Abstract
In this study, the protective effects of chrysin (CR) on lead acetate (PbAc)-induced renal toxicity in Sprague-Dawley rats were investigated with biochemical, histopathological, and immunohistochemical methods. In the study, rats were given orally at 30 mg/kg/body weight (BW) PbAc after CR of 25 and 50 mg/kg/BW was administered to them orally (a total of 7 administrations for 7 days). The results showed that CR reduced urea and creatinine levels by alleviating PbAc-induced kidney damage. It was determined that CR decreases PbAc-induced lipid peroxidation due to its antioxidant properties and increases catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) activities, and glutathione (GSH) levels. It was also detected that CR protects DNA from the toxic effects of PbAc and reduces 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels. Biochemical and immunohistochemical findings demonstrated that CR had anti-inflammatory and antiapoptotic effects and reduced nuclear factor kappa-B (NF-κB), interleukin-33 (IL-33), prostaglandin-E2 (PGE-2), tumor necrosis factor-α (TNF-α), p53 levels, and the activities of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which were increased with PbAc administration. Moreover, CR was found to increase the levels of aquaporin-1 (AQP-1) and nephrine in PbAc-induced kidney tissue. CR decreased the contents of lead (Pb), zinc (Zn), iron (Fe), sodium (Na), and copper (Cu) and increased those of potassium (K) calcium (Ca) in renal tissue. These results indicated that CR considerably alleviates kidney toxicity caused by PbAc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb), an environmental pollutant and toxic agent, is among the heavy metals [1, 2]. Contaminated food, water, and air pollution are major sources of Pb toxicity [3, 4]. According to the World Health Organization, Pb is among the 10 most dangerous substances for public health [5]. Pb excretion is very difficult and can be stored in soft tissues, bones, and other important organs for a long time [6, 7]. In addition, it has toxic effects on many tissues, especially kidney [6, 8, 9]. Although the mechanism of Pb toxicity in the kidneys cannot be understood precisely, studies suggest that oxidative stress has an important contribution to Pb toxicity [10, 11]. Moreover, studies have reported that Pb can induce apoptosis by causing mitochondrial degradation and DNA damage [12, 13] (Fig. 1).
In the treatment of toxicity caused by heavy metals, including Pb, chelators are used to excrete heavy metal from the body [7, 14]. It has been reported that chelators used in lead treatment, along with some undesirable side effects [15], did not have much effect on the lead accumulated in tissue [16, 17]. Therefore, research has focused on various alternative approaches to the treatment of Pb toxicity, particularly plant-based drugs [7].
Herbal products and their active components protect the tissues and organs from the attacks reactive oxygen species (ROS) and prevent the formation of oxidative stress [18, 19]. Thus, these substances are often among the research topics in metal detoxification [19]. Flavonoids, plant secondary metabolites, are the phenolic compounds which are abundant in foods and have antioxidant, antibacterial, anti-cancer, anti-mutagenic, and anti-inflammatory properties [20,21,22,23,24,25]. Chrysin (CR), whose chemical name is 5,7-dihydroxiflavone, is one of the flavonoids commonly used as a traditional medicine and found in many plant extracts, honey, and propolis [26, 27]. Much research demonstrates that CR has antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, anti-allergic, antiapoptotic properties [28,29,30]. CR has antioxidant properties since the hydroxyl groups in its structure have an elimination effect on free radicals [31, 32]. Furthermore, CR has been shown to have anti-inflammatory effect by lowering levels of certain cytokines, prostaglandin E (PGE), cyclooxygenase-2 (COX-2), and nitric oxide (NO) [33, 34]. The daily intake of CR is 0.5–3 g for people [35]. However, CR has been reported to cause toxicity even at low doses in the fish liver cell line [36].
There is no definite information in the literature on whether CR has a protective effect against kidney damage caused by lead, a toxic heavy metal. That’s why the present study was conducted to investigate the protective effects of CR on kidney damage caused by PbAc through using some biochemical and histopathological methods.
Materials and methods
Drugs and chemicals
All chemicals used in the experiment, including lead acetate (lead (II) acetate trihydrate [Pb(CH3CO2)2 3H2O], cas no: 6080-56-4, purity: 99.5–102.0%, mp: 75°C) and chrysin (5,7-dihydroxyflavone [C15H10O4], cas no: 480–40-0, purity 97%, mp: 284–286°C) were of the highest purity and obtained from Sigma-Aldrich Chemical Company (St Louis, MO, USA).
Animals
Thirty-five Sprague-Dawley male rats purchased from Atatürk University Medical Experimental Application and Research Center were used in the experiment. The animals were about 10–12 weeks old and weighed 250–270 g when the experiment began. The environment in which they were kept had 24 ± 1 ° C (room temperature) temperature, 45 ± 5% humidity, and 12-h light/dark cycles. They were fed ad libitum with standard laboratory feed and tap water. Rats were adapted to the environment for 1 week before drug administration. Approval was obtained from the Local Ethics Committee for Animal Experiments of Ataturk University in order to make the applications (Approval no: 2019-12/163).
Treatment protocol
PbAc and CR doses were determined with reference to previous studies [37, 38]. In this study, 5 different groups were formed with 7 male rats in each group. The groups were designed as follows:
-
Group 1 (control group): saline was given orally to rats for 7 days.
-
Group 2 (CR-50 group): 50 mg/kg/BW CR was given orally to rats for 7 days.
-
Group 3 (PbAc group): 30 mg/kg/BW PbAc was given orally to rats for 7 days.
-
Group 4 (PbAc + CR-25 group): 30 mg/kg/BW PbAc was given orally to rats 30 min after 25 mg/kg/BW CR the administration for 7 days.
-
Group 5 (PbAc + CR-50 group): 30 mg/kg/BW PbAc was given orally to rats 30 min after the administration of 50 mg/kg/BW CR for 7 days.
Collection of samples
Twenty-four hours after the last drug administration, the animals’ body weights were measured and then they decapitated under mild isoflurane (IsoFlo; Abbott, Queenborough, UK) anesthesia. As soon as they were decapitated, blood samples were collected from their Vena jugularis. Blood samples were collected in anticoagulant free tubes and centrifuged at 1200×g for 15 min. Blood serum was used to determine renal function. After the kidneys from rats were washed with ice-cold physiologic saline (0.85% NaCl), one of them was stored at − 80 ° C for biochemical analysis and the other in 10% buffered formalin solution for histological examination until used.
Determination of serum urea and creatinine levels
Serum urea [39] and creatinine [40] levels were analyzed with a commercial kit (Diasis Diagnostic Systems, Istanbul, Turkey) according to the manufacturer’s instructions.
Preparation of tissue homogenates
To obtain homogenate from the kidneys, the tissues were diluted 1:20 v/w with phosphate-buffered saline (PBS; pH 7.4). The resulting mixture was rapidly homogenized with a tissue lysate device (TissueLyser II, Qiagen). The homogenate was then centrifuged at + 4°C and 3000 rpm for 30 min. The supernatant was used for biochemical analysis.
Determination of lipid peroxidation and antioxidant enzyme activities in kidney tissue
The level of lipid peroxidation was determined by analyzing the amount of malondialdehyde (MDA) at 532 nm according to the method developed by Placer et al. [41]. The amount of MDA was expressed as nmol/g tissue. Superoxide dismutase activity was measured by the method designed by Sun et al. [42]. The results were expressed as U/g protein. The measurement of catalase activity was performed according to the method of Aebi [43]. The results were expressed as catal/g protein. Glutathione peroxidase (GPx) activity was measured according to the method developed by Lawrence, Burk [44]. Results were expressed as U/g protein. The method developed by Sedlak, Lindsay [45] was used to determine the level of glutathione. Results were expressed as nmol/g of tissue. Total protein analysis was performed according to the method developed by Lowry et al. [46] using bovine serum albumin (BSA) as standard.
Determination of AQP-1 levels in renal tissue
Aquaporin-1 (AQP-1) levels was performed by using rat enzyme-linked immunosorbent assay (ELISA) kit (cat. no: 201-11-0566; assay range: 0.15–40 ng/ml) obtained from Sunred Biological Technology Company.
Determination of p53 levels in renal tissue
p53 levels were analyzed with rat ELISA kit according to the manufacturer’s instructions (Sunred, Shanghai, China) (cat. no: 201-11-0072; assay range: 0.05–10 ng/ml). The color intensity at the end of the procedures was read at 450 nm with the ELISA microplate reader (Bio-Tek, Winooski, VT, USA).
Determination of inflammatory response levels in kidney tissue
Interleukin-33 (IL-33) (cat. no: 201-11-3102; assay range: 1.5 - 400 ng/L), COX-2 (cat. no: 201-11-0297; assay range: 0.5–150 ng/ml), PGE-2 (cat. no: 201-11-0505; assay range: 0.05–15 ng/ml), nuclear factor kappa-B (NF-κB) (cat. no: 201-11-0288; assay range: 0.08–20 ng/ml), and inducible nitric oxide synthase (iNOS) (cat. no: 201-11-0741; assay range: 0.8–200 ng/ml) in renal tissue were measured by ELISA to determine the degree of inflammatory response levels in accordance with the manufacturer’s instructions (Sunred, Shanghai, China).
Determination of DNA damage level in kidney tissue
The degree of DNA damage in kidney tissue was determined by measuring the level of 8-hydroxy-2′-deoxyguanosine (8-OHdG) using a commercial kit (Sunred, Shangai, China) (cat. no: 201-11-0032; assay range: 0.05–20 ng/ml).
Histopathological examination of kidney tissue
Necropsy was performed for histopathological evaluation of the renal tissues and tissue samples were fixed in 10% formalin solution for 48 h. Tissues were embedded in paraffin blocks with routine-tracking procedures. From each block were taken 4-μm-thick cross-sections. All preparations were stained with hematoxylin-eosin (HE) and examined with a light microscope (Leica DM 1000, Germany).
Immunohistochemical examination of kidney tissue
Cross-sections obtained from kidney tissues were transferred to adhesive (poly-L-Lysin) slides for immunperoxidase examination and passed through xylol and alcohol series. After washing with phosphate-buffered saline (PBS), endogenous peroxidase was inactivated for 10 min at 3% H2O2 (Merck, K50505100 830 1.08600.1000). In the microwave oven set at 500 watts, the antigen in the tissues was released by exposure to retrieval solution (abcam, ab93678) for 2 × 5 min. Tissues were incubated with nephrin and tumor necrosis factor-α (TNF-α) antibodies (catalog no. Ab-216692, Abcam, UK, sc-52B83, Santa Cruz, USA) for 30 min,in an incubator set at 37 °C. Immunohistochemistry procedures were followed according to the kit instructions (AbcamHRP/DAB Detection IHC kit). 3-3 ’Diaminobenzidine (DAB) was used as chromogen. Background staining was performed with hematoxylin. Immuno-positivity of the samples was expressed as none (-), mild (+), moderate (++), and severe (+++).
Determination of elemental content of kidney tissue
In this study, ICP-MS NexION® 2000 (PerkinElmer® Inc., USA) device with quartz nebulizer gasifier, cyclonic spray chember, and integrated auto-sampler was used for the element analysis of samples. The washing solution containing 1% hydrochloric acid ultra-pure water was prepared using 18.3 MΩ ultra-pure water and the ICP-MS method was performed. In the preparation of the sample, 0.2 g were weighed, and transferred to the microwave oven teflon cups and added 10 mL nitric acid. ICP-MS calibration solutions were prepared by dilution with commercially available multi-element standards of 1% (nitric acid, ultra-pure water). In addition, ICP-MS calibration was performed before each measurement. With a peristaltic pump, the samples were sent to the cyclonic spray chamber with argon gas flow. ICP-MS NexION instrument software was used to control the instrument, including calibration, interferences, data collection, and data analysis. In addition to argon gas, helium gas was used to prevent interference.
Statistical analysis
Statistical analysis of biochemical data was done with one-way ANOVA test in IBM SPSS program (version 20.0; IBM Co, North Castle, NY). Tukey’s multiple comparison test was used for comparisons between the groups. All values were expressed as mean ± standard error (SEM), and p < 0.05 was considered significant. Kruskal-Wallis test, which is one of the nonparametric tests, was applied to the data obtained from histopathological examinations in order to determine the differences between the groups. The comparison of binary groups was done using Mann-Whitney U test. SPSS 13.0 package program was used for these statistical analyzes.
Results
Analysis results of serum urea and creatinine levels
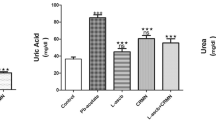
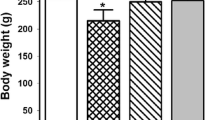
The results indicated that PbAc increases serum urea and creatinine levels by disrupting kidney function (P < 0.05). CR was found to reduce serum urea and creatinine levels by decreasing kidney toxicity caused by PbAc in a dose-dependent manner (25 and 50 mg/kg/BW). It was determined that there was no significant difference between the control and CR groups. Furthermore, it was observed that the rats who lost weight with PbAc administration reached the weight of the animals in the control group with CR treatment. Serum urea and creatinine levels and body weights of all groups are presented in Table 1.
Analysis of lipid peroxidation and antioxidant markers in kidney tissue
It was determined that PbAc administration increased MDA levels by causing lipid peroxidation in kidney tissue and decreased the activities of antioxidant enzymes (SOD, CAT and GPx) and GSH levels. On the other hand, it was found that CR administration decreased lipid peroxidation; thus, MDA levels reduced. It was also detected that CR treatment significantly increased SOD, CAT, and GPx activities and GSH levels compared to PbAc group (P < 0.05). Lipid peroxidation and the levels of antioxidant markers in kidney tissue are given in Table 2.
Analysis results of AQP-1 levels in kidney tissue
AQP-1 levels are given in Fig. 2. The results demonstrated that PbAc decreased AQP-1 levels due to the damage to the kidneys. Furthermore, CR administration was observed to significantly increase AQP-1 levels compared to PbAc group by alleviating kidney damage P < 0.05.
Analysis results of p53 levels in kidney tissue
p53 levels analyzed by ELISA method are given in Fig. 3. The results demonstrated that PbAc made cells undergo apoptosis by increasing the p53 levels. CR was found to protect the cells against apoptosis by suppressing p53 expression.
Analysis of inflammatory response levels in kidney tissue
Analysis results of inflammatory markers of kidney tissue are presented in Table 3. The results showed that PbAc led to inflammation by significantly increasing IL-33, PGE-2, COX-2, NF-κB, and iNOS levels compared to the control group. Nevertheless, CR was found to reduce PGE-2, COX-2, NF-κB, and iNOS levels in a dose-dependent manner, thereby alleviating inflammation in the kidney. It was found that IL-33 levels did not make a significant difference between PbAc + CR-25 and PbAc + CR-50 groups.
DNA damage level
As stated in Fig. 4, it was observed that there were increases in 8-OHdG levels since PbAc damaged DNA. CR was observed to protect DNA from damage caused by PbAc and to limit the rise of 8-OHdG levels.
Histopathological findings
Histopathological examination showed that the kidney tissues of the rats in the control and CR-50 groups had normal appearance (Fig. 5a–b). Mononuclear cell infiltration in the interstitium of the PbAc group, severe hydropic degeneration and necrosis in the tubules, severe hyperemia in the vessels, and hyaline cylinders in some tubulus lumens were observed (Fig. 5c). Hydropic degeneration and mild coagulation necrosis, mild mononuclear cell (MNH) infiltration, and hyperemia in interstitial areas were detected in moderate tubulus epithelium in the PbAc + CR-25 group (Fig. 5d). In the PbAc + CR-50 group, the lesions were very mild and statistically significant P ˂ 0.05 differences were found when compared to the PbAc group (Fig. 5e). Histopathological findings of all groups are summarized in Table 4.
a–e Histopathological examination of rat kidney tissue. a–b Control and CR-50 group: kidney tissue with normal histological structure; c PbAc group: tubular epithelium with hydropic degeneration (arrowheads), necrosis (thin arrows), mononuclear cell infiltration in interstitial areas (stars) and severe hyperemia (arrows) in vessels, hyaline cylinders in tubulus lumens (thick arrows). d PbAc + CR-25: mild mononuclear cell infiltration in interstitial areas (star), tubular epithelium with moderate degeneration (arrowheads), mild necrosis (thin arrow), hyaline cylinders in tubulus lumens (thick arrows). e PbAc + CR-50: tubular epithelium with mild hydropic degeneration (arrowheads), H&E; bar, 20 μm
Immunohistochemical findings
As a result of immunohistochemical examination of the renal tissues, severe nephrin expression was observed in the tubulus epithelium in the control and CR-50 groups, but TNF-α expression was not observed (Fig. 6, 7a–b). Negative nephrin expression in tubular epithelium in PbAc group and severe TNF-α expression in interstitial tissues, perivascular, and glomeruli were determined (Fig. 6, 7c). Nephrine was slightly expressed in renal tubule epithelium of the PbAc + CR-25 group (Fig. 6d) and TNF-α expression was moderate in the interstitial area (Fig. 7d). In the PbAc + CR-50 group, nephrine expression was severe in the tubulus epithelium (Fig. 6e). Mild TNF-α expression was detected in the interstitial area (Fig. 7e). In this group, expression levels of immunohistochemical markers were statistically significant (P ˂ 0.05) differences when compared with PbAc group. Immunohistochemical findings are summarized in Table 4.
a–e Nephrin expression in the rat kidney tissue. a–b Control and CR-50 group: severe nephrin expression (arrowheads) in tubular epithelium; c PbAc group: negative nephrin expression; d PbAc + CR-25: mild nephrin expression (arrowheads) in tubulus epithelia; e PbAc + CR-50, moderate nephrin expression in tubular epithelium (arrowheads), IHC-P; bar, 20 μm
a–e TNF-α expression in the rat kidney tissue. a–b Control and CR-50 group, negative TNF-α expression; c PbAc group: severe TNF-α expression (arrowheads) at interstitial intervals; d PbAc + CR-25: moderate TNF-α expression (arrowheads); e PbAc + CR-50: mild TNF-α expression at interstitial intervals (arrowheads), IHC-P; bar, 20 μm
Levels of elements in kidney tissue
According to the data obtained by ICP-MS method, the PbAc and PbAc + CR-25 groups were found to have the highest Pb accumulation in kidney tissue compared to the control group (P < 0.05). It was determined that 50 mg/kg/BW administration of CR reduced Pb accumulation caused by PbAc. The groups with the highest K level were found to have CR administration with PbAc. In the PbAc and CR groups, K levels were significantly lower than the control group (P < 0.05), but there was no significant difference between them (P > 0.05). The PbAc group had the highest Na levels in the kidney tissue compared to the control group. In the CR-50 group, Na levels decreased significantly (P < 0.05), but there was no significant difference between CR administration with PbAc and control groups (P > 0.05). PbAc + CR-25 and PbAc + CR-50 were the groups with the highest Ca levels compared to the control group. While there was no significant difference between CR-50 and PbAc groups (P > 0.05), it was found that Ca levels decreased compared to the control group (P < 0.05). It was observed that CR decreased significantly Fe levels in kidney tissue compared to control group (P < 0.05). Cu and Zn levels were significantly higher in the PbAc group than in the control group (P < 0.05); nevertheless, it was found that CR administration reduced this increase caused by PbAc from the control group to low levels. Levels of all elements in the kidney tissue in different groups are given in Table 5.
Discussion
The protective effects of naturally occurring antioxidant substances against heavy metal toxicity have been the subject of many studies, and researchers have achieved promising results [4, 47, 48]. In this study, the protective effects of CR against kidney damage of rats exposed to PbAc orally were investigated. Biochemical, histopathological, and immunohistochemical results showed that PbAc caused damage in the kidneys. However, it was determined that the administration of CR to rats before PbAc administration had a positive effect on these results and had a protective effect against kidney damage.
The kidneys are highly vulnerable to toxic damage because they are exposed directly to blood plasma through their open fenestrae [49]. That’s why, it is thought that one of the organs primarily affected by toxic substances is kidneys and kidney function disorders occur. The measurements of serum urea and creatinine levels are frequently used to evaluate kidney function [50]. It is known that an increase in serum urea and creatinine levels is associated with kidney failure [51]. Abdel-Moneim et al. [49] reported that PbAc caused an increase in serum urea and creatinine levels possibly because it led to kidney dysfunction and kidney failure. In this study, it was detected that there was a significant increase in serum urea and creatinine levels in animals treated with PbAc. In addition, as a result of histopathological examinations, it was observed that PbAc caused severe hydropic degeneration and necrosis in the tubules. It was determined that CR administration alleviates PbAc-induced lesions in tissues and reduces urea and creatinine levels to normal levels.
The disruption of the balance between the antioxidant system in the body and the production of reactive oxygen species causes oxidative stress [52, 53]. The possible mechanism of Pb toxicity is thought to be oxidative stress [15]. Lipid peroxidation provides important contributions in determining renal cell damage. MDA is the degradation product of lipid peroxidation. On the other hand, SOD, CAT, and GPx are antioxidant enzymes that provide antioxidant defense in the body [54]. Studies reported that Pb, by linking to SH- groups of antioxidant enzymes, decreased the activities of these enzymes and caused the depletion of GSH, a non-enzymatic antioxidant and lipid peroxidation. Therefore, in the treatment of Pb toxicity, it is aimed both to remove Pb from the body and to prevent the occurrence of oxidative stress by cleaning the reactive oxygen species [15]. In the current study, it was seen that PbAc increased MDA levels by causing lipid peroxidation. It was observed that CH reduced lipid peroxidation, increased enzymatic and non-enzymatic markers and protected membrane integrity in kidney tissue due to the antioxidant properties of hydroxyl groups in its structure.
Aquaporins, which are water channel proteins, are transmembrane glycoproteins that allow the entry or release of water across the permeable epithelium, such as renal tubular epithelium [55, 56]. To date, many types of AQP have been cloned and characterized. The expression and/or physiological regulation of AQP-1, AQP-2, AQP-3, AQP-4, AQP-6, AQP-7, AQP-8, and AQP-11 are well documented in the kidney [57] AQP1 is abundantly expressed in the apical and basolateral areas of the proximal tubular cells and in the descending limb cells of the Henle loop. AQP1 helps quickly reabsorption of large quantities of filtered water [58]. It has been reported that toxicity and dysfunction in kidneys considerably affect the levels of AQPs and that AQP-1 levels in the kidney tissues of rats with different toxic agents dramatically decrease [19, 55, 59]. Nephrin is an important structural protein of the glomerular filtration barrier and is responsible for ultrafiltration [60, 61]. Decrease in nephrine levels causes glomerular dysfunction and proteinuria [60]. Preservation of nephrine expression is thought to be a potential therapeutic approach to alleviate podocyte loss and glomerular damage in glomerular disease [62]. In the present study, it was determined that PbAc had toxic effects in the kidneys, which led to kidney dysfunction and significantly reduced AQP-1 and nephrin levels. CR was found to reduce the toxic effects of PbAc and alleviate kidney dysfunction and increase AQP-1 and nephrin levels.
The mechanism of lead toxicity is quite complicated. However, according to the studies done, it has been reported that one of Pb’s toxicity mechanisms is the apoptosis pathway [63]. Apoptosis induced by various chemicals or environmental stimuli occurs under the control of many genes, including p53 [64]. Under normal conditions, p53 expression is kept in a low levels due to the extremely short half-life of the polypeptide. Nonetheless, in the cases where ROS levels increase and following the damage to DNA, p53 protein levels increase significantly in a short time [63]. PbAc can directly damage DNA, or by causing oxidative stress, it can indirectly damage cells and DNA [65]. This explains the situation in the current study that PbAc causes severe damage to the kidneys by inducing an increase in p53 levels. However, it has been determined that CR reduces p53 levels by alleviating oxidative stress and DNA damage due to its antioxidant effect, thus protecting kidneys from PbAc toxicity.
There is growing evidence of the link between oxidative stress and inflammatory response. Oxidative stress provides important contributions to the inflammation process. It has been reported that oxidant molecules affect all phases of the inflammatory process, such as the release of endogenous danger signal molecules, their perception by natural immune cells from the Toll-like receptors (TLRs) and NOD-like receptor (NLRs) families, and the activation of signal pathways that initiate an adaptive cellular reaction to these signals [66]. The responses initiated by TLRs are transmitted by activation of NF-κB [67]. Therefore, oxidative stress activates NF-κB and initiates inflammation mechanism. This is one of the strongest evidence supporting the link between oxidative stress and inflammation in disease progression [68]. NF-κB stimulates the release of pro-inflammatory cytokines, particularly TNF-α. In addition, expression of iNOS and COX-2 proteins is regulated by NF-κB. Therefore, suppression of NF-κB is of great therapeutic importance [19, 69]. Liu et al. [70] reported that Pb affects kidney tissue and causes NF-κB activation and inflammation. Flavonoids play an important role in the regulation of cellular functions such as cell cycle signals and modulation of inflammatory pathways [71]. Rehman et al. [72] showed that CR effectively inhibited the increase in ferric nitrilotriacetate-mediated TNF-α, COX-2, iNOS, and PGE2 expressions. Kandemir et al. [73] reported that paracetamol-induced inflammation in kidney tissue improves CR and decreases IL-33 levels. Similar to the literature, in the present study, PbAc increased NF-κB, IL-33, PGE-2, COX-2, and iNOS levels in renal tissue due to oxidative stress. As a result, inflammation in the kidney tissue occurred. CR reduced the inflammation caused by PbAc and decreased NF-κB, IL-33, PGE-2, COX-2, and iNOS levels significantly compared to PbAc group. Immunohistochemical examination revealed that TNF-α was strongly expressed in the PbAc-treated group, while CR decreased TNF-α expression.
DNA is a highly sensitive macromolecule to oxidative damage [74]. 8-OHdG is a widely used biomarker for determining oxidative damage in DNA [75]. ROS is thought to play an active role in the formation of 8-OHdG [19, 75]. According to the data obtained from this study, PbAc increased the formation of 8-OHdG by causing oxidative damage in DNA. Also, CR improved PbAc-induced oxidative DNA damage with antioxidant properties, approximating the formation of 8-OHdG to that of the control group. Similarly, Rani et al. [76] reported that CR significantly reduced the 8-OHdG level dose-dependently.
In the studies evaluating the effectiveness of antioxidants as chelating agents, although antioxidants are reported to be not as effective as traditional chelators [77, 78], there are the studies showing that flavonoids have chelating properties in addition to their antioxidant properties [79]. It has been reported that CR is also capable of metal chelation [80]. In our study, we found that administration of 25 mg/kg/BW of CR did not make a significant difference in the amount of Pb in renal tissue compared to the PbAc group, but that of 50 mg/kg/BW reduced chelation of Pb significantly.
There is sufficient information that heavy metals, including Pb, may have adverse effects on the concentrations of essential metals. However, the information about the effect of electrolytes in the body is insufficient [81]. Xia et al. [82] reported that Pb has no effect on the amount of Cu and Zn in kidney tissue. Aksu et al. [83] stated that PbAc administration increases zinc accumulation in the kidney and has no effect on Cu and Fe levels and also increases Zn level with the use of phenolic compounds. In our study, it was found that PbAc significantly increased Cu, Zn, and Na amounts in kidney tissue and decreased K, Ca, and Fe amounts in comparison to the control group. However, it was seen that K and Ca increased; Fe, Cu, and Zn decreased in the kidneys of the rats given CR with PbAc, and Na did not make a significant difference compared to the control group. Given that Zn homeostasis is provided by the kidneys, the information that PbAc accumulates in the kidneys along with damage to the kidneys confirms our data [83]. Also, Pb causes Fe absorption to reduce by linking to similar areas with Fe [84]. This explains why PbAc reduces the amount of Fe in the kidney in the current study.
Conclusion
Our findings confirmed that PbAc led to toxicity in the kidneys because of inflammation and apoptosis associated with oxidative stress. It was also detected that toxicity decreased AQP-1 levels. However, it was concluded that the antioxidant, anti-inflammatory and antiapoptotic properties of CR also apply to PbAc-induced nephrotoxicity, and that CR is a promising compound in the treatment of renal toxicity. Still, the mechanism of this effect of CR needs to be supported by further studies.
References
Phyu MP, Tangpong J (2014) Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem Toxicol 70:151–156. https://doi.org/10.1016/j.fct.2014.04.035
Khalil SR, Khalifa HA, Abdel-Motal SM, Mohammed HH, Elewa YHA, Mahmoud HA (2018) Spirulina platensis attenuates the associated neurobehavioral and inflammatory response impairments in rats exposed to lead acetate. Ecotoxicol Environ Saf 157:255–265. https://doi.org/10.1016/j.ecoenv.2018.03.068
Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T (2016) The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: role of oxidative stress. Food Chem Toxicol 96:263–272. https://doi.org/10.1016/j.fct.2016.07.009
Khalil SR, Elhady WM, Elewa YHA, Abd El-Hameed NE, Ali SA (2018) Possible role of Arthrospira platensis in reversing oxidative stress-mediated liver damage in rats exposed to lead. Biomed Pharmacother 97:1259–1268. https://doi.org/10.1016/j.biopha.2017.11.045
Shojaeepour S, Fazeli M, Oghabian Z, Pourgholi L, Mandegary A (2018) Oxidative stress in opium users after using lead-adulterated opium: the role of genetic polymorphism. Food Chem Toxicol 120:571–577. https://doi.org/10.1016/j.fct.2018.07.061
Song XB, Liu G, Liu F, Yan ZG, Wang ZY, Liu ZP, Wang L (2017) Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis 8(6):e2863. https://doi.org/10.1038/cddis.2017.262
Abd El-Hack ME, Abdelnour SA, Abd El-Moneim AEE, Arif M, Khafaga A, Shaheen H, Samak D, Swelum AA (2019) Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ Sci Pollut Res Int 26(23):23209–23218. https://doi.org/10.1007/s11356-019-05805-8
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243. https://doi.org/10.1002/tox.21994
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev 11(1):2–22
Matovic V, Buha A, Ethukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. https://doi.org/10.1016/j.fct.2015.02.011
Farmand F, Ehdaie A, Roberts CK, Sindhu RK (2005) Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ Res 98(1):33–39. https://doi.org/10.1016/j.envres.2004.05.016
Liu J, Jia DY, Cai SZ, Li CP, Zhang MS, Zhang YY, Yan CH, Wang YP (2015) Mitochondria defects are involved in lead-acetate-induced adult hematopoietic stem cell decline. Toxicol Lett 235(1):37–44. https://doi.org/10.1016/j.toxlet.2015.03.007
Dobrakowski M, Pawlas N, Kasperczyk A, Kozlowska A, Olewinska E, Machon-Grecka A, Kasperczyk S (2017) Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 36(7):744–754. https://doi.org/10.1177/0960327116665674
Caglayan C, Kandemir FM, Darendelioglu E, Yildirim S, Kucukler S, Dortbudak MB (2019) Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 56:60–68. https://doi.org/10.1016/j.jtemb.2019.07.011
Alcaraz-Contreras Y, Mendoza-Lozano RP, Martinez-Alcaraz ER, Martinez-Alfaro M, Gallegos-Corona MA, Ramirez-Morales MA, Vazquez-Guevara MA (2016) Silymarin and dimercaptosuccinic acid ameliorate lead-induced nephrotoxicity and genotoxicity in rats. Hum Exp Toxicol 35(4):398–403. https://doi.org/10.1177/0960327115591373
Andersen O, Aaseth J (2016) A review of pitfalls and progress in chelation treatment of metal poisonings. J Trace Elem Med Biol 38:74–80. https://doi.org/10.1016/j.jtemb.2016.03.013
BaSalamah MA, Abdelghany AH, El-Boshy M, Ahmad J, Idris S, Refaat B (2018) Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep 8(1):4853. https://doi.org/10.1038/s41598-018-23258-w
Benzer F, Kandemir FM, Kucukler S, Comakli S, Caglayan C (2018) Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: by modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch Physiol Biochem 124(5):448–457. https://doi.org/10.1080/13813455.2017.1422766
Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G (2019) Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol 54:69–78. https://doi.org/10.1016/j.jtemb.2019.04.007
Kuzu M, Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Turk E (2018) Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother 106:443–453. https://doi.org/10.1016/j.biopha.2018.06.161
Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ (2017) Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem 41(5):e12398
Benzer F, Kandemir FM, Ozkaraca M, Kucukler S, Caglayan C (2018) Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol 32(2). https://doi.org/10.1002/jbt.22030
Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Mahamadu A, Dortbudak MB (2018) Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed Pharmacother 105:981–991. https://doi.org/10.1016/j.biopha.2018.06.048
Kandemir FM, Ozkaraca M, Küçükler S, Caglayan C, Hanedan B (2018) Preventive effects of hesperidin on diabetic nephropathy induced by streptozotocin via modulating TGF-β1 and oxidative DNA damage. Toxin Rev 37(4):287–293
Celik H, Kucukler S, Comakli S, Ozdemir S, Caglayan C, Yardim A, Kandemir FM (2019) Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of oxidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology 76:126–137. https://doi.org/10.1016/j.neuro.2019.11.004
Hanedan B, Ozkaraca M, Kirbas A, Kandemir FM, Aktas MS, Kilic K, Comakli S, Kucukler S, Bilgili A (2018) Investigation of the effects of hesperidin and chrysin on renal injury induced by colistin in rats. Biomed Pharmacother 108:1607–1616. https://doi.org/10.1016/j.biopha.2018.10.001
Samarghandian S, Farkhondeh T, Azimi-Nezhad M (2017) Protective effects of chrysin against drugs and toxic agents. Dose-Response 15(2):1559325817711782. https://doi.org/10.1177/1559325817711782
Aksu EH, Ozkaraca M, Kandemir FM, Omur AD, Eldutar E, Kucukler S, Comakli S (2016) Mitigation of paracetamol-induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia 48(10):1145–1154. https://doi.org/10.1111/and.12553
Tahir M, Sultana S (2011) Chrysin modulates ethanol metabolism in Wistar rats: a promising role against organ toxicities. Alcohol Alcohol 46(4):383–392. https://doi.org/10.1093/alcalc/agr038
Eldutar E, Kandemir FM, Kucukler S, Caglayan C (2017) Restorative effects of chrysin pretreatment on oxidant-antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: an experimental and biochemical study. J Biochem Mol Toxicol 31(11). https://doi.org/10.1002/jbt.21960
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118. https://doi.org/10.1016/j.ejphar.2014.01.065
Aksu EH, Kandemir FM, Kucukler S, Mahamadu A (2018) Improvement in colistin-induced reproductive damage, apoptosis, and autophagy in testes via reducing oxidative stress by chrysin. J Biochem Mol Toxicol 32(11):e22201. https://doi.org/10.1002/jbt.22201
Zheng X, Meng WD, Xu YY, Cao JG, Qing FL (2003) Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett 13(5):881–884. https://doi.org/10.1016/s0960-894x(02)01081-8
Ha SK, Moon E, Kim SY (2010) Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-kappaB and JNK activations in microglia cells. Neurosci Lett 485(3):143–147. https://doi.org/10.1016/j.neulet.2010.08.064
Mani R, Natesan V (2018) Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 145:187–196. https://doi.org/10.1016/j.phytochem.2017.09.016
Tsuji PA, Walle T (2008) Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem Biol Interact 171(1):37–44. https://doi.org/10.1016/j.cbi.2007.08.007
Asad A, Hamid S, Qama K (2018) Effect of Lead acetate on basement membrane of seminiferous tubules of adult rat testis and protective effects of Ficus carica: a histological study. J Coll Physicians Surg Pak 28(10):731–734 3010
Temel Y, Kucukler S, Yildirim S, Caglayan C, Kandemir FM (2020) Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedeberg's Arch Pharmacol 393(3):325–337. https://doi.org/10.1007/s00210-019-01741-z
Talke H, Schubert GE (1965) Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr 43:174–175. https://doi.org/10.1007/BF01484513
Newman D (1999) Renal function and nitrogen metabolites. Tietz textbook of clinical chemistry:1204-1270
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16(2):359–364. https://doi.org/10.1016/0003-2697(66)90167-9
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71(4):952–958. https://doi.org/10.1016/0006-291x(76)90747-6
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Moustafa GG, Khalil S, Hussein MM, Labib M (2012) The cytotoxic and ultrastrctural perturbations of aluminum exposed nile catfish with special reference to the mitigating effect of vitamin C
Khalil SR, Hussein MM (2015) Neurotransmitters and neuronal apoptotic cell death of chronically aluminum intoxicated Nile catfish (Clarias gariepinus) in response to ascorbic acid supplementation. Neurotoxicology 51:184–191. https://doi.org/10.1016/j.neuro.2015.09.008
Abdel-Moneim AM, El-Toweissy MY, Ali AM, Allah AAMA, Darwish HS, Sadek IA (2015) Curcumin ameliorates lead (Pb2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168(1):206–220
Qi SS, Zheng HX, Jiang H, Yuan LP, Dong LC (2020) Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 10(3). https://doi.org/10.3390/biom10030398
Soussi A, Gargouri M, Akrouti A, El Feki A (2018) Antioxidant and nephro-protective effect of Juglans regia vegetable oil against lead-induced nephrotoxicity in rats and its characterization by GC-MS. EXCLI J 17:492–504. https://doi.org/10.17179/excli2018-1235
Köksal E, Bursal E, Gülçin İ, Korkmaz M, Çağlayan C, Gören AC, Alwasel SH (2017) Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int J Food Prop 20(3):514–525
Taslimi P, Kandemir FM, Demir Y, Ileriturk M, Temel Y, Caglayan C, Gulcin I (2019) The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol:e22313. doi:https://doi.org/10.1002/jbt.22313
Zheng HX, Qi SS, He J, Hu CY, Han H, Jiang H, Li XS (2020) Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor beta1/Smad expression. J Agric Food Chem 68(15):4399–4410. https://doi.org/10.1021/acs.jafc.0c00680
Gao J, Wang X, Chang Y, Zhang J, Song Q, Yu H, Li X (2006) Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal Biochem 350(2):165–170. https://doi.org/10.1016/j.ab.2006.01.003
Kishore BK, Krane CM, Di Iulio D, Menon AG, Cacini W (2000) Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int 58(2):701–711. https://doi.org/10.1046/j.1523-1755.2000.00216.x
Matsuzaki T, Yaguchi T, Shimizu K, Kita A, Ishibashi K, Takata K (2017) The distribution and function of aquaporins in the kidney: resolved and unresolved questions. Anat Sci Int 92(2):187–199. https://doi.org/10.1007/s12565-016-0325-2
Pallone TL, Kishore BK, Nielsen S, Agre P, Knepper MA (1997) Evidence that aquaporin-1 mediates NaCl-induced water flux across descending vasa recta. Am J Phys 272(5 Pt 2):F587–F596. https://doi.org/10.1152/ajprenal.1997.272.5.F587
Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G (2019) Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res Int 26(22):22562–22574. https://doi.org/10.1007/s11356-019-05505-3
Zhang X, Williams MC, Rentsendorj O, D’Agnillo F (2018) Reversible renal glomerular dysfunction in guinea pigs exposed to glutaraldehyde-polymerized cell-free hemoglobin. Toxicology 402:37–49
Moraes A, Magalhães V (2018) Renal tubular damage caused by cylindrospermopsin (cyanotoxin) in mice. Toxicol Lett 286:89–95
Li X, Chuang PY, D’Agati VD, Dai Y, Yacoub R, Fu J, Xu J, Taku O, Premsrirut PK, Holzman LB (2015) Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J Am Soc Nephrol 26(10):2361–2377
Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH (2008) Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol 46(5):1488–1494. https://doi.org/10.1016/j.fct.2007.12.016
Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, Saglam YS (2018) Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother 102:517–530
Paulis MG, Hassan OA, Abbass MF, Mohammad MAH (2018) Structural and lipid peroxidation effects of lead on rat hippocampus and its attenuation by hydrogen rich water. J Chem Neuroanat 91:55–62. https://doi.org/10.1016/j.jchemneu.2018.04.004
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2):203–230. https://doi.org/10.1515/hsz-2013-0241
Khalil SR, Mohammed WA, Zaglool AW, Elhady WM, Farag MR, El Sayed SAM (2019) Inflammatory and oxidative injury is induced in cardiac and pulmonary tissue following fipronil exposure in Japanese quail: mRNA expression of the genes encoding interleukin 6, nuclear factor kappa B, and tumor necrosis factor-alpha. Environ Pollut 251:564–572. https://doi.org/10.1016/j.envpol.2019.05.012
Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardo FP, Pomara C, Riezzo I, Fineschi V (2016) Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J Cell Mol Med 20(4):601–612
Kaulmann A, Legay S, Schneider YJ, Hoffmann L, Bohn T (2016) Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion. Mol Nutr Food Res 60(5):992–1005
Liu B, Zhang H, Tan X, Yang D, Lv Z, Jiang H, Lu J, Baiyun R, Zhang Z (2017) GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget 8(26):42226–42237
Gargouri M, Magné C, Dauvergne X, Ksouri R, El Feki A, Metges M-AG, Talarmin H (2013) Cytoprotective and antioxidant effects of the edible halophyte Sarcocornia perennis L.(swampfire) against lead-induced toxicity in renal cells. Ecotoxicol Environ Saf 95:44–51
Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Qamar W, Ali F, Sultana S (2013) Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett 216(2-3):146–158
Kandemir F, Kucukler S, Eldutar E, Caglayan C, Gülçin I (2017) Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: a multi-biomarker approach. Sci Pharm 85(1):4
Esplugas R, LLovet MI, Bellés M, Serra N, Vallvé JC, Domingo JL, Linares V (2018) Renal and hepatic effects following neonatal exposure to low doses of Bisphenol-A and 137Cs. Food Chem Toxicol 114:270–277
Caglayan C, Temel Y, Kandemir FM, Yildirim S, Kucukler S (2018) Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res 25(21):20968–20984
Rani N, Bharti S, Bhatia J, Tomar A, Nag T, Ray R, Arya DS (2015) Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutr Metab 12(1):11
Gurer H, Ozgunes H, Neal R, Spitz DR, Ercal N (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128(3):181–189. https://doi.org/10.1016/s0300-483x(98)00074-2
Flora SJ, Pande M, Mehta A (2003) Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 145(3):267–280. https://doi.org/10.1016/s0009-2797(03)00025-5
Gautam P, Flora SJ (2010) Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition 26(5):563–570. https://doi.org/10.1016/j.nut.2009.06.008
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–2788. https://doi.org/10.3390/ijerph7072745
Fiati Kenston SS, Su H, Li Z, Kong L, Wang Y, Song X, Gu Y, Barber T, Aldinger J, Hua Q, Li Z, Ding M, Zhao J, Lin X (2018) The systemic toxicity of heavy metal mixtures in rats. Toxicol Res (Camb) 7(3):396–407. https://doi.org/10.1039/c7tx00260b
Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W (2010) Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 130(2):414–420
Aksu D, Sağlam Y, Yildirim S, Aksu T (2017) Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol (Noisy le Grand) 63 (10)
Kwong WT, Friello P, Semba RD (2004) Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ 330(1-3):21–37
Funding
This study was funded by the Unit of Scientific Research Projects in Munzur University. (Project No: YLMUB017-24)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kucukler, S., Benzer, F., Yildirim, S. et al. Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: a Biochemical and Histopathological Approach. Biol Trace Elem Res 199, 1501–1514 (2021). https://doi.org/10.1007/s12011-020-02268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02268-8