group were significantly increased (P < 0.05) compared with those of the control (Fig. 4A). Mn decreased (P < 0.05) the heat stress–induced mRNA expression of NRF2 and Keap1 but increased the mRNA expression of SOD2 (P < 0.05). After heat treatment, the protein levels of NRF2 increased notably (P < 0.05) (Fig. 4B–D), and Mn significantly attenuated this change (P < 0.05). Additionally, the protein levels of SOD2 decreased under heat stress, and Mn treatment group significantly enhanced the SOD2 expression level (P < 0.05) in heat-stressed chick embryonic myocardial cells (Fig. 4C, D).
Manganese inhibits oxidative stress by activating the Nrf2/SOD2 signaling pathway. Primary chick embryonic myocardial cells were treated with Mn for 24 h and heat stressed at 42 °C for 4 h. (A) qRT-PCR was used to analyze the mRNA levels of target genes. (B–D) Western blotting was used to analyze the protein expression levels of Nrf2 (C) and SOD2 (D). GAPDH was used as an internal control. The data shown represent the mean ± SD of at least three independent experiments, and different lowercase letters indicate significant differences (P < 0.05)
Correlation Analysis
To further clarify the correlation of apoptosis and ROS production, intracellular Ca2+ concentration, ER stress levels in heat-stressed chick embryonic myocardial cells, we conducted a Pearson correlation coefficient analysis (Table 2). Data showed strong correlation between the protein expression of bax (marker of apoptosis) and ROS, intracellular Ca2+ concentration, the protein expression of GRP78 (marker of ER stress) (P < 0.05), but no significant correlation with the protein expression of CHOP. Additionally, there was a significant correlation between ROS and intracellular Ca2+concentration (P < 0.05), and between intracellular Ca2+concentration and the protein expression of GRP78 (P < 0.01). However, there was no significant correlation between ROS and the expression of ER stress–related genes.
Discussion
Heat stress is a difficult problem that is often encountered in the concentrated feeding of poultry, which can lead to great loss of growth and reproduction. Heat stress causes intracellular ROS accumulation, which leads to disruptions in the antioxidant mechanism of the body, resulting in oxidative stress [3, 4, 21]. Therefore, heat stress is widely recognized as a major extracellular stimulus [22]. In addition, heat stress leads to protein denaturation, especially that of various enzymes, and the disruption of normal physiological processes, leading to apoptosis [23]. Heat stress and apoptosis are closely linked. Studies have shown widespread heat stress–induced apoptosis in several cell types, such as cells of the spleen, lymph node, thymus, and small bowel and cells of lymphoid origin in the lamina propria [24], which triggers ER stress, disordered Ca2+ balance, and mitochondrial p53 translocation [5, 6, 8]. Mn is an essential trace mineral that has a variety of beneficial effects on animals. As a cofactor of MnSOD, Mn plays an antioxidant role by inhibiting or scavenging excessive ROS in the body [25]. In the present study, we showed that Mn attenuated heat stress–induced apoptosis in cultured primary chick embryonic myocardial cells. After 4 h of heat stress, ROS increased dramatically. Generally, basal levels of ROS contribute to normal cellular function, but excessive ROS are induced by extreme environments, including irradiation, external stimulation, or environmental pollution, and could induce oxidative stress and cell death [26]. We also observed that the apoptosis rates of heat-treated cells were significantly increased compared with those of the control. We believe that heat stress and heat-induced ROS production may jointly promote cell death [27,28,29]. In this study, consistent with other reported studies [13, 30], heat stress stimulated the enzymatic activity of MnSOD in primary chick embryonic myocardial cells, and we hypothesized that MnSOD activity was a defense measure against heat stress. We found that the enzymatic activity of MnSOD was significantly increased; additionally, the expression levels of HSP70 and Bax were decreased after Mn treatment, suggesting that Mn could alleviate the level of heat stress by improving MnSOD activity and reducing cell apoptosis. Organic Mn showed better antiapoptotic effects than inorganic Mn, which may be due to the higher bioavailability of organic Mn.
The ER is the main site of protein synthesis, secretion, modification, and transport and is the storage site of Ca2+ in cells, playing an important role apoptosis [31]. Ca2+ is an important second messenger in cells that affects various physiological and pathological activities by translating extracellular stimuli into intracellular signaling pathways [32]. Increased cytoplasmic Ca2+ concentrations disrupt Ca2+ homeostasis and induce caspase activation and subsequent apoptosis [33, 34]. In this study, Mn dramatically decreased the accumulation of intracellular Ca2+ and the heat stress–induced expression levels of apoptosis-related genes and ER stress activation markers Bax, GRP78, and CHOP in primary chick embryonic myocardial cells. Other studies with mouse granulosa cells [11] and human umbilical vein endothelial cells [7] have also shown that the inhibition of ER stress and Ca2+ disorder can effectively reduce apoptosis. A prior study reported that Ca2+ depletion in the ER was the main cause of apoptosis and ER stress; conversely, blocking ER Ca2+ outflow could promote oxidative phosphorylation and maintain intracellular adenosine triphosphate (ATP) levels, consequently supporting cell growth [35]. Based on previous findings, we hypothesized that the inhibitory effect of Mn on ER stress and Ca2+ accumulation would be beneficial for alleviating heat stress–induced apoptosis in primary chick embryonic myocardial cells.
NRF2 can activate the endogenous antioxidant response as a key transcription factor that regulated the intracellular antioxidant stress response [36]. After oxidative stimulation, NRF2 enters the nucleus, binds to the oxidative response element, and then stimulates antioxidant function by activating downstream antioxidant genes such as HO-1 Rbx1 and TrxR1 [37, 38]. There have been few reports about the interaction between MnSOD and Nrf2-mediated signaling pathways. In this study, we investigated the expression of NRF2 and SOD2 in primary chick embryonic myocardial cells that were pretreated with Mn for 24 h and subjected to heat stress for 4 h. The data show that as a type of oxidative stress, heat stress can increase the expression of NRF2 and decrease the expression of SOD. As expected, Mn treatment decreased the expression of NRF2 and increased the expression of SOD. In addition, the protective effect of Mn on primary chick embryonic myocardial cells under heat stress was confirmed. Based on this comprehensive analysis, we believe that the NRF2/SOD2 signaling pathway is involved in the mitigating effect of Mn on heat stress–induced apoptosis in primary chick embryonic myocardial cells.
In conclusion, our results reveal that Mn mitigated heat stress–induced apoptosis by inhibiting ROS generation, intracellular Ca2+ accumulation, and the ER stress pathway. Moreover, Mn may activate or enhance the NRF2/SOD2 signaling pathway and thus can protect myocardial cells against oxidative stress during chick embryonic development. These findings suggest that Mn may be used as a potential preventive or therapeutic agent for heat stress–associated loss of production performance in broiler.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
References
Vanmuylder N, Evrard L, Daelemans P, Van RJ, Dourov N (2000) Immunohisto chemical expression of heat shock proteins HSP27, HSP70, HSP90 and HSP110 in salivary gland tumors: a study of 50 cases. Ann Pathol 20(3):190–195
Slimen IB, Najar T, Ghram A et al (2014) Reactive oxygen species, heart stress and oxidative-induced mitoehondrial damage. A review. Int J Hyperthermia 30(30):513–523
]Yang L, Tan GY, Fu YQ, et al (2010) Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C: Toxicol Pharmacol 151(2):204–208
Zeng T, Li JJ, Wang DQ et al (2014) Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperones 19(6):895–901
Gu ZT, Li L, Wu F, Zhao P, Yang H et al (2015) Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci Rep 5:11497
Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo SF, Yuan FF, Liu ZF, Tong HS, Su L (2014) Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci Rep 4(3):4469
Li L, Tan H, Gu Z et al (2014) Heat stress induces apoptosis through a Ca2+-mediated mitochondrial apoptotic pathway in human umbilical vein endothelial cells. Plos One 9(12):e111083
Yu B, Wen LL, Xiao B, Han F, Shi YX (2014) Single prolonged stress induces ATF6 alpha-dependent endoplasmic reticulum stress and the apoptotic process in medial frontal cortex neurons. BMC Neurosci 15:115
Yang Y, Li C, Dai Z et al (2014) Ursolic acid prevents endoplasmic reticulum stress-mediated apoptosis induced by heat stress in mouse cardiac myocytes. J Mol Cell Cardiol 67(67):103–111
Zhang M, Li SW, Pang KY, Zhou ZL (2019) Endoplasmic reticulum stress affected chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult Sci 98:1111–1120
Xiong YJ, Yin QR, Jin EH, Chen HT, He SJ (2020) Selenium attenuates chronic heat stress- induced apoptosis via the inhibition of endoplasmic reticulum stress in mouse granulosa cells. Molecules 25:557
Xiong YJ, Yin QR, Li J, He SJ (2020) Oxidative stress and endoplasmic reticulum stress are involved in the protective effect of Alpha lipoic acid against heat damage in chicken testes. Animals 10:384
Qin SZ, Liao XD, Lu L, Zhang LY, Xi L, Guo YL, Luo XG (2017) Manganese enhances the expression of the manganese superoxide dismutase in cultured primary chick embryonic myocardial cells. J Integr Agric 16(9):2038–2046
Li SF, Lu L, Liao XD, Gao TQ, Wang FN, Zhang LY, Xi L, Liu SB, Luo XG (2016) Manganese elevates manganese superoxide dismutase protein level through protein kinase C and proteintyrosine kinase. Biometals 29(2):265–274
Zhu YW, Lu L, Liao XD, Li WY, Zhang LY, Ji C, Xi L, Liu HS, Odle J, Luo XG (2017) Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and antiapoptotic abilitie. Oncotarget 8(52):89665
Liao XD, Zhu YW, Lu L (2019) Maternal manganese activates anti-apoptotic-related gene expressions via miR-1551 and miR-34c in embryonic hearts from maternal heat stress(Gallus gallus). J Therm Biol 8:190–199
Tombal B, Denmeade SR, Isaacs JT (1999) Assessment and validation of a microinjection method For kinetic analysis of [Ca2+] in individual cells undergoing apoptosis. Cell Calcium 25:19–28
Lynch K, Fernandez G, Pappalardo A, Peluso JJ (2000) Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium Levels through a protein kinase Cdelta-dependent pathway. Endocrinology 141:4209–4217
Niture SK, Kaspar JW, Shen J et al (2010) Nrf2 signa-ling and cell survival. Toxicol Appl Pharmacol 244(1):37
Ji Q, Gao JB, Zheng Y et al (2017) Inhibition of microRNA-153 protects neurons against ischemia/reperfusion injury in an oxygen-glucose deprivation and reoxygenation cellular model by regulating Nrf2/HO-1 signaling. J Biochem Mol Toxicol 31(7):e21905
Gu XH, Hao Y, Wang XL (2012) Overexpression of hest shock protein 70 and its relationship to intestine under acute heat stress in broilers:2.intesinal oxidative stress. Poultry Science 91(4):790–799
Khan I, Lee KL, Xu LG, Mesalam A, Chowdhury MMR, Joo MD et al (2017) Improvement of in vitro-produced bovine embryo treated with coagulansin-a under heat-stressed condition. Reproduction 153(4):421–431
Matsuki S et al (2003) Suppression of cytochrome c release and apoptosis in testeswith heat stress by minocycline. BBRC 312:843–849
Roberts GT et al (2008) Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke a study in baboon model. Arterioscl Throm Vas 28:1130–1136
Oberley LW, Buettner GR (1979) Role of superoxide dismutase in cancer: a review. Can Res 39(4):1141–1149
Bhandary B, Marahatta A, Kim HR, Chae HJ (2012) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14:434–456
McAnulty SR, McAnulty L, Pascoe DD, Gropper SS, Keith RE et al (2005) Hyperthermia increases exercise-induced oxidative stress. Int J Sports Med 26:188–192
Burdon RH, Gill VM, Rice-Evans C (1987) Oxidative stress and heat shock protein induction in humancells. Free Radic Res Commun 3:129–139
Skibba JL, Powers RH, Stadnicka A, Cullinane DW, Almagro UA et al (1991) Oxidative stress as a precursor to the irreversible hepatocellular injury caused by hyperthermia. Int J Hyperthermia 7:749–761
Zhu YW, Lu L, Li WX et al (2015) Effect of dietary manganese on antioxidant status and expression levels of heat-shock proteins and factors in tissues of laying broiler breeders under normal and high environmental temperatures. Br J Nutr 114(12):1–10
Kupsco A, Schlenk D (2015) Oxidative stres, unfolded proteinresponse, and apoptosis in developmental toxicity. Int Rev Cell Mol Biol 317:1–66
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21
Breitwieser GE (2006) Calcium sensing receptors and calcium oscillations: calcium as a first messenger. Curr Top Dev Biol 73:85–114
Hajnoczky G, Davies E, Madesh M (2003) Calcium signaling and apoptosis. Biochem Biophys Res Commun 304:445–454
Dubois C, Vanden AF, Sehgal P et al (2013) Differential effects of thapsigargin analogues on apoptosis of prostate cancer cells:complex regulation by intracellular calcium. FEBS J 280(21):5430–5440
Kundu JK, Surh YJ (2010) Nrf2-Keapl signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res 27(10):999–1013
Calvert JW, Jha S, Gundewar S et al (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105(4):365–374
Bae SH, Sung SH, Oh SY et al (2013) Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab 17(1):73–84
Funding
This research was funded by National Natural Science Foundation of China (No. 03119023); Youth Science and Technology Fund Scheme in Gansu (No. 20JR5RA013); Special Funds for Talents of Gansu Agricultural University (No. 2017RCZX-18).
Author information
Authors and Affiliations
Contributions
Conceptualization, Shizhen Qin; investigation, Rui Wang; writing – original draft, Rui Wang and Shijiao Qin; writing – review and editing, Yanli Guo; supervision, Defu Tang; project administration, Zhaoguo Shi; funding acquisition, Shizhen Qin.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, S., Wang, R., Tang, D. et al. Manganese Mitigates Heat Stress–Induced Apoptosis by Alleviating Endoplasmic Reticulum Stress and Activating the NRF2/SOD2 Pathway in Primary Chick Embryonic Myocardial Cells. Biol Trace Elem Res 200, 2312–2320 (2022). https://doi.org/10.1007/s12011-021-02810-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02810-2