Abstract
This study aimed to observe the influence of selenium (Se) deficiency on sperm quality and selenoprotein expression in rats. Four-week male Wista rats were randomly divided into three groups: Se-A, Se-L, and Se-D (respectively for Se- adequate, low, and deficient group). After 9 weeks, the rats were sacrificed by anesthesia, with the cauda epididymidis quickly fetched for sperm count, motility, and deformity. Meanwhile the blood, liver, brain, heart, and testis were collected for Se and biochemical analysis. It was found that the rats in Se-D had poor growth, while the Se concentrations in blood, liver, and heart for Se-D decreased significantly, compared with Se-A and Se-L (p < 0.01). But no significant difference was observed in testis and brain and also no statistical significance for sperm count. The sperm motility for Se-A (63.07%) was significantly higher than Se-L (53.91%) and Se-D (54.15%). Deformities were observed in both Se-L and Se-D. Both glutathione peroxidases (GPxs) and selenoprotein-P (SEPP1) levels in plasma and tissues of Se-D were significantly lower than those of Se-A and Se-L (p < 0.01). The SEPP1 levels in heart and brain of Se-L were lower than Se-A (p < 0.01). There was no statistical difference for GPx1 between Se-A and Se-L. The GPx4 level in testis of Se-L was lower than Se-A (p < 0.05). However, the SEPP1 in plasma, liver, testis, and the GPx3 level in plasma of Se-L were higher than those of Se-A (p < 0.05 or p < 0.01). Our results show that dietary Se deficiency could reduce GPx4 and SEPP1 expression in testis, which further influence sperm motility and may cause sperm deformity. Selenoprotein expression in some tissues of Se-L was higher than that of Se-A, but sperm quality and GPx4 expression in testis were not improved for Se-L. Low active pseudoselenoproteins might be synthesized in low-Se condition. The underlying mechanism needs to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Selenium (Se) is an essential micronutrient for mammals to maintain overall health [1]. Se deficiency has been recognized as a contributing factor to pathophysiological conditions, such as heart disease [2], neuromuscular disorders [3], reproduction [4], and numerous other disorders. Previous studies demonstrated the essential role of Se in spermatogenesis and male fertility in mammals [5]. Se exerts its nutritional functions in the form of amino acid Sec inserted into a group of selenoproteins. Several review literatures reported a strong implication of Se and selenoproteins in mammalian reproduction [6, 7]. The well-characterized selenoproteins with essential functions are selenoprotein-P (SEPP1) and glutathione peroxidases (GPxs), while the latter includes GPx1 and GPx4.
GPx4 is an intracellular selenoprotein that directly reduces peroxidized phospholipids production in cell membranes, also known as phospholipid hydroperoxide GPx [8]. It is distinctly expressed in testes, comprised of cytosolic protein, mitochondrial protein (mGPx4) and nuclear protein (nGPx4), and constituting over 50% of mitochondrial capsule in midpiece of mature sperm [9]. In the early stage of spermatogenesis, GPx4 is believed to protect the developing sperm from oxidative stress-induced DNA damage. In the later phase, through cross-linkage with proteins in midpiece region, however, it provides the integrity to the sperm midpiece by becoming a structural component of mitochondrial sheath circumventing the flagellum, which is an essential component for sperm stability and motility [10]. Similarly, SEPP1 plays an essential role in male reproductive functions. It serves as a transport protein of Se, expressed in vesicle like structures in the basal region of the Sertoli cells [11]. Besides, SEPP1 mRNA was also expressed in Leydig cells of rats. The largest loss of Se was manifested in SEPP1 KO models, where a 77% decrease of Se was observed [12].
The foregoing evidences highlight that Se performs significant functions in the male reproductive system regulated by selenoproteins, especially GPX4 and SEPP1. Therefore, it is advisable to perform more studies focusing on the elucidation of additional roles played by GPX4 and SEPP1 in male reproductive functions. In the present study, we observed the influences of different dietary Se (adequate, low, and deficient) on the sperm quality, Se content of blood and tissues, and selenoproteins level, to investigate the important role of Se in male fertility via GPx4 and SEPP1.
Materials and methods
Experimental groups and diet regimes
Thirty-six of four-week SPF male Wista rats (Beijing HFK Bioscience Co., LTD, China) were feed with a standard housing environment. Following 1-week adaptation period, the rats were randomly divided (n = 12 each) into three groups: Se-adequate (Se-A), Se-low (Se-L), and Se-deficient (Se-D). According to the literatures [27, 28], Se supplements for Se-D and Se-A groups were 0.01 (no sodium selenite added) and 0.3 mg Se/kg, respectively. We took the middle value 0.15 mg Se/kg as Se-L. The diet fed to the animals in this study was supplied by Beijing HFK Bioscience Co., LTD, China. The license key of animal fodder was SCXK(Beijing)2014-0008. The rats of Se-A group were fed with the basal feed (1022). The rats of Se-L and Se-D groups were fed with custom-made feed (M1003G). Its composition was given in Table 1 and Table 2. The actual measured Se levels in this study were 0.37, 0.13, and 0.01 mg Se/kg for Se-A, Se-L, and Se-D groups, respectively. The weights were recorded once a week. After feed for 9 weeks, the rats were sacrificed by anesthesia, and the cauda epididymidis were quickly fetched for sperm count, motility, and deformity detections. Blood was taken from the abdominal aorta. The liver, testis, heart, and brain tissues were collected for Se, SEPP1, and GPx4 analysis. All procedures used in this study were approved by the Animal Care and Use Committee of National Institute of Nutrition and Health, Chinese Centre for Disease Prevention and Control.
Detection of sperm count, motility and deformity
Sperm count and motility: The method was based on an epididymal sperm count protocol [13]. The sperm solution was immediately prepared after sample collection (n = 10–12), and the cells were loaded into a 100-mm-deep hemocytometer chamber (Thermo Fisher). The videos of the 4 corner squares of the chamber were recorded using a microscope (Nikon TS100) at 4003 magnification for 10 s. We then evaluated the sperm to assess their progressive motility (PM), no-progressive motility (NPM), and total motility (PM + NPM) with reference to the methods recommended by the WHO.
Deformity analysis: The methods for pathologic slide preparation and morphology analysis were obtained from the study by Seed et al [14]. Duplicate slides were prepared for each sperm sample (n = 6/group). We then randomly selected 200 sperm cells from each slide, and the cells were counted at 4003 amplification. The deformity rate (percentage) was calculated as (number of deformed sperm/total number of sperm) x100%.
Se detection of whole blood and tissues in rats
For the analysis of whole blood and tissue Se, 1 g of a previously heated (25 °C) and shaken blood and tissue samples were weighed into 10-mL Teflon microwave vessels and 2 mL of 65% HNO3 was added. During digestion, the samples were digested at 120 °C for 10 min, after which the temperature was ramped to 120 °C (within 8 min), and then to 160 °C for 10 min, and finally to 175 °C for 20 min using a CEM MARS Xpress microwave system (CEM, Matthew, NC, USA). The cooled, digested samples were diluted to 10 mL with ultrapure water and analyzed for total Se content by inductively coupled plasma mass spectrometry (ICP-MS).
Double-antibody sandwich ELISA assay for SEPP1 and GPx
After cutting samples, check the weight and add cold PBS (PH7.4), and maintain samples at 2–8 °C after melting. The samples were homogenized by hand or grinders and centrifugated for 20 min at the speed of 2000–3000 rpm. The supernatant was collected for detection. The double-antibody sandwich ELISA assay for quantification of SEPP1 and GPx was used according to the instructions of a validated SEPP1/GPx-specific ELISA kit [15, 16]. Setting five standards for drawing calibration curve, the absorbance was measured using a microplate reader at a wavelength of 450 nm.
Statistical analysis
Data were analyzed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). All values are expressed as Mean ± SEM. Comparisons between groups were statistically evaluated by Student’s test or one-way ANOVA with a post hoc Fisher’s test. A probability of p < 0.05 was considered to be statistically significant.
Results
Weight change of Rats
Their average weights were weekly given in Fig. 1. For the forgoing 6 weeks, the weight gains of the Se-A, Se-L, and Se-D groups were 207.88, 204.05, and 198.24 g, respectively. During the next 3 weeks, the weight gains became 41.74, 56.41, and 55.25 g, respectively. Compared with the Se-A and Se-L groups, the weight gain of rats in Se-D group was slow for the forgoing 6 weeks, but fast for the next 3 weeks.
Se concentrations in blood, liver, testis, brain, and heart of rats
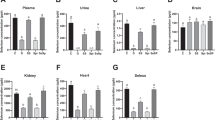
Compared with Se-A group, Se concentrations in blood and liver were significantly decreased for Se-L and Se-D groups (p < 0.05 or p < 0.01), as compared with Se-L group for Se-D group (p < 0.01). Se concentration in heart for Se-D group was lower than Se-L group (p < 0.01) (Fig. 2). Se concentrations in blood, liver, and heart were significantly decreased in Se-D group. There were no significant differences for Se concentrations in testis and brain.
Sperm density, motility, and deformity
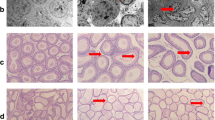
As shown in Table 3, there was no statistic difference for sperm density. The sperm motility in Se-A is 63.07%, significantly higher than that in Se-L (53.91%) and Se-D (54.15%) groups. There were observed deformities in Se-L and Se-D groups, but no deformity in Se-A group was found (Fig. 3). Both sperm motility and morphology were affected for Se-L and Se-D groups.
Double-antibody sandwich ELISA assay for SEPP1 and GPxs level in plasma and tissues
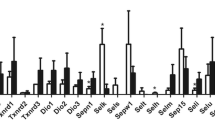
As shown in Fig. 4, the SEPP1 levels in plasma and tissues of Se-D group were significantly lower than those in Se-A and Se-L groups (p < 0.01). The SEPP1 levels in heart and brain of Se-L group were lower than Se-A group (p < 0.01). However, the SEPP1 levels in plasma, liver, and testis of Se-L group were higher than those of Se-A (p < 0.05 or p < 0.01).
The GPx levels in plasma, liver, and testis of Se-D group were significantly lower than Se-A and Se-L groups (p < 0.01). There was no difference for GPx1 in liver between Se-L and Se-A group. The GPx4 level in testis of Se-L was lower than Se-A (p < 0.05). However, the GPx3 level in plasma of Se-L was higher than that of Se-A (p < 0.01).
The GPx3 level in plasma and the SEPP1 levels in plasma, liver, and testis for Se-L group were significantly higher than those of Se-A group, but the GPx4 level in testis of Se-L was lower than that of Se-A.
Discussion
Selenium (Se) is an important micronutrient for animal and human health. More and more relevant evidences suggest that Se is essentially required for spermatogenesis and male fertility, presumably because of its vital role in modulation of antioxidant defense mechanisms and other essential biological pathways [17, 18]. It was shown that the alterations in Se levels might perturb the redox status and could lead to oxidative stress, adversely affecting male fertility by altering the expression of biologically important markers and activity of antioxidant enzymes [19, 20].
Se concentration and GPx4 activity (in testis) were significantly reduced when mice fed with Se-deficient dietary (0.02 ppm) for 4 months [21]. Se deficiency resulted in a significant decrease in body and testicle weights of the chicks [22]. Our result showed that weight gain was slow for Se-deficient rats. Compared with Se-adequate and Se-low groups, Se concentrations in blood, liver, and heart were significantly decreased in Se-deficient group, but not in testis and brain, probably because of the prior Se utilization for different tissues in Se-deficiency condition [23]. Accordingly, both sperm motility and morphology were affected when the rats were fed with Se-deficient fodder for 8 weeks. These results were in line with previous reports that sperm from Se-deficient (0.02 ppm) mice demonstrated vitiated chromatin condensation and declined in vitro fertilization ability compared to the Se-sufficient (0.2 ppm) mice [24].
As an essential component of selenoproteins, Se plays both structural and enzymic roles, being well known for its catalytic and antioxidative functions. GPx4 is the major selenoprotein expressed by germ cells in the testis, having multiple functions and representing the pivotal link between selenium, sperm quality, and male fertility. The deletion of mGPx4 causes male infertility, conferring the vital role of selenium in mammalian male fertility [25]. Homozygous expression of GPx4 with a targeted substitution of selenocysteine to serine causes early embryonic death but male subfertility in heterozygous mice [26]. Zhou et al. reported Se deficiency showed a lower expression of sensitive antioxidant selenoproteins (GPx1 and Txnrds), but excessive doses of Se-impaired sperm quality and this were linked with reduced mRNA expression of nGPx4 [27]. Our result showed that liver GPx1, testis GPx4, and plasma GPx3 activities were all significantly decreased for Se-D groups compared with Se-A and Se-L group, as reported in literatures that dietary selenium deficiency resulted the mRNA levels of some selenoprotein genes and the activities of GPxs decreased both in the spleen of pigs [28] and chicken aorta vessels [29].
Recently, another fertility-related marker, the selenoprotein P (SEPP1), was highlighted [30]. SEPP1 is a plasma protein, required for selenium supply to the testis. Liver-derived SEPP1 can bind apolipoprotein E receptor 2 on epithelial cell (Sertoli cells) membranes in testes to deliver selenium to organs [31], while testicular SEPP1 is locally and exclusively expressed in Leydig cells [32]. Our result showed that, in plasma and tissues, SEPP1 concentrations of Se-D group were significantly less than that of Se-A and Se-L groups, supplying more evidence for SEPP1 as a fertility-related marker.
It was reported that up to 10% selenocysteine (Sec) sites were occupied by cysteine (Cys) in selenoprotein (Thioredoxin Reductase 1, TR1) with a lower activity in mice fed with normal amounts of dietary selenium [33]. And then the same research group found that Cys might be inserted in place of various Sec residues in SEPP1 in human plasma [34]. And more, someone reported that Sec/Cys (U46C) mutant rescued cell death of GPx4-/- cells, whereas the Sec/Ser (U46S) mutant failed [35]. In this study, we have noticed that SEPP1 expressions of Se-L group in several tissues (plasma, liver and testis) were significantly higher than that of Se-A together without an optimal activity of GPx4 and the normal form of sperm. As shown in Fig. 5, the de novo biosynthesis of Cys was found to be decoded by the same codon UGA as that of SeCys [36]. Hence, we speculated there was a physiological adaptation to low-Se by replacing SeCys with Cys to synthesize low active pseudoselenoproteins and maintain the basic physiological functions. That is to say, physiological adaptation to low-Se might not safeguard the normal function of sperm. In the next study, we will further verify the replacement between Sec and Cys residue in SEPP1 from mice feed with different amounts of dietary selenium and human tissue from people in different Se-level areas.
This schematic illustrates the de novo synthesis of SeCys and Cys with the presence of codon UGA. Modified from Reference [36]
References
Duntas LH, Benvenga S (2015) Selenium: an element for life. Endocrine 48(3):756–775. https://doi.org/10.1007/s12020-014-0477-6
Djalalinia S, Khosravi M, Hasani M et al (2019) Effects of selenium supplementation on cardiometabolic risk factors, inflammatory, and antioxidant markers: a systematic review and meta-analysis protocol. Int J Prev Med 10:213. https://doi.org/10.4103/ijpvm.IJPVM_509_17
Kuršvietienė L, Mongirdienė A, Bernatonienė J, Šulinskienė J, Stanevičienė I (2020) Selenium anticancer properties and impact on cellular redox status. Antioxidants (Basel) 9(1):80. https://doi.org/10.3390/antiox9010080
Mistry HD, Broughton Pipkin F, Redman CW, Poston L (2012) Selenium in reproductive health. Am J Obstet Gynecol 206(1):21–30. https://doi.org/10.1016/j.ajog.2011.07.034
Maiorino M, Roveri A, Ursini F, Brigelius-Flohé R, Flohé L (2006) Selenium and male reproduction. In: Hatfield DL, Berry MJ, Gladyshev VN (eds) Selenium: Its Molecular Biology and Role in Human Health, 2nd edn. Springer Science+Business Media, LLC, pp 323–332 Chap 25
Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz M, Iqbal Z (2014) Role of selenium in male reproduction-a review. Anim Reprod Sci 146:55–62. https://doi.org/10.1016/j.anireprosci.2014.01.009
Qazi IH, Angel C, Yang H, Pan B, Zoidis E, Zeng CJ, Han H, Zhou GB (2018) Selenium, selenoproteins, and female reproduction: a review. Molecules 23(12):3053. https://doi.org/10.3390/molecules23123053
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34(2):145–169. https://doi.org/10.1016/s0891-5849(02)01197-8
Foresta C, FlohéL GA, Roveri A, Ursini F, Maiorino M (2002) Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod 67(3):967–971. https://doi.org/10.1095/biolreprod.102.003822
Brigelius-Flohé R, Flohé L (2020) Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal 33(7):498–516. https://doi.org/10.1089/ars.2019.7905
Olson GE, Winfrey VP, NagDas SK, Hill KE, Burk RF (2007) Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem 282(16):12290–12297. https://doi.org/10.1074/jbc.M611403200
Kehr S, Malinouski M, Finney L, Vogt S, Labunskyy VM, Kasaikina MV, Carlson BA, Zhou Y, Hatfield DL, Gladyshev VN (2009) X-ray fluorescence microscopy reveals the role of selenium in spermatogenesis. J Mol Biol 389(5):808–818. https://doi.org/10.1016/j.jmb.2009.04.024
Wang Y (2003) Epididymal sperm count. Curr Protoc Toxicol, Chapter 16: Unit16.6. https://doi.org/10.1002/0471140856.tx1606s14
Seed J, Chapin RE, Clegg ED et al (1996) Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI Risk Sci ence Institute Expert Working Group on Sperm Evaluation. Reprod Toxicol 10:237–244. https://doi.org/10.1016/0890-6238(96)00028-7
Wang Q, Sun LC, Liu YQ, Lu JX, Han F, Huang ZW (2016) The synergistic effect of serine with selenocompounds on the expression of SEPP1 and GPx in HepG2 cells. Biol Trace Elem Res 173(2):291–296. https://doi.org/10.1007/s12011-016-0665-8
Hybsier S, Schulz T, Wu Z, Demuth I, Minich WB, Renko K, Rijntjes E, Kohrle J, Strasburger CJ, Steinhagen-Thiessen E, Schomburg L (2017) Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated elisa for selenoprotein p. Redox Biol:403–414. https://doi.org/10.1016/j.redox.2016.12.025
Boitani C, Puglisi R (2008) Selenium, a Key Element in Spermatogenesis and Male Fertility. Adv Exp Med Biol 636:65–73. https://doi.org/10.1007/978-0-387-09597-4_4
Qazi IH, Angel C, Yang H, Zoidis E, Pan B, Wu Z, Ming Z, Zeng C-J, Meng Q, Han H, Zhou G (2019) Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences. Antioxidants (Basel) 8(8):268. https://doi.org/10.3390/antiox8080268
Shalini S, Bansal MP (2008) Dietary selenium deficiency as well as excess supplementation induces multiple defects in mouse epididymal spermatozoa: understanding the role of selenium in male fertility. Int J Androl 31(4):438–449. https://doi.org/10.1111/j.1365-2605.2007.00789.x
Mintziori G, Mousiolis A, Duntas LH, Goulis DG (2020) Evidence for a manifold role of selenium in infertility. Hormones (Athens) 19(1):55–59. https://doi.org/10.1007/s42000-019-00140-6
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21(3):351–357. https://doi.org/10.1016/j.nut.2004.05.028
Li M, Zhang Y, Li S (2020) Effects of selenium deficiency on testis development and autophagy in chicks. Ital J Anim Sci:753–761. https://doi.org/10.1080/1828051X.2020.1786739
Hill KE, Zhou J, Austin LM, Motley AK, Ham AJ, Olson GE, Atkins JF, Gesteland RF, Burk RF (2007) The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J Biol Chem 282(15):10972–10980. https://doi.org/10.1074/jbc.M700436200
Sánchez-Gutiérrez M, García-Montalvo E, Izquierdo-Vega J, Del Razo L (2008) Effect of dietary selenium deficiency on the in vitro fertilizing ability of mice spermatozoa. Cell Biol Toxicol 24(4):321–329. https://doi.org/10.1007/s10565-007-9044-8
Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumüller C, Deutsch MJ, Walch A, Hrabé de Angelis M, Wurst W, Ursini F, Roveri A, Maleszewski M, Maiorino M, Conrad M (2009) Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 23(9):3233–3242. https://doi.org/10.1096/fj.09-132795
Ingold I, Aichler M, Yefremova E, Roveri A, Buday K, Doll S, Tasdemir A, Hoffard N, Wurst W, Walch A, Ursini F, Friedmann Angeli JP, Conrad M (2015) Expression of a catalytically inactive mutant form of glutathione peroxidase 4 (gpx4) confers a dominant-negative effect in male fertility. J Biol Chem 290(23):14668–14678. https://doi.org/10.1074/jbc.M115.656363
Zhou JC, Zheng S, Mo J, Liang X, Xu Y, Zhang H, Gong C, Liu XL, Lei XG (2017) Dietary selenium deficiency or excess reduces sperm quality and testicular mRNA abundance of nuclear glutathione peroxidase 4 in rats. J Nutr 147(10):1947–1953. https://doi.org/10.3945/jn.117.252544
Lu Z, Wang P, Teng T, Shi B, Shan A, Lei XG (2019) Effects of dietary selenium deficiency or excess on selenoprotein gene expression in the spleen tissue of pigs. Animals (Basel) 912(12):1122. https://doi.org/10.3390/ani9121122
Du Q, Yao H, Yao L, Zhang Z, Lei X, Xu S (2016) Selenium deficiency influences the expression of selenoproteins and inflammatory cytokines in chicken aorta vessels. Biol Trace Elem Res 173(2):501–513. https://doi.org/10.1007/s12011-016-0676-5
Michaelis M, Gralla O, Behrends T, Scharpf M, Endermann T, Rijntjes E, Pietschmann N, Hollenbach B, Schomburg L (2014) Selenoprotein P in seminal fluid is a novel biomarker of sperm quality. Biochem Biophys Res Commun 443(3):905–910. https://doi.org/10.1016/j.bbrc.2013.12.067
Burk RF, Hill KE (2009) Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 1790(11):1441–1447. https://doi.org/10.1016/j.bbagen.2009.03.026
Koga M, Tanaka H, Yomogida K, Tsuchida J, Uchida K, Kitamura M, Sakoda S, Matsumiya K, Okuyama A, Nishimune Y (1998) Expression of selenoprotein-P messenger ribonucleic acid in the rat testis. Biol Reprod 58(1):261–265. https://doi.org/10.1095/biolreprod58.1.261
Xu XM, Turanov AA, Carlson BA, Yoo MH, Everley RA, Nandakumar R, Sorokina I, Gygi SP, Gladyshev VN, Hatfield DL (2010) Targeted insertion of cysteine by decoding UGA codons with mammalian selenocysteine machinery. Proc Natl Acad Sci 107(50):21430–21434. https://doi.org/10.1073/pnas.1009947107
Turanov AA, Everley RA, Hybsier S, Renko K, Schomburg L, Gygi SP, Hatfield DL, Gladyshev VN (2015) Regulation of selenocysteine content of human selenoprotein p by dietary selenium and insertion of cysteine in place of selenocysteine. PLoS One 10(10):e0140353. https://doi.org/10.1371/journal.pone.0140353
Mannes AM, Seiler A, Bosello V, Maiorino M, Conrad M (2011) Cysteine mutant of mammalian gpx4 rescues cell death induced by disruption of the wild-type selenoenzyme. FASEB J 25(7):2135–2144. https://doi.org/10.1096/fj.10-177147
Vindry C, Ohlmann T, Chavatte L (2018) Translation regulation of mammalian selenoproteins. Biochim Biophys Acta Gen Subj. https://doi.org/10.1016/j.bbagen.2018.05.010
Acknowledgment
This work was supported by the youth science fund of National Institute of Nutrition and Health, Chinese Centre for Disease Control and Prevention (Grant No. NINH2018001).
The authors’ responsibilities were as follows
QW and SZ completed the experiments and wrote the paper; YQL and FH contributed to the detection of Se; LLS and CH participated in the animal experimental; WPM and JZC provided valuable advice on the writing; ZWH designed the experiments and revised the paper critically for important content; and all authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Q., Zhan, S., Liu, Y. et al. Low-Se Diet Can Affect Sperm Quality and Testicular Glutathione Peroxidase-4 activity in Rats. Biol Trace Elem Res 199, 3752–3758 (2021). https://doi.org/10.1007/s12011-020-02515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02515-y