Abstract
Selenium (Se) deficiency induces testicular functional disturbances, but the molecular mechanism remains unclear. In the present study, 1-day-old broiler chickens were maintained for 55 days with a normal diet (0.2 mg/kg) and a Se-deficient diet (0.033 mg Se/kg). Then, the messenger RNA (mRNA) levels of selenoproteins, heat shock proteins (HSPs), and inflammatory factors were examined. Se deficiency led to decreased selenoproteins (Gpx1, Selk, and Selh) and HSPs (HSP40, HSP60, and HSP90) (P < 0.05). However, the expression levels of Gpx2, Sepn1, Seli, Selpb, Sepx1, HSP27, and inflammatory factors (iNOS, TNF-α, COX-2, and HO-1) were increased by Se deficiency (P < 0.05). Gpx1, Selk, and Selh showed positive correlation with HSP40, HSP60, and HSP90, but negative correlation with HSP27, HSP70, iNOS, TNF-α, COX-2, and HO-1. However, Gpx2, Spen1, Seli, Selpb, and Sepx1 showed positive correlation with inflammatory factors and HSP27 and HSP70. Selenoproteins showed different correlation with HSPs and inflammatory factors and were classified into different groups in response to Se deficiency. The results suggested that selenoproteins play different roles in chicken testes, and we think that Gpx1 and Selk may play a special role in chicken testes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) plays important roles in the biological functions in animals, such as regulating the metabolism, antioxidant defense, reproduction, and detoxification [1–3]. Se deficiency can result in types of diseases and structural and functional disturbances in animals [4]. In chickens, the normal function of the muscles [5, 6], liver [7], immune system [8], kidneys [9], intestine [10], erythrocytes [11], pancreas [12], thyroids [13], and adipose tissue [14] was shown to be influenced by Se deficiency in previous studies. Se deficiency induced oxidative stress, inflammatory response, and disorder of the selenoproteins [15–17], but the possible mechanism remains elusive.
The physiological functions of Se are considered to be mediated through selenoproteins [18]. There are 25 selenoprotein genes in chickens [19]. Selenoproteins play various roles such as redox regulation [20–23], Se transport [24], inflammatory response [25], immune response [26], the conversion of T4 to T3 [20, 27], calcium homeostasis regulation [28, 29], and cell cycle regulation [30–32]. In addition, the disturbance of selenoproteins showed a close correlation with inflammatory response, cytokines, heat-shock stress, apoptosis, and oxidative stress. Thus, the biological roles of selenoproteins have attracted the attention of many researchers. In previous studies, Se deficiency influenced the expressions of selenoproteins in different chicken tissues, including the muscles [5, 6], liver [7], erythrocytes [11], pancreas [12], thyroids [13], and adipose tissue [14]. Selenoproteins preserved different expression patterns and responses to Se deficiency, so they may preserve different status in different organs. However, which selenoprotein plays the main role in the specific organ still need to be validated.
Se also plays an important role in the testes tissues. The Se level in the male gonads is maintained by regulation mechanisms, and the supply of sufficient amounts of Se to the testes has priority over the supply to other tissues [1]. However, Se deficiency also gives rise to testicular structural and functional disturbances [33]. Even though Se plays an important role in chickens, few studies show the effect of Se deficiency on chicken testes. There are still some questions that need to be answered: (1) Is there a similar effect of Se deficiency on the expression of selenoproteins in chicken testes compared to other tissues? (2) Which selenoprotein plays the primary roles in chicken testes in response to Se deficiency? (3) Are levels of selenoproteins correlated with disturbance of testes function? In the present study, we used a Se-deficient chicken model to evaluate these questions.
Materials and Methods
Birds and Diets
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. A total of 180 male broiler chickens (1-day old; Weiwei Co. Ltd., Harbin, China) were randomly divided into two groups (90 chickens per group). The Se-deficient group (−Se) was fed a Se-deficient corn-soy basal diet (corn and soy were produced in the Se-deficient region of Heilongjiang Province, China, and the diet contained 0.033 mg of Se/kg). The Se-adequate group (Con) was fed the same basal diet supplemented with Se at 0.2 mg/kg (sodium selenite). The feeding experiment lasted for 55 days and the experimental animals were given free access to feed and water. On day 55, the chickens were killed with sodium pentobarbital and their testes were removed. The tissues were blotted, rinsed with ice-cold sterile deionized water, frozen immediately in liquid nitrogen, and stored at −80 °C until analysis.
Quantitative Real-Time PCR Analysis of Selenoprotein messenger RNA Levels
The total RNA was isolated from chicken testes using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Shanghai, China). The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 10 pg of total RNA using dN12 primers and AccuPower RocketScript RT PreMix (Bioneer, Alameda, CA, USA) according to the manufacturer”s instructions. Synthesized cDNA was diluted 5 times with sterile water and stored at −80 °C before use.
Primer Premier Software (Premier Biosoft International, Palo Alto, CA, USA) was used to design specific primers based on known chicken sequences (Table 1). General PCRs were first performed to confirm the specificity of the primers. The PCR products were electrophoresed on 2% agarose gels, extracted, cloned into the pMD18-T vector (TaKaRa, China), and sequenced. Quantitative real-time PCR (qPCR) was performed on a BIO-RAD C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA). Reactions were performed in a 20 μL reaction mixture containing 10 μL of AccuPower 2X Greenstar qPCR Master Mix (Bioneer), 2 μL of diluted cDNA, 1 μL of each primer (10 μM), and 6 μL of PCR-grade water. The PCR procedure consisted of 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. A dissociation curve was run for each plate to confirm the production of a single product. The amplification efficiency for each gene was determined using the DART-PCR program [34]. The mRNA relative abundance was calculated as previously described [35] to account for gene-specific efficiencies and was normalized to the mean expression of GAPDH.
Statistical Analysis
Statistical analysis of all data was performed using SPSS for Windows (version 19, SPSS Inc., USA). Differences between the Se-deficient group and the control group were considered to be significant at P < 0.05. The data are expressed as mean ± SD. In addition, principal component analysis (PCA) was used to define the most important parameters, which could be used as key factors for individual variations using the Statistics 6.0 program (version 19, SPSS Inc., Chicago, IL, USA).
Results
The Effect of Se Deficiency on the Expression of Selenoproteins
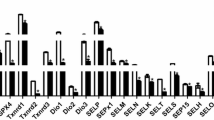
Twenty-five selenoproteins were detected by real-time PCR. As shown in Fig. 1, the expression levels of selenoproteins were different. Selk, Sepw1, Sep15, and Selu were highly expressed in chicken testes, but Gpx3, Txnrd3, Selh, Selt, Sepn1, Selm, Seli, Selpb, and SPS2 were less expressed in chicken testes. Se deficiency decreased Gpx1, Selk, and Selh (P < 0.05), but increased Gpx2, Spen1, Seli, Selpb, and Sepx1 (P < 0.05). Among the affected selenoproteins, the expression of Gpx1, Selk, and Selh were decreased by Se deficiency by 81.00, 88.69, and 99.41%, respectively. Thus, these selenoproteins were significantly decreased by Se deficiency.
The Effect of Se Deficiency on the Gene Expression Levels of HSPs
The expression levels of HSPs were examined. The results (Fig. 2) showed that Se deficiency decreased the expression of HSP40, HSP60, and HSP90, but increased HSP27 (P < 0.05). Among these affected HSPs, the expression of HSP40, HSP60, and HSP90 was decreased by Se deficiency by 88.75, 99.92, and 97.00%, respectively.
The Effect of Se Deficiency on the Gene Expression Levels of Inflammatory Factors
We also examined the expression of some inflammatory factors including iNOS, TNF-α, NF-κB, PTGE, COX-2, and HO-1. The results (Fig. 3) showed that Se deficiency induced the expression of 4 inflammatory factors, iNOS, TNF-α, COX-2, and HO-1 (P < 0.05), but had no effect on the expression of NF-κB and PTGE. Thus, Se deficiency increased the inflammatory response in chicken testes.
Principal Component Analysis
All the parameters were distinguished on ordination plots (the first and second principle components 63.73 and 17.77%, respectively) using PCA (Fig. 4). The observed relationships among the parameters were confirmed and quantified according to Spearman’s test. There were complicated correlations between different selenoproteins, HSPs, and inflammatory factors. For the affected selenoproteins, Gpx1, Selk, and Selh showed positive correlation with HSP40, HSP60, and HSP90 but negative correlation with HSP27, HSP70, iNOS, TNF-α, COX-2, and HO-1. However, Gpx2, Spen1, Seli, Selpb, and Sepx1 showed positive correlation with inflammatory factors and HSP27 and HSP70. In addition, Gpx1, Gpx3, Selh, Sels, Selt, Sepw1, Selk, HSP40, HSP60, and HSP90 were grouped in the same rotating component matrix. Sep15, SPS2, Txnrd2, HSP70, PTGE, and NF-κB were grouped in the same matrix, and other genes were grouped in the other matrix (Fig. 4). Thus, in response to Se deficiency selenoproteins exhibited significantly different patterns, and showed different correlations with HSPs and inflammatory factors.
Discussion
Se plays crucial roles in the biological function of the testes. Under normal conditions, Se is maintained at stable levels in the testes due to their priority of using Se over other tissues [1]. However, a prolonged Se-deficient diet also induces structural and functional disturbances in the testes [36]. As tissues show different responses to Se deficiency, it is necessary to detect the effect of Se deficiency on various tissues and evaluate Se’s role in these tissues. In the present study, we examined the effect of Se deficiency on the expression of selenoproteins in chicken testes. The results showed that Se deficiency decreased the expression of only 3 selenoproteins (Gpx1, Selk, and Selh), which were less influenced than in other tissues (12 selenoproteins were decreased in the pancreas [12], 19 in the muscles [6], 24 in the erythrocytes [11], 24 in the thyroids [13], 25 in the adipose tissue [14], 14 in the kidneys [9], and 19 in the liver [7]). Thus, in chicken testes, the response of selenoproteins to Se deficiency is not as significant as that in other tissues.
In chickens, 25 selenoproteins have been identified. According to their structure and function these selenoproteins are classified into several functional families, such as the Gpx family (including Gpx1-Gpx6), the Txnrds family (including Txnrd1, Txnrd2, and Txnrd3), the Dios family (including Dio1, Dio2, and Dio3) [19], and the Rdx family (Selm, Sep15, Selt, Sepw1, Selh and Selv) [21]. These typical selenoproteins play important roles in antioxidant defense, redox regulation, and regulation in injuries and disease [20, 24]. By examining the expression of these selenoproteins in different chicken tissues, we found that selenoproteins showed different expression patterns and responses to Se deficiency and had different status and play varying roles in different organs. In chicken muscles, Gpx3, Gpx4, and Sepw1 were highly expressed and may play crucial roles. By excessively decreasing the expression of some selenoproteins such as Dio1, Selu, Selpb, and Sepp1, the muscles may conserve limited Se and maintain the levels of some crucial selenoproteins [6]. Similarly, in adipose tissues, all 25 selenoproteins were significantly decreased in a time-dependent manner [14], which suggests that adipose tissues may play special roles in regulating the Se levels by decreasing its selenoprotein levels and sacrificing itself to contribute to the limited Se level. In addition, in the erythrocytes, 24 selenoproteins were decreased by Se deficiency, and Gpxs, Txnrd1, Selp, and SPS2 were highly expressed compared to the other selenoproteins, which suggested that Gpxs, Txnrd1, Selp, and SPS2 possibly play more important role than the other selenoproteins [11]. Tissues such as the kidneys [9], pancreas [12], and liver [7] also showed an extensive decrease in selenoproteins. However, in chicken testes only three selenoproteins (Gpx1, Selk, and Selh) were significantly decreased and five selenoproteins (Gpx2, Sepn1, Seli, Selpb, and Sepx1) were increased. Thus, the response of the testes to Se deficiency is significantly different from that of other tissues. Under normal conditions, Selk, Sepw1, and Sep15 were the most highly expressed selenoproteins; Selk decreased by about 89%. In addition, Gpx1 is a crucial antioxidant in various tissues. GPx1 is an important member of the family of closely related antioxidant enzymes encoded in humans by the GPX1 genes. Gpx1 was discovered in the erythrocytes, where it removes H2O2 formed by the dissociation of oxyhemoglobin into O2 − and methemoglobin [37]. During depletion, Se is rapidly mobilized from Gpx1 stores, whereas the expression of other selenoproteins such as Gpx4, Gpx2, Dio2, Dio3, and Txnrd is hardly affected or may even be increased, as in the case of Dio1 [24]. Thus, we surmise that Selk and Gpx1 may play primary roles in chicken testes. The higher expression levels of Gpx2, Sepn1, Seli, Selpb, and Sepx1 in Se-deficient testes may show that these selenoproteins also play some special roles in chicken testes.
The relationship between selenoproteins and several types of injuries, molecular response, inflammation, and apoptosis have been extensively reported in previous studies. Gpx1, Gpx3, Gpx4, Selk, Selh, Sepp1, and Sepw1 play important roles in chicken myoblasts and show a special correlation with oxidative stress [23]. Gpx1, Gpx2, Gpx3, Dio1, Selk, Seli, Sepx1, and SPS2 have a strong correlation with NO and iNOS, which indicates that these selenoproteins may play crucial roles in inflammatory responses in the chicken pancreas [12]. In chicken erythrocytes, Txnrd1, Sels, Selu, Sepx1, and SPS2 were highly expressed and may play key roles in regulating the expression of cytokines, IL-2, IL-4, IL-8, IL-10, IL-12β, IFN-γ, and TGF-β4 [11]. In the present study, selenoproteins, Gpx1, Gpx3, Selk, Sepw1, Selh, Sels, and Selt have higher correlation with HSPs. Sep15, SPS2, and Txnrd2 highly correlated with HSP70 and inflammatory factors, NF-κB and PTGE, and other selenoproteins have close relationships with HSP27, COX-2, and HO-1. Thus, the selenoproteins were classified into different groups and may play different roles in the testes in response to Se deficiency. Among the affected selenoproteins, Gpx1, Selk, and Selh play important roles in regulating HSPs stress, and Gpx2, Sepn1, Seli, Selpb, and Sepx1 may be closely related to regulating inflammatory response. Combining with the highly expressed levels of Selk and significant change in Gpx1, and Selh expression levels, we suggested that Gpx1, Selk, and Selh play crucial roles in chicken testes.
In summary, we assessed 25 selenoproteins in chicken testes and found that Gpx1, Selk, and Selh were decreased, but Gpx2, Sepn1, Selpb, Seli, and Sepx1 were increased. These selenoproteins showed different correlations with HSPs and inflammatory factors and can be classified into different groups in response to Se deficiency. The results showed that the response of selenoproteins to Se deficiency in chicken testes is significantly different from that in other tissues, and we surmise that Gpx1, Selk, and Selh may play crucial roles in chicken testes.
References
Behne D, Hofer T, von Berswordt-Wallrabe R et al (1982) Selenium in the testis of the rat: studies on its regulation and its importance for the organism. J Nutr 112:1682–1687
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4:593–599
Seema P, Swathy SS, Indira M (2007) Protective effect of selenium on nicotine-induced testicular toxicity in rats. Biol Trace Elem Res 120:212–218
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63:52–59
Yao HD, Wu Q, Zhang ZW et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143:613–619
Yao H, Zhao W, Zhao X et al (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161:318–327
Liu CP, Fu J, Lin SL et al (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159:192–198
Yu D, Zhang Z, Yao H et al (2015) The role of selenoprotein W in inflammatory injury in chicken immune tissues and cultured splenic lymphocyte. Biometals 28:75–87
Zhang JL, Xu B, Huang XD et al (2016) Selenium deficiency affects the mRNA expression of inflammatory factors and selenoprotein genes in the kidneys of broiler chicks. Biol Trace Elem Res 171:201–207
Yu J, Yao H, Gao X et al (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163:144–153
Luan Y, Zhao J, Yao H et al (2015) Selenium deficiency influences the mRNA expression of selenoproteins and cytokines in chicken erythrocytes. Biol Trace Elem Res 171:427–436
Zhao X, Yao H, Fan R et al (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161:341–349
Lin SL, Wang CW, Tan SR et al (2014) Selenium deficiency inhibits the conversion of thyroidal thyroxine (T) to triiodothyronine (T) in chicken thyroids. Biol Trace Elem Res 161:263–271
Liang Y, Lin SL, Wang CW et al (2014) Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res 160:41–48
Xu S-W, Yao H-D, Zhang J et al (2012) The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res 151:225–233
Wu Q, Yao HD, Tan SR et al (2014) Possible correlation of selenoprotein W with inflammation factors in chicken skeletal muscles. Biol Trace Elem Res 161:167–172
Ghazi Harsini S, Habibiyan M, Moeini MM et al (2012) Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res 148:322–330
Kieliszek M, Blazejak S (2013) Selenium: significance, and outlook for supplementation. Nutrition 29:713–718
Mariotti M, Ridge PG, Zhang Y et al (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7:e33066
Bellinger FP, Raman AV, Reeves MA et al (2009) Regulation and function of selenoproteins in human disease. Biochem J 422:11–22
Dikiy A, Novoselov SV, Fomenko DE et al (2007) SelT, SelW, SelH, and Rdx12 genomics and molecular insights into the functions. Biochemistry 46:6871–6882
Yao HD, Wu Q, Zhang ZW et al (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830:3112–3120
Yao HD, Liu W, Zhao WC et al (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4:64032
Pappas AC, Zoidis E, Surai PF et al (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151:361–372
Martinez A, Santiago JL, Varade J et al (2008) Polymorphisms in the selenoprotein S gene: lack of association with autoimmune inflammatory diseases. BMC Genomics 9:329
Verma S, Hoffmann FW, Kumar M et al (2011) Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186:2127–2137
Sunde RA, Raines AM, Barnes KM et al (2009) Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep 29:329–338
Lescure A, Rederstorff M, Krol A et al (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790:1569–1574
Yao H, Fan R, Zhao X et al (2016) Selenoprotein W redox-regulated Ca2+ channels correlate with selenium deficiency-induced muscles Ca2+ leak. Oncotarget 7:57618–57632
Hawkes WC, Alkan Z (2011) Delayed cell cycle progression from SEPW1 depletion is p53- and p21-dependent in MCF-7 breast cancer cells. Biochem Biophys Res Commun 413:36–40
Hawkes WC, Alkan Z (2012) Delayed cell cycle progression in selenoprotein W-depleted cells is regulated by a mitogen-activated protein kinase kinase 4-p38/c-Jun NH2-terminal kinase-p53 pathway. J Biol Chem 287:27371–27379
Hawkes WC, Printsev I, Alkan Z (2012) Selenoprotein W depletion induces a p53- and p21-dependent delay in cell cycle progression in RWPE-1 prostate epithelial cells. J Cell Biochem 113:61–69
Bekpinar S, Tugrul Y (1995) Influence of selenium supplementation in non-toxic doses on testis lipid peroxide and antioxidant levels in chronic alcohol-fed rats. Alcohol Alcohol 30:645–650
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31:e73
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408
Behne D, Duk M, Elger W (1986) Selenium content and glutathione peroxidase activity in the testis of the maturing rat. J Nutr 116:1442–1447
Hawkes WC, Alkan Z (2010) Regulation of redox signaling by selenoproteins. Biol Trace Elem Res 134:235–251
Acknowledgements
This study was supported by the Research Fund for the Doctoral Program of Higher Education (20122325110018), the National Natural Science Foundations of China (31472104, 31272626, and 30871902), and the National Science and Technology Support Program (2013BAD20B04). The authors thank the members in the Veterinary Internal Medicine Laboratory at the College of Veterinary Medicine, Northeast Agricultural University for their help in analyzing the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All authors have read the manuscript and agreed to submit it in its current form for consideration for publication
Rights and permissions
About this article
Cite this article
Gao, Y., Zhang, J., Huang, X. et al. Glutathione Peroxidase 1, Selenoprotein K, and Selenoprotein H May Play Important Roles in Chicken Testes in Response to Selenium Deficiency. Biol Trace Elem Res 179, 271–276 (2017). https://doi.org/10.1007/s12011-017-0953-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0953-y