Abstract
Lead is a biohazardous metal that is commonly involved in human illness including renal injury. Although it is a non-redox reactive metal, lead-induced renal injury is largely based on oxidative stress. The current work aimed at exploring the possible protective effect of γ-glutamyl cysteine (γGC) against lead-induced renal injury. Rats were allocated to normal and γGC control groups, lead-treated group, and lead and γGC-treated group. γGC alleviated lead-induced renal injury as evidenced by attenuation of histopathological aberration, amelioration of oxidative injury as demonstrated by significant reduction in lipid and protein oxidation, elevation of total antioxidant capacity, and glutathione level. The activity of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) was significantly elevated. γGC significantly decreased levels of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β and the activity of the apoptotic marker caspase-3. In addition, γGC reduced kidney lead content, enhanced weight gain, and improved renal function as demonstrated by reduced serum levels of urea and creatinine. Importantly, γGC upregulated proliferating cell nuclear antigen (PCNA) expression, denoting enhanced renal regenerative capacity. Together, our findings highlight evidence for alleviating effects of γGC against lead-induced renal injury that is potentially mediated through diminution of oxidative tissue injury, reduction of inflammatory response, attenuation of apoptosis, and enhancement of renal regenerative capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead is a non-biodegradable environmental pollutant that is commonly involved in acute and chronic human illness [1, 2]. Although lead has been banned from many environmental sources such as gasoline and paints, other industrial sources such as mining, crystal and ceramic industry, and smelting still represent important pollution sources [3]. Industrial waste-contaminated food and water represent major exposure sources of general population to lead [4]. Lead can be absorbed through gastrointestinal tract and skin and can also be inhaled in polluted air [4–7]. Exposure to lead has been associated with a broad range of biological alterations in renal tissues including oxidative tissue injury, histopathological changes, apoptotic cell death, inflammatory responses, as well as reduced excretory renal function (accumulation of metabolic waste products such as urea and creatinine) [8]. In experimental animals, lead toxicity is also associated with diminished ability to gain weight [8]. The exact mechanism of lead-induced renal toxicity is not clearly understood; however, oxidative stress has been proven as a major underlining mechanism [4, 5, 7, 9–14]. Although it is a non-redox reactive metal, lead induces oxidative stress and subsequent oxidative tissue injury through generation of reactive oxygen species (ROS) such as superoxide anion radical, hydroxyl radical, and hydrogen peroxide [1, 3, 15]. In addition, lead inhibits activity of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [1, 3, 16, 17]. Moreover, lead depletes the biologically important non-enzymatic antioxidant reduced glutathione. [3, 15, 18]. Antioxidants, thus, may represent a potential measures for reducing lead-induced renal tissue injury. γ-Glutamyl cysteine (γGC) is a dipeptide with reported antioxidant activity owning to its thiol group of its cysteine residue [19–25]. It has been reported that γGC plays important roles in detoxification of reactive oxygen species through its action as a cofactor for glutathione peroxidase 1 [26]. In addition, γGC is a substrate for glutathione synthetase for synthesis of the known non-enzymatic antioxidant reduced glutathione [26]. The aim of the current work was to explore the potential alleviating effect of γGC dipeptide against lead-induced renal tissue injury using rats as an experimental mammalian model.
Material and Methods
Chemicals and Kits
γ-Glutamyl cysteine, lead acetate, thiobarbituric acid, trichloroacetic acid, phenylmethanesulfonyl fluoride (PMSF), and dinitrophenyl hydrazine (DNPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All Other chemicals were of high purity. Total antioxidant capacity, superoxide dismutase, catalase, and glutathione peroxidase kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). TNF-α, IL-6, and IL-1β kits were purchased from RayBiotech (Norcross, GA, USA). Caspase-3 colorimetric assay kit was purchased from R&D systems (Minneapolis, MN, USA). Primary and secondary antibodies for proliferating cell nuclear antigen (PCNA) and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals, Experimental Design, and Treatment Protocol
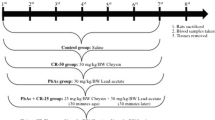
Thirty-two male Wistar rats that were matched for age and weight (210 g on average) were obtained from King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. The rats were housed in polypropylene cages, four rats per cage, under standard environmental conditions (temperature 23 ± 2 °C, humidity 60 ± 10 % and 12-h light/dark cycle). Standard commercial rat chow and water were provided ad libitum. All procedures related to animal care, treatments, and sampling were conducted according to the guidelines of Taif University research ethical committee. After 10 days of acclimatization to our laboratory conditions, rats were randomly allocated to four groups of eight rats each. In group 1 (normal control, C), rats were given distilled water orally by gastric gavage; in group 2 (γ-glutamyl cysteine control, G), animals received γ-glutamyl cysteine (γGC)150 mg/kg b.w./day ip for 1 week; in group 3 (Lead only-treated group, L), rats received 300 mg/kg b.w. lead acetate as a daily dose for 1 week orally by gastric gavage; in group 4 (lead and γ-glutamyl cysteine-treated group, L + G), animals were treated the same way as group 3; however, they were also injected with a daily dose of 150 mg/kg b.w. ip of γGC for 1 week starting with the first dose of lead. Lead and γ-glutamyl cysteine doses were consistent with previous studies [22, 27].
Sample Preparation
On the day after the last dose of γGC, rats in all groups were weighed and euthanized by exsanguination under pentobarbital sodium anesthesia (65 mg/kg, ip) [21, 28] to collect blood and kidneys. Blood samples were collected via cardiac puncture in plain tubes to separate serum for determination of serum urea and creatinine. Both kidneys were quickly removed, rinsed in ice-cold saline, weighed, and divided into two parts for homogenization and histopathological examination. The kidney tissue samples designated for homogenization were divided into two parts; the first part was homogenized 10 % w/v in phosphate-buffered saline (PBS). Homogenates were then centrifuged for 15 min at 10,000×g, 4 °C, and the supernatant was used for determination of total protein and the other biochemical parameters. The second part was homogenized in PBS containing 1 % sodium dodecyl sulfate (SDS) and 1 mM PMSF and manipulated as described in “Determination of PCNA Expression Level in Kidney Tissues” section for Western blotting analysis of PCNA. The kidney tissue samples designated for histopathological examination were processed as described in the following “Histopathological Examination” section.
Measured Parameters
Histopathological Examination
The kidney tissues were fixed in 10 % formol saline for 1 day followed by dehydration using serial dilutions of alcohols (methyl, ethyl, and absolute ethyl alcohols). The kidney tissues were then cleared in xylene and embedded in paraffin at 56 °C in a hot air oven for 24 h. Paraffin blocks were then sectioned using Leitz 1512 microtome (Ramsey, MN, USA) at a thickness of 4 μm. After collection on glass slides, deparaffinization, and staining with hematoxylin and eosin (H&E), tissue sections were examined under light microscope [29].
Determination of Lead Content of Kidney Tissues
Lead content of the kidney tissues was determined using flame atomic absorption spectrophotometer based on previously mentioned method [5, 30]. Briefly, the kidney tissue samples were dried at 60 °C then combusted at 450 °C for 24 h. The samples were then dissolved in nitric acid (1 mM) and analyzed at 283 nm.
Determination of Serum Creatinine and Urea
Serum levels of creatinine and urea were determined using QuantiChrom™ Creatinine and QuantiChrom™ Urea Assay Kits (BioAssay Systems, Hayward, CA), respectively, according to the manufacturer’s protocol. Creatinine assay depends on the ability of creatinine in samples to react with picrate in Jaffe’s reagent to form a red-colored complex that can be measured spectrophotometrically at 510 nm [31]. Urea assay is based on Jung method that forms a colored complex with urea which can be measured spectrophotometrically at 520 nm [32].
Determination of Caspase-3 Activity in Kidney Tissues
Caspase-3 activity in the supernatant of the kidney tissue homogenates was determined using R&D systems colorimetric kit (Minneapolis, MN, USA) according to the manufacturer’s instructions as previously described [33]. The principle of the assay depends on the ability of caspase-3 in samples to cleave the colorimetric substrate acetyl-Asp-Glu-Val-Asp-p-nitroaniline with the release of p-nitroaniline (pNA) chromophore which can be monitored colorimetrically at 405 nm. The activity of caspase-3 in the tested sample is directly proportional to the level of pNA. The level of pNA in different samples was monitored using BioTek ELx800 microplate reader, and the activity of caspase-3 was expressed as fold change.
Determination of PCNA Expression Level in Kidney Tissues
Protein expression level of PCNA was determined in the kidney tissue homogenates using Western blotting analysis as described previously [20, 34]. Briefly, 0.2 g of kidney tissue was homogenized in 1 ml PBS containing 1 % SDS and 1 mM PMSF. After centrifugation (10,000×g, for 2 min at room temperature), protein concentration in the supernatant was determined using Bio-Rad DC protein assay kit. The samples were then subjected to electrophoresis (10 % SDS-PAGE, 40 μg per lane) and semi-dry transfer to a nitrocellulose membrane (Protran, PerkinElmer, Shelton, CT). Blocked nitrocellulose membranes were incubated overnight at 4 °C with monoclonal mouse anti-rat PCNA primary antibody, PC10, (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:2000, rinsed, and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Bio-Rad) at a dilution of 1:3000. PCNA bands were detected by SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) using X-ray films. The apparent molecular weights of PCNA bands were estimated by comparison to molecular weight markers. Actin was used as a loading control (sc-1616-R primary antibody, Santa Cruz Biotechnology, Santa Cruz, CA). Band density was quantitated using ImageJ image processing program (ImageJ, National Institutes of Health, USA).
Determination of Antioxidant Enzyme Activities
The activity of the antioxidant enzymes SOD, CAT, and GPx in kidney tissues was determined using Cayman chemicals kits according to the manufacturer’s instructions. Briefly, SOD kit employs hypoxanthine-xanthine oxidase-generated superoxide anion and a tetrazolium salt to generate a colored formazan dye that can be monitored at 450 nm. SOD in tissue samples inhibits the formation of formazan dye as a result of dismutation of superoxide anion [35]. SOD activity was expressed in units per milliliter kidney tissue homogenates where one unit is the amount of SOD needed to show 50 % dismutation of the superoxide anion. Catalase assay kit measures catalase activity based on its ability to inhibit oxidation of methanol by hydrogen peroxide through decomposition of hydrogen peroxide (peroxidative properties). The amount of oxidized methanol (formaldehyde) produced is thus inversely proportional to catalase activity in samples. Formaldehyde level was measured colorimetrically at 540 nm using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole as a chromogen, and catalase activity was expressed in nanomoles hydrogen peroxide per minute per milliliter tissue homogenate [36]. GPX assay relies on the ability of GPx to reduce hydrogen peroxide using reduced glutathione. The reduced glutathione is then regenerated using glutathione reductase in the presence of NADPH. The decline in absorbance of NADPH which can be monitored at 340 is proportional to GPx activity in samples [37].
Measurement of Reduced Glutathione Level
Kidney tissue content of reduced glutathione (GSH) was evaluated spectrophotometrically using 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) in accordance with Ellman’s method [38, 39]. Briefly, tissue homogenates were deprotonated with 10 % w/v TCA. The supernatant that was separated by centrifugation (10,000×g for 10 min at 4 °C) was reacted with 10 mM DTNB, and the absorbance of the produced color was measured at 412 nm.
Determination of Total Antioxidant Capacity
Total antioxidant capacity of kidney tissues was evaluated using Cayman total antioxidant assay kit in accordance with the manufacturer’s instructions as described previously [39]. The procedure is based on the fact that antioxidants in kidney tissue homogenates inhibit the oxidation of the oxidizable substrate ABTS (2,2-azino-di-[3-ethylbenzthiazoline sulfonate]). The level of oxidized ABTS was measured at 405 nm.
Measurement of Lipid Peroxidation
The level of lipid peroxides in kidney tissue homogenates was evaluated by measuring level of thiobarbituric acid reactive substances (TBARS) as described previously [33, 40]. Briefly, equal volumes of both kidney tissue homogenate and thiobarbituric acid solution (0.5 % w/v prepared in 20 % w/v trichloroacetic acid) were mixed and heated at 95 °C for 30 min. The reaction was then stopped by sudden cooling on ice. The samples were then centrifuged (10,000×g for 15 min, 4 °C) to separate supernatants, and absorbance of the supernatants was measured spectrophotometrically at 532 nm.
Measurement of Protein Carbonyl Content
The level of protein oxidation in kidney tissues was determined by evaluating the protein carbonyl content using dinitrophenyl hydrazine (DNPH) as described previously [41, 42]. Briefly, DNPH was allowed to react with proteins in kidney tissue samples followed by protein precipitation by addition of trichloroacetic acid. After separation, protein precipitates were then dissolved in guanidine-HCl and absorbance of the solution was measured at 370 nm. Extinction coefficient of 22,000 M−1 cm−1 was used to calculate protein carbonyl levels.
Determination of Proinflammatory Cytokines
The concentrations of proinflammatory cytokines TNF-α, IL-6, and IL-1β in kidney tissues of different experimental groups were measured using RayBiotech ELISA kits according to manufacturer’s instructions as described previously [33]. Briefly, tested samples or standard cytokines were incubated with anti-proinflammatory cytokine antibodies that were pre-coated to microplates. The samples were then discarded, and biotinylated antibodies were added. After incubation had been ended, biotinylated antibody solution was discarded and incubation with HRP-conjugated streptavidin was started. Absorbance of the color generated by adding 3,3′,5,5′-tetramethylbenzidine solution was measured spectrophotometrically at 450 nm.
Statistical Analysis
Multiple comparisons among different experimental groups were analyzed for statistical significance by one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons posttest. Data were presented as mean ± standard deviation (SD). Differences were considered significant at p < 0.05. SigmaPlot 12 statistics software (Systat Software, Inc., San Jose, CA) was used to create graphs and execute the statistical analysis.
Results
γ-Glutamyl Cysteine Reduces Kidney Lead Content and Improves Body Weight Gain
Administration of lead acetate resulted in significant increase in kidney lead content in lead only-treated group (L) compared to both normal (C) and γ-glutamyl cysteine (G) controls (Fig. 1a). Lead administration also resulted in diminished body weight gain and increased ratio of kidney/body weight compared to control groups (Fig. 1b–c). Treatment with γ-glutamyl cysteine dipeptide, however, significantly reduced kidney lead content and improved weight gain (Fig. 1).
Renal lead content and gravimetric analysis. a Lead content was determined in kidney tissues of different experimental groups: normal control (C), γGC control (G), lead only-treated group (L), and both lead and γGC-treated group (L + G). b Body weight of rats in different experimental group as measured just before sample collection. c Relative kidney to body weights. The average weights of both kidneys along with the body weight were used to calculate relative kidney/body weight according to the following formula: (average kidneys weight/body weight) × 100. Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
γ-Glutamyl Cysteine Alleviates Histopathological Alteration of Kidney Tissues
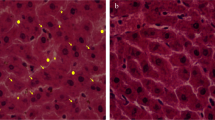
Kidney tissue samples from different experimental groups were subjected to H&E staining followed by histopathological examination to evaluate the probable modulating effect of γGC against lead-induced kidney histopathological aberrations. Representative photomicrograph of the lead only-treated group (L) showed degradation in the lining epithelium of the tubules with narrowing of the tubular lumen (Fig. 2(B)). Hemorrhage was also observed in the capsular and subcapsular area as well as between tubules (Fig. 2(B’)). γ-Glutamyl cysteine administration attenuated the observed histopathological alterations (Fig. 2(C)). Normal control (C) and γ-glutamyl cysteine control (G) groups showed normal histological architecture of kidney tissue (Fig. 2(A, D)).
Histopathological alterations in renal tissues and the alleviating effect of γ-glutamyl cysteine. A representative photomicrographs from different rat groups: normal control (C, panel A), γGC control (G, panel D), lead only-treated group (L, panels B and B’), and both lead and γGC-treated group (L + G, panel C). L group shows degeneration in lining tubular epithelial cells with narrowing of the tubular lumen (white and black arrows respectively in panel B) besides hemorrhage in capsular and subcapsular area (black arrows in panel B’) compared to both normal and γGC controls that show normal histological structure of glomeruli (G) and tubules (T). A representative photomicrograph from L + G group shows mitigated histopathological changes compared to L group (panel C). The representative photomicrographs of sections taken from kidney tissues were stained with hematoxylin and eosin, magnification ×40
γ-Glutamyl Cysteine Decreased Serum Levels of Creatinine and Urea
Serum levels of creatinine and urea are routinely used for monitoring renal glomerular function. In the current study, serum levels of creatinine and urea were determined to assess renal function in different rat groups. Our results showed that lead administration significantly increased serum levels of creatinine and urea in lead only-treated group (L). However, γ-glutamyl cysteine administration significantly reduced levels of both creatinine and urea (Fig. 3).
Serum creatinine and urea. Serum levels of creatinine (a) and urea (b) were determined at the end of the experiment in different experimental groups. Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
γ-Glutamyl Cysteine Modulates Oxidative Stress Markers in Kidney Tissues
To evaluate the possible alleviating effect of γ-glutamyl cysteine against lead-induced oxidative stress and oxidative tissue injury, markers of oxidative stress and oxidative tissue injury were evaluated. Levels of reduced glutathione GSH and total antioxidant capacity (TAC) (Fig. 4a–b) as well as activity of the antioxidant enzymes CAT, GPx, and SOD (Fig. 5) were significantly reduced in the lead only-treated group (L) compared to the control groups. Moreover, the lipid peroxidation marker malondialdehyde (MDA) and the protein oxidation marker protein carbonyl content (PCC) were significantly elevated in the lead only-treated group (L) when compared to the control groups (Fig. 4c–d). γGC restored levels of GSH and PCC back to the normal control levels (Fig. 4a, d). γGC treatment also significantly enhanced the activity of the antioxidant enzymes and significantly elevated TAC (Fig. 5). Moreover, γGC significantly reduced level of the lipid peroxidation marker MDA (Fig. 4c).
Oxidative stress markers in kidney tissues. Reduced glutathione GSH (a), total antioxidant capacity (TAC) (b), lipid peroxidation marker MDA (c), and protein oxidation marker PCC (d) were evaluated in kidney tissues of different rat groups. Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
Activity of antioxidant enzymes in kidney tissues. Activity of catalase (CAT) (a), glutathione peroxidase (GPx) (b), and superoxide dismutase (SOD) (c) were measured in kidney tissue homogenates of different rat groups. Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
γ-Glutamyl Cysteine Reduces Levels of Proinflammatory Cytokines in Kidney Tissues
In addition to the oxidative stress and oxidative tissue injury, the results of the current work showed that lead administration resulted in significant elevation of IL-1β, TNF-α, and IL-6 levels in kidney tissues of lead only-treated group L when compared to the control groups (Fig. 6). The results also revealed that γGC administration significantly reduced levels of all tested proinflammatory cytokines.
Levels of proinflammatory cytokines in kidney tissues. Levels of IL-1β (a), TNF-α (b), and IL-6 (c) were determined at the end of the experiment in kidney tissue homogenates of different experimental groups. Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
γ-Glutamyl Cysteine Reduces Caspase-3 Activity and Enhances PCNA Expression
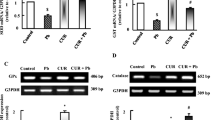
To evaluate the possible ameliorating effect of γGC against lead-induced apoptosis, activity of the apoptotic marker caspase-3 was determined in kidney tissues of different experimental groups. The results indicated that while lead administration elevated caspase-3 activity 3.5-fold, γGC administration restored caspase-3 activity back to the normal control level (Fig. 7a). To evaluate the regenerative capacity of kidney tissues after lead-induced tissue injury, proliferating cell nuclear antigen (PCNA) protein expression level was monitored. The results revealed that while lead administration slightly induced protein expression of PCNA, γGC administration robustly induced PCNA expression in the lead + γGC-treated (L + G) group (Fig. 7b).
Caspase-3 activity and PCNA expression in kidney tissues. a Activity of the apoptotic marker caspase-3. b Upper panel: Western blotting analysis of proliferating cell nuclear antigen PCNA protein level in kidney tissues of different rat groups. b Lower panel: quantitation of PCNA protein expression level (normalized to the loading control actin). Data are presented as mean ± standard deviation. Asterisks indicate significant difference from both C and G. Number signs indicate significant difference from L, p < 0.05 (n = 8)
Discussion
Among different body organs, kidney represents a major target of lead toxicity [4, 5, 43]. Lead accumulates in renal tissue and induces kidney hypertrophy that is manifested by increased kidney weight [5, 8]. Lead also reduces the ability of the exposed animal to gain weight, leading to increased kidney/body weight ratio [4, 5, 8]. Consistent with these data, our results revealed that lead administration resulted in increased lead contents of kidney tissues and reduced body weight gaining ability besides increased kidney/body weight ratio (Fig. 1). Previous studies have shown that lead-induced renal injury is evidenced by renal histopathological alteration and reduced renal excretory function [44, 45]. In concert with these data, the results of the current work demonstrated that animals in lead only-treated group (L) showed histopathological aberrations that include degradation and swelling in the lining epithelium of renal tubules with narrowing of the tubular lumen (Fig. 2(B)). Hemorrhage was also observed in the capsular and subcapsular area as well as between tubules in lead only-treated group (Fig. 2(B’)). In contrast, normal control (C) and γGC control (G) showed normal histological architecture of renal tissues (Fig. 2(A, D)). Serum levels of creatinine and urea were significantly elevated in lead only-treated group (L) when compared to normal control group (C), indicating deterioration of excretory renal functions as a result of lead exposure (Fig. 3). The results of the current study revealed that combined administration of lead and γGC (L + G) resulted in enhanced weight gaining ability and reduced kidney/body weight ratio compared to the lead only-treated group L (Fig. 1b–c). γGC also attenuated the observed histopathological alteration (Fig. 2(C)) and decreased levels of serum creatinine and urea (Fig. 3), indicating improved excretory renal functions.
Although it is a non-redox reactive metal, accumulating evidence has indicated that lead-induced renal tissue injury is basically mediated through induction of oxidative stress and subsequent oxidative tissue injury [4, 8, 18, 19, 46]. Lead-induced oxidative stress is mediated through generation of reactive oxygen species [8] and attenuation of both enzymatic and non-enzymatic antioxidant defense [8, 18, 46]. Lead inhibits antioxidant enzymes including SOD, CAT, and GPx through interaction with their thiol groups and replacement of the divalent co-factors that are required for their enzymatic activity [1, 3, 16, 17]. Lead also depletes reduced glutathione (GSH), the critical non-enzymatic antioxidant that works as a co-enzyme for glutathione peroxidase. Lead-induced inhibition of glutathione reductase and glutathione S-transferase inhibits GSH regeneration and significantly contributes to GSH depletion [3, 15, 18]. Consistent with these data, our results revealed that lead administration induced oxidative stress as manifested by significant reduction in GSH level and total antioxidant capacity in renal tissues of lead only-treated group (L) when compared to normal control group (Fig. 4a–b) besides significant reduction in antioxidant enzyme activity including CAT, SOD, and GPx (Fig. 5). The levels of lipid peroxidation marker MDA and protein oxidation marker PCC were also significantly elevated (Fig. 4b–c) in lead only-treated group compared to the normal control group. γGC administration restored GSH level and significantly attenuated lead-induced reduction in total antioxidant capacity (TAC) compared to the lead only-treated group (Fig. 4a–b). Because thiol group is a major target of lead and is considered as a major underlining cause of lead-induced oxidative stress [3], thiol group of cysteine residue of γGC may mediate the observed alleviating effect against lead-induced oxidative stress. γGC is the immediate precursor of the GSH [26] which may explain, at least in part, its ability to restore level of GSH to the normal control levels. In addition, glutamyl cysteine administration reduced levels of lipid peroxidation and protein oxidation compared to the lead only-treated group (Fig. 4c–d). Decreasing level of protein oxidation was more robust than that of lipid peroxidation which may be explained by the peptide nature of γGC that permit higher association with protein than with lipids. Moreover, γGC administration significantly increased activity of the antioxidant enzymes CAT, SOD, and GPx compared to the lead only-treated group (Fig. 5), highlighting the ameliorative effect of γGC against lead-induced oxidative stress. The ability of γGC to increase the antioxidant enzymes activity may be, at least in part, due to rescuing sulfhydryl groups of the antioxidant enzymes by that of γGC.
In addition to oxidative stress, induction of inflammatory responses has been also implicated in lead-induced renal tissue injury [8]. Lead exposure has been associated with increased levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1-β (IL-1β) [8, 47]. In addition, MAPKs and NF-κB have been implicated in lead-induced kidney injury [8, 48, 49]. In concert with these studies, the current work demonstrated that lead only-treated group, L, showed significant elevation of proinflammatory cytokines including IL-1β, TNF-α, and IL-6 when compared to the normal control group, C (Fig. 6). Administration of γGC, however, significantly reduced levels of these proinflammatory cytokines compared to lead only-treated group (Fig. 6), signifying the alleviating effect of γGC against lead-induced renal injury. Lead-induced ROS-mediated renal tissue injury has also been associated with caspase-dependent renal cellular apoptosis [4, 8, 11, 50]. During the course of apoptosis, mitochondrial cytochrome C is released to cytosol under the influence of pro-apoptotic signals. Cytochrome C activates initiator caspases caspase-8 and 9 and, eventually, the main caspase, caspase-3 [21, 33, 51]. Our results demonstrated that lead administration significantly increased caspase-3 activity in renal tissues of lead only-treated group when compared to the normal control group (Fig. 7a). γGC administration significantly attenuated activity of caspase-3 when compared to the lead only-treated group (Fig. 7a), highlighting the anti-apoptotic activity of γGC and reinforcing its alleviating effect against lead-induced renal injury.
PCNA is a homo-trimetric protein that is highly expressed in proliferating cells [20, 34]. It has many critical functions including DNA repair and cell proliferation, and it is considered as a tissue regeneration marker [52–54]. Previous studies have revealed that PCNA expression is enhanced after oxidative tissue damage, possibly as a compensatory mechanism to regenerate the damaged tissues [55, 56]. Consistent with this finding, our results showed that PCNA expression was enhanced in lead-only treated group (L) by 1.25-fold compared to the normal control group (Fig. 7b). Although the oxidative tissue damage markers (MDA and PCC) were significantly lower in L + G group than in L group (Fig. 4c–d), the expression of the regenerative marker PCNA is higher in L + G group than in L group (Fig. 7b), indicating the ability of γGC to enhance renal tissue regeneration through mechanisms other than the regular compensatory mechanisms. Unlike L + G group, γGC alone (G) was not able to enhance PCNA expression, denoting that the mechanism of γGC-induced PCNA expression is downstream to tissue damage and possibly synergizes with the regular compensatory mechanisms to enhance PCNA expression and consequently to enhance renal tissue regeneration. While the current study demonstrated the ability of γGC to enhance PCNA expression, further studies are required to investigate the detailed mechanisms by which γGC enhances the expression of PCNA. Previous studies have reported that metal chelators reduce tissue content of lead [57, 58]. The current study demonstrated that γGC administration decreased renal tissue lead content (Fig. 1a). γGC dipeptide contains glutamic acid residue that has been reported to exhibit metal chelation properties [59]. Together, these data are consistent with possible lead chelation competency of γGC. However, additional studies are warranted to investigate its potential lead chelation properties and to explore the mechanisms of reduction in kidney lead content.
In conclusion, the current work accentuate, for the first time, evidence for the alleviating effect of γGC dipeptide against lead-induced renal tissue injury which is potentially mediated through attenuation of oxidative stress, amelioration of the inflammatory response, reduction of renal cellular apoptosis, and enhanced tissue regeneration and possibly through lead chelation.
Abbreviations
- CAT:
-
Catalase
- DNPH:
-
2,4-Dinitrophenyl hydrazine
- DTT:
-
Dithiothreitol
- γGC:
-
γ-Glutamyl cysteine
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- H&E:
-
Hematoxylin and eosin
- IL-1β:
-
Interleukin-1-β
- IL-6:
-
Interleukin-6
- MDA:
-
Malondialdehyde
- PBS:
-
Phosphate-buffered saline
- PCC:
-
Protein carbonyl content
- PCNA:
-
Proliferating cell nuclear antigen
- PMSF:
-
Phenylmethanesulfonyl fluoride
- ROS:
-
Reactive oxygen species
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity
- TBARS:
-
Thiobarbituric acid reactive substance
- TCA:
-
Trichloroacetic acid
- TNF-α:
-
Tumor necrosis factor-α
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
References
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. doi:10.1016/j.tox.2011.03.001
Bhatti P, Stewart PA, Hutchinson A, Rothman N, Linet MS, Inskip PD, Rajaraman P (2009) Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol Biomark Prev 18(6):1841–1848. doi:10.1158/1055-9965.EPI-09-0197
Matovic V, Buha A, Ethukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. doi:10.1016/j.fct.2015.02.011
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol 258(3):330–342. doi:10.1016/j.taap.2011.11.015
Abdel Moneim AE, Dkhil MA, Al-Quraishy S (2011) The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 194:250–255. doi:10.1016/j.jhazmat.2011.07.097
Mudipalli A (2007) Lead hepatotoxicity & potential health effects. Indian J Med Res 126(6):518–527
Pande M, Flora SJ (2002) Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 177(2–3):187–196
Liu CM, Sun YZ, Sun JM, Ma JQ, Cheng C (2012) Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-kappaB pathway. Biochim Biophys Acta 1820(10):1693–1703. doi:10.1016/j.bbagen.2012.06.011
Jurczuk M, Brzoska MM, Moniuszko-Jakoniuk J (2007) Hepatic and renal concentrations of vitamins E and C in lead- and ethanol-exposed rats. An assessment of their involvement in the mechanisms of peroxidative damage. Food Chem Toxicol 45(8):1478–1486. doi:10.1016/j.fct.2007.02.007
Pulido MD, Parrish AR (2003) Metal-induced apoptosis: mechanisms. Mutat Res 533(1–2):227–241
Liu CM, Ma JQ, Sun YZ (2010) Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ Toxicol Pharmacol 30(3):264–271. doi:10.1016/j.etap.2010.07.002
Sivaprasad R, Nagaraj M, Varalakshmi P (2002) Lipoic acid in combination with a chelator ameliorates lead-induced peroxidative damages in rat kidney. Arch Toxicol 76(8):437–441. doi:10.1007/s00204-002-0350-x
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162(2):81–88
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 29(10):927–945
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47–58. doi:10.2478/v10102-012-0009-2
Kasperczyk A, Machnik G, Dobrakowski M, Sypniewski D, Birkner E, Kasperczyk S (2012) Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 301(1–3):79–84. doi:10.1016/j.tox.2012.07.002
Othman AI, El Missiry MA (1998) Role of selenium against lead toxicity in male rats. J Biochem Mol Toxicol 12(6):345–349
Ahamed M, Siddiqui MK (2007) Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta 383(1–2):57–64. doi:10.1016/j.cca.2007.04.024
Sharma S, Raghuvanshi BP, Shukla S (2014) Toxic effects of lead exposure in rats: involvement of oxidative stress, genotoxic effect, and the beneficial role of N-acetylcysteine supplemented with selenium. J Environ Pathol Toxicol Oncol 33(1):19–32
Salama SA, Arab HH, Omar HA, Maghrabi IA, Snapka RM (2014) Nicotine mediates hypochlorous acid-induced nuclear protein damage in mammalian cells. Inflammation 37(3):785–792. doi:10.1007/s10753-013-9797-6
Salama SA, Al-Harbi MS, Abdel-Bakky MS, Omar HA (2015) Glutamyl cysteine dipeptide suppresses ferritin expression and alleviates liver injury in iron-overload rat model. Biochimie 115:203–211. doi:10.1016/j.biochi.2015.06.006
Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RS (2008) Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cereb Blood Flow Metab 28(3):540–550. doi:10.1038/sj.jcbfm.9600551
Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA (2003) Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res 74(6):917–927. doi:10.1002/jnr.10810
Drake J, Kanski J, Varadarajan S, Tsoras M, Butterfield DA (2002) Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J Neurosci Res 68(6):776–784. doi:10.1002/jnr.10266
Lok J, Leung W, Zhao S, Pallast S, van Leyen K, Guo S, Wang X, Yalcin A, Lo EH (2011) Gamma-glutamylcysteine ethyl ester protects cerebral endothelial cells during injury and decreases blood-brain barrier permeability after experimental brain trauma. J Neurochem 118(2):248–255. doi:10.1111/j.1471-4159.2011.07294.x
Quintana-Cabrera R, Fernandez-Fernandez S, Bobo-Jimenez V, Escobar J, Sastre J, Almeida A, Bolanos JP (2012) Gamma-glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nature Communications 3:718. doi:10.1038/ncomms1722
Reddy YA, Chalamaiah M, Ramesh B, Balaji G, Indira P (2014) Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sci Technol 51(5):908–914. doi:10.1007/s13197-011-0568-9
Brown KE, Mathahs MM, Broadhurst KA, Weydert J (2006) Chronic iron overload stimulates hepatocyte proliferation and cyclin D1 expression in rodent liver. Transl Res 148(2):55–62. doi:10.1016/j.trsl.2006.03.002
Banchroft JD, Stevens A, Turner DR (eds) (1996) Theory and practice of histological techniques. Fourth edn. Churchil Livingstone New York, London, San Francisco, Tokyo
Jones DT, Hopkin SP (1998) Reduced survival and body size in the terrestrial isopod Porcellio scaber from a metal-polluted environment. Environ Pollut 99(2):215–223
Zhang SX, Wang JJ, Lu K, Mott R, Longeras R, Ma JX (2006) Therapeutic potential of angiostatin in diabetic nephropathy. J Am Soc Nephrol 17(2):475–486. doi:10.1681/ASN.2005020217
Ji H, Bachmanov AA (2007) Differences in postingestive metabolism of glutamate and glycine between C57BL/6ByJ and 129P3/J mice. Physiol Genomics 31(3):475–482. doi:10.1152/physiolgenomics.00013.2007
Arab HH, Salama SA, Eid AH, Omar HA, Arafa el SA, Maghrabi IA (2014) Camel’s milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem Toxicol 69:294–302. doi:10.1016/j.fct.2014.04.032
Salama SA, Snapka RM (2012) Amino acid chloramine damage to proliferating cell nuclear antigen in mammalian cells. Vivo 26(4):501–517
MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A 93(21):11853–11858
Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte Jr DW (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184(2):193–199
Ursini F, Maiorino M, Gregolin C (1985) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839(1):62–70
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Salama SA, Omar HA, Maghrabi IA, Alsaeed MS, El-Tarras AE (2014) Iron supplementation at high altitudes induces inflammation and oxidative injury to lung tissues in rats. Toxicol Appl Pharmacol 274(1):1–6. doi:10.1016/j.taap.2013.10.034
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Salama S, Omar H, Maghrabi I, AlSaeed M, EL-Tarras A (2014) Iron supplementation at high altitude induces inflammation and oxidative injury to lung tissues in rats (708.7). The FASEB Journal 28 (1 Supplement)
Hawkins CL, Morgan PE, Davies MJ (2009) Quantification of protein modification by oxidants. Free Radic Biol Med 46(8):965–988. doi:10.1016/j.freeradbiomed.2009.01.007
Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W (2010) Protective effect of smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 130(2):414–420. doi:10.1016/j.jep.2010.05.025
El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA (2009) Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol 47(9):2209–2215. doi:10.1016/j.fct.2009.06.019
Sujatha K, Srilatha C, Anjaneyulu Y, Amaravathi P (2011) Lead acetate induced nephrotoxicity in wistar albino rats, pathological, immunohistochemical and ultra structural studies. Int J Pharm Bio Sci 2(2):B459–B469
Gurer-Orhan H, Sabir HU, Ozgunes H (2004) Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 195(2–3):147–154
Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B (2007) Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293(2):F616–F623. doi:10.1152/ajprenal.00507.2006
Mishra KP, Singh VK, Rani R, Yadav VS, Chandran V, Srivastava SP, Seth PK (2003) Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology 188(2–3):251–259
Ramesh GT, Manna SK, Aggarwal BB, Jadhav AL (1999) Lead activates nuclear transcription factor-kappaB, activator protein-1, and amino-terminal c-Jun kinase in pheochromocytoma cells. Toxicol Appl Pharmacol 155(3):280–286. doi:10.1006/taap.1999.8624
Xu J, Ji LD, Xu LH (2006) Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3. Toxicol Lett 166(2):160–167. doi:10.1016/j.toxlet.2006.06.643
Arab HH, Salama SA, Omar HA, Arafa el SA, Maghrabi IA (2015) Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One 10(3):e0122417. doi:10.1371/journal.pone.0122417
Kunduzova OR, Bianchi P, Pizzinat N, Escourrou G, Seguelas MH, Parini A, Cambon C (2002) Regulation of JNK/ERK activation, cell apoptosis, and tissue regeneration by monoamine oxidases after renal ischemia-reperfusion. Faseb j 16(9):1129–1131. doi:10.1096/fj.01-1008fje
Theocharis SE, Skopelitou AS, Margeli AP, Pavlaki KJ, Kittas C (1994) Proliferating cell nuclear antigen (PCNA) expression in regenerating rat liver after partial hepatectomy. Dig Dis Sci 39(2):245–252
Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y (1999) Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol 30(5):911–915
El-Haleem MR, Kattaia AA, El-Baset SA, Mostafa HE (2015) Alleviative effect of myricetin on ochratoxin A-induced oxidative stress in rat renal cortex: histological and biochemical study. Histol Histopathol. doi:10.14670/HH-11-689
Siddiqi A, Hasan SK, Nafees S, Rashid S, Saidullah B, Sultana S (2015) Chemopreventive efficacy of hesperidin against chemically induced nephrotoxicity and renal carcinogenesis via amelioration of oxidative stress and modulation of multiple molecular pathways. Exp Mol Pathol 99(3):641–653. doi:10.1016/j.yexmp.2015.11.012
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–2788. doi:10.3390/ijerph7072745
Tandon SK, Singh S, Prasad S, Srivastava S, Siddiqui MK (2002) Reversal of lead-induced oxidative stress by chelating agent, antioxidant, or their combination in the rat. Environ Res 90(1):61–66
Sajadi S (2010) Metal ion-binding properties of L-glutamic acid and L-aspartic acid, a comparative investigation. Nat Sci 2(02):85
Acknowledgments
We greatly thank Prof. Adel Kholoussy (Professor of Pathology, Cairo University, Egypt) for his contribution in the histopathological examination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures related to animal care, treatments, and sampling were conducted according to the guidelines of Taif University research ethical committee.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Salama, S.A., Arab, H.H., Maghrabi, I.A. et al. Gamma-Glutamyl Cysteine Attenuates Tissue Damage and Enhances Tissue Regeneration in a rat Model of Lead-Induced Nephrotoxicity. Biol Trace Elem Res 173, 96–107 (2016). https://doi.org/10.1007/s12011-016-0624-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0624-4