Abstract
Insulin resistance is a very common associate of polycystic ovary syndrome (PCOS). Pathophysiology in relation with the essential elements including copper, magnesium, zinc, manganese, chromium, and calcium has been reported in women with insulin resistance. This prospective study was designed to explore whether the women with PCOS do exhibit altered serum element levels in association with/without insulin resistance. One hundred and thirty-two women with PCOS and forty-six control women were studied. Women with PCOS were further divided based on the presence of insulin resistance (insulin resistant: n = 50; non-insulin resistant: n = 82). In all women, basal levels of gonadotropins, prolactin, testosterone, insulin, glucose, and the six different elements were measured. Serum levels of testosterone (p < 0.001), luteinizing hormone (p < 0.05), and fasting insulin (p < 0.004) were significantly higher in the PCOS population compared to controls as well as PCOS women without insulin resistance. Women with PCOS exhibited a significantly high calcium (p < 0.04) and lower manganese levels (p < 0.002) when compared to controls. However, the PCOS women with insulin resistance exhibited significantly lower serum levels of magnesium and chromium (p < 0.04), in addition to higher levels of zinc and copper (p < 0.04). The differences in calcium (p < 0.03) and manganese levels (p < 0.0001) became aggravated with the presence of insulin resistance when compared to control as well as PCOS women without insulin resistance. In PCOS-associated insulin resistance, circulating serum magnesium (r = −0.31; p < 0.03) and chromium (r = −0.38; p < 0.006) status significantly correlated with fasting insulin levels. We conclude that imbalanced element status may be a key foundation for insulin resistance in PCOS. The findings in this study should be investigated with further trials in order to obtain new insights into PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anatomical and functional integrity of the reproductive system is influenced by genetic, physiological, and environmental factors. Nutrition represents one such environmental influence. It is generally acknowledged that not only under- and over-nutrition, but deficiency or excess of single nutrients may also compromise the integrity of the reproductive performance [1–3]. Polycystic ovary syndrome (PCOS), probably the most common endocrine disorder in women of reproductive age, is traditionally characterized by chronic anovulation, functional hyperandrogenism, and/or polycystic ovaries on ultrasound examination [4]. The syndrome has enigmatic pathophysiologic and molecular basis in which environmental factors act on a genetic background. Insulin resistance (IR) is an important ‘upstream’ driver for the reproductive and metabolic abnormalities in many women with PCOS predisposing the population to the development of glucose intolerance and ultimately type 2 diabetes mellitus (T2DM) [5].
Data on mineral levels in women with PCOS have not been adequately addressed. Decreased magnesium level was reported in women with increased testosterone levels [6] and/or IR [7]. Serum zinc levels were found to be lower in diabetic patients than in controls in one study [8], while another study showed no significant differences between them [9]. High-ionized [10] and/or increased coronary artery calcium [11] is often associated with the long-term complications of PCOS such as T2DM, IR, and/or cardiovascular problems. Indeed, Pasquali et al. demonstrated that calcium channel blockers regulate insulin secretion [12], which can reduce the degree of hyperinsulinemia, and ameliorate the state of IR in particularly men with obesity and hypertension [13].
The routinely provided therapies are usually targeted against the prevalent endocrine disorder [4]. Alternative treatment options aimed at improving glucose control include nutritional supplement of chromium [14]. Although a clear link between chromium and glucose metabolism is documented, evidence for its interaction in insulin-resistant state is ambiguous. However, the data from wildlife studies and laboratory studies with rodents, ungulates, and nonhuman primates revealed a significant role of endocrine-disrupting chemicals in the pathogenesis of several female reproductive disorders, including PCOS [15]. Excessive generation of reactive oxygen species (ROS) is a common feature in patients with PCOS [16, 17]. Manganese, zinc, and copper are essential micronutrients incorporated into many metalloenzymes and proteins responsible in cell metabolism and regulatory pathways of oxidative stress [18]. We thought that there might be a relationship between these trace elements and PCOS linked to the oxidative stress. In the current study, we aimed to investigate serum levels of some essential elements (copper, zinc, magnesium, manganese, calcium, and chromium) in patients with PCOS.
Materials and Methods
Subjects
This prospective study was conducted at Institute of Reproductive Medicine, Kolkata, India, on patients selected from the women attending the infertility clinic between June 2010 and July 2011. The investigation was performed with approval from the Research Ethics Board of the Institute. One hundred and eighty-eight women younger than 30 years of age having primary infertility for 5–10 years were initially enrolled demonstrating one/more following signs like oligomenorrhea, hirsutism, or acne. Cases with severe endometriosis (n = 13), history of previous pelvic surgery (n = 2), baseline (day 2) FSH > 12 mIU/ml (n = 3), or type 2 diabetes mellitus (n = 38) were excluded. The remaining women diagnosed with PCOS (n = 132) were included in the study. The diagnosis of PCOS was based on the presence of two out of the following three features: chronic anovulation/oligomenorrhea, biochemical hyperandrogenemia and polycystic ovary morphology (>12 follicles) on ultrasound. Other common causes of hyperandrogenism (prolactinoma, congenital adrenal hyperplasia, Cushing syndrome, and virilizing ovarian/adrenal tumors) were excluded in accordance with the criteria proposed in 2003 by the Rotterdam ESHRE/ASRM sponsored PCOS Consensus Workshop Group [19]. All were healthy, euthyroid, and had normal renal function as demonstrated by normal creatinine clearance values.
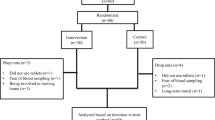
Women with PCOS were sub-stratified into two groups on the basis of insulin resistance. The first group comprised women who demonstrated IR with polycystic ovary morphology (n = 50) and the other without IR (n = 82; Fig. 1). Forty-six age- and weight-matched control women having their infertility attributed to male factors were selected as the control group. They had normal ovulatory cycles (mean ± SD) of 28 ± 2 days and normal ovarian morphology on ultrasound. Controls had no signs of hyperandrogenism and none of the women studied had galactorrhea or any systemic disease that could possibly influence their reproductive physiology. Furthermore, no women reported use of any medication that could possibly alter their hypothalamic–pituitary–gonadal axis. Necessary measures were taken to make the assessments free from the influence of factors known to have modulatory effects on the metabolism of the concerned trace elements and it was assured that no subjects were on a course of multivitamin supplementation for last 2 months. All subjects gave fully informed consent.
Enrolment and distribution of study cohorts. One hundred and eighty-eight women younger than 30 years of age having primary infertility for 5–10 years were initially enrolled representing one/more following signs like oligomenorrhea, hirsutism or acne. Cases with severe endometriosis (n = 13), history of previous pelvic surgery (n = 2) or baseline (day 2) FSH > 12 mIU/ml (n = 3) and diabetes mellitus (n = 38) were excluded. From the selected study population women with PCOS (n = 132) were diagnosed. Women with PCOS were sub-stratified into two groups on the basis of insulin resistance (IR). The first group comprised women who confirmed IR with polycystic ovary morphology (n = 50) and the other without IR (n = 82)
Biochemical Evaluations
Blood samples were collected between the second and the fourth day of the spontaneous or steroid challenged (gestagen; medroxyprogesterone acetate: Meprate; Serum Institute of India, Mumbai, India) menstrual cycles of the ovulating women and of women who presented only with hyperinsulinemia with a spontaneous bleeding of the anovulatory population, at 9 AM after an overnight fast. On the same day the transvaginal ultrasound examination was performed. Follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, prolactin, testosterone (total), and insulin were assayed in serum with automated chemiluminescence assay system using an Immulite® platform (Diagnostic Products Corporation, Los Angeles, CA, USA). The intra- and inter-assay coefficients of variation were <10 % for all assays performed. Glucose levels were measured using VITROS dry chemistry system® (Ortho-clinical diagnostics, France). All samples were assayed in duplicate.
Criteria for Diagnosis of Insulin Resistance
IR was determined by homeostasis model assessment (HOMA2-IR > 2.1) using the Oxford Diabetes Trials Unit calculator (http://www.dtu.ox.ac.uk; University of Oxford, UK). (HOMA2-IR = (fasting insulin × fasting glucose)/22.5). Patients with HOMA2-IR greater than 2.1were classified as IR. In order to identify “IR”, the cut-off value of HOMA2-IR was set at 2.1. In Indian population Homa2-IR value >1.93 is considered as insulin resistant [20] while in a Chinese population the cut-off for HOMA2-IR was set at 2 [21]. Hence, as a measure of abundant caution, the limit has been fixed at a slightly higher value.
Determination of Copper, Manganese, Zinc, Calcium, Magnesium, and Chromium
In order to determine elements levels, venous blood samples were collected on day 2; serum was separated by centrifugation for 5 min at 5,000 rpm at 4 °C and stored at −70 °C until analyzed. Serum levels of copper, manganese, zinc, calcium, magnesium, and chromium were measured by atomic absorption spectrophotometer (AA240 Varian version 5.0; Agilent Technologies. Inc, USA) at the division of atomic absorption spectrometry, Bose Institute (Kolkata, India). Flame atomization procedure was used for each of the elements measured at required specific wavelengths (calcium, 422.7 nm; magnesium, 285.2 nm; chromium, 357.9 nm; manganese, 279.5 nm; copper, 324.8 nm; and zinc, 213.9 nm). Necessary measures were taken to make the assessments free from the influence of factors having modulatory effects on the metabolism of the concerned trace elements during the 7 days prior to sample collection.
Statistical Analysis
The Kolmogorov–Smirnov test was used to test the normality of distribution. The data were expressed as mean ± SEM and ‘n’ refers to the number of determinations. Student’s t test was used to analyse differences between the experimental and control observations. Bivariate correlation analysis (calculation of the Pearson coefficient) was used to assess the correlation of mineral levels with other parameters. All analyses were performed by Graph-pad In stat software. p < 0.05 was considered to be statistically significant.
Results
The anthropometric and basal hormonal features of four groups are summarized in Table 1. Serum luteinizing hormone (p < 0.05) and testosterone (p < 0.001), levels were significantly higher in women with PCOS, compared to controls. An elevated LH/FSH ratio (>2:1) was found in 56.06 % of the subjects with PCOS (74/132). Fasting insulin (p < 0.004) levels and HOMA2-IR (p < 0.05) values were significantly higher in women with PCOS, compared to the control group. Serum element levels were comparable between the PCOS and control population, except serum calcium (p > 0.04) and manganese (p < 0.002) levels, which were significantly higher in the former (Fig. 2). Furthermore, mean serum calcium/magnesium ratio in the PCOS population (3.31 ± 0.35) was significantly higher (p < 0.02) than that of the control group (2.41 ± 0.14; Fig. 3). Around 55 % of the PCO patients had a calcium/magnesium up above the highest level observed in the control group. Most strikingly, 25 % of this study population having IR represents the population having higher calcium/magnesium. Fasting glucose levels were significantly higher (p < 0.001) in PCOS women with IR compared to controls.
Bar diagram representing the levels (ppm) of minerals investigated in PCOS women (n = 132), PCOS women with (n = 50)/without (n = 82) insulin resistance with comparison to controls (n = 46). a–f Depict copper, manganese, zinc, calcium, magnesium, and chromium levels, respectively. IR insulin resistance, NIR non-insulin resistance
Since a number of women with PCOS exhibited some form of hyperinsulinemia, they were further divided into women with or without IR based on the insulinemic response according to the HOMA2-IR values. Eighty-one PCOS subjects were classified as hyper-insulinemic (61 %), of which 50 patients turned out to be insulin resistant. The remaining 51 (39 %) constituted the normo-insulinemic population and 82 subjects were categorized as the non-IR cohort. It deserves to mention that all the control subjects were normo-insulinemic. Further analysis revealed that the majority of women with elevated insulin concentrations belong to the group with normal luteinizing hormone/follicle-stimulating hormone ratio. HOMA2-IR level (2.83 ± 0.54) was significantly higher (p < 0.02) among insulin-resistant PCOS population compared to controls (Table 1).
In order to determine possible connection between the studied metals in insulin-resistant women with PCOS, we found the significantly lower levels of serum magnesium (p < 0.04), manganese (p < 0.0001), and chromium (p < 0.04) whereas, higher values of serum calcium (p < 0.03), copper (p < 0.04), and zinc (p < 0.04) levels. No noteworthy variations were perceived in the non-IR PCOS subset (Fig. 2).
No significant correlation between mineral levels and testosterone, HOMA2-IR, and insulin values was observed. However, magnesium (r = −0.31; p < 0.03) and chromium (r = −0.38; p < 0.006) levels were significantly correlated with fasting insulin levels in insulin-resistant PCOS women.
Discussion
The mechanistic links between obesity, hyperinsulinemia, and anovulation have been investigated to a larger extent. Many publications concerned the physiological levels of elements in PCOS, however, a number of questions pertaining to the pathogenesis of the disease still remain unclear. In women with PCOS, a correlation in some elements concentrations has been reported [22–24] in most but not all [24]. In the present study, women with PCOS had lower manganese, magnesium, and chromium with increased calcium, copper, and zinc concentrations than controls. However, women with PCOS in association with IR had significantly lower (p < 0.04) serum magnesium levels than controls. Moreover, a significant negative correlation (r = −0.31; p < 0.03) reinforced the idea that PCOS-associated IR results in reduced magnesium concentrations. Besides this observation of ours contradicts the cross-sectional study by Sharifi et al. [25] and they reported that the magnesium deficiency is not associated with IR. Nevertheless, hypo-magnesia is associated with type 2 diabetes mellitus and other components of metabolic syndrome [26]. This may raise a question whether women with magnesium deficiency in PCOS are likely to proceed towards type 2 diabetes mellitus or metabolic syndrome.
It is well known that insulin secretion is a calcium-dependent process, while calcium levels in turn are interrelated to those of magnesium. Some recent studies showed that an elevation in calcium is required for both first- and second-phase insulin secretions [27, 28]. Magnesium deficiency can secondarily lead to changes in cellular calcium levels [29]. Furthermore, increased intracellular calcium has also been reported to induce diabetes [30] as well as IR [31]. Under these circumstances, magnesium deficiency as observed in PCOS-IR patients may lead to a reduction in insulin action by increasing free calcium levels. In the present study a significant rise (p < 0.04) in serum calcium levels in women with PCOS was observed compared to controls and PCOS without IR. Interestingly, mean serum levels of calcium/magnesium in the PCOS population were also significantly higher (p < 0.02) than that of the control group. An elevated level of the ratio of the bivalent elements might be a reflection of elevated extracellular calcium possibly in conjunction with the impairments of voltage-gated calcium channels. For instance, one study has showed that an increased serum calcium level led to an increased cystolic calcium level, which induced contraction of arterial smooth muscle [32] leading to a possibility of coronary atherosclerosis, a long-term sequel of the syndrome. Our study has clearly shown the fact that PCOS subjects had higher calcium levels which aggravated in alliance with IR (p < 0.03). Hence, we postulate that PCOS-associated IR results in decreased magnesium as well as higher calcium concentrations.
Cellular chromium potentiates insulin signaling by increasing insulin receptor kinase activity [33]. In this study population, a significant lower chromium level in IR-PCOS patients suggests possible impairment in the downstream mechanism of insulin. A strong negative correlation (r = −0.38; p < 0.006) among chromium in insulin-resistant PCOS patients further confirmed this observation and raised question regarding the rate of insulin sensitivity in the insulin-resistant subgroup.
The role of zinc in the prevention or clinical management of PCOS and/or diabetes is unclear [34]. However, few studies indicate a favorable outcome in diabetic patients following zinc supplementation while others did not achieve similar results [9]. In the current study, the level of zinc in the PCOS group was on a rising trend, although not statistically different from that of controls. Interestingly, PCOS in association with IR demonstrated a significant increase in the level of zinc when compared to controls (p < 0.04). Zinc has been reported to inhibit 5 α-reductase, which catalyzes the transformation of testosterone to its non-aromatizable form, di-hydro testosterone [35]. Thus, elevation in zinc levels may also help to reduce PCOS-associated hyperandrogenemia by limiting the transformation of testosterone to its active form DHT. However, zinc is also one of the co-factors of antioxidant enzymes such as catalase and superoxide dismutase (SOD). Since, role of antioxidants and oxidative stress in PCOS patients have been known for a long time, elevated zinc levels may have an association with the increased oxidative stress, often seen in PCOS patients [36, 37].
Copper is one of the essential trace elements and is a cofactor of many enzymes involved in redox reactions, such as cytochrome c oxidase or superoxide dismutase. In the present investigation, higher serum copper level was observed in PCOS patients than the controls which significantly increased with the association of insulin resistance (p < 0.04). In addition to its enzymatic roles, copper similarly can induce oxidative stress by catalyzing the formation of reactive oxygen species and decreasing glutathione levels [38]. Many studies conferred the role of increased oxidative stress resulting from high generation of ROS in the pathogenesis of PCOS [16, 17]. Although, SOD level was not measured in this study, but recently Zhang et al. [39] demonstrated that higher SOD levels was evident in PCOS patients. This might give a cue towards an association between copper and PCOS.
Manganese too is an essential element protecting against oxidative stress and is a cofactor for the metalloenzyme like Mn-containing SOD (MnSOD). It neutralizes the highly reactive superoxide ions to less reactive hydrogen peroxide (H2O2), which is followed by its immediate conversion to H2O by catalase and other peroxidases in the mitochondrial matrix [21]. In the present study, the mean serum manganese levels (ppm) of PCOS patients (0.230 ± 0.012) were significantly lower (p < 0.002) corresponding to the controls. Since oxidative stress is increased in PCOS, it is possible that serum manganese level is decreased as a result of consumption in the antioxidant defense system including MnSOD.
The selected subjects were infertile for 5–10 years which probably raises the question of a potential systematic bias which may be an error, or deviation from the truth, in results or inferences. However, the PCOS population chosen may be considered as a limitation to the present study. Because of the study design and large size, we used serum measurements of glucose as an indication of glucose metabolism rather than the glucose tolerance test (GTT), which is a more precise method of glucose tolerance. Furthermore, HOMA2-IR, a surrogate marker for IR was used as a criterion for IR determination without using standard GTT. This will remain as a limitation [40] in the present study. Further studies are warranted to explore the effect of these elements clinically because alteration in the mineral status has not been evaluated against different treatment regimens advised. In addition to this, the levels of SOD, catalase, and cytochrome-c oxidase may also need to be evaluated to know their possible relationships with PCOS.
In conclusion, a possible association is perceptible in our study between serum elements and women with PCOS. The association attains a connotative level with the involvement of IR in it. Additional studies evaluating the effects of these elements supplementation would be required to confirm the hypothesis as well as to ascertain whether insulin resistance in the presence of magnesium deficiency can directly lead to hypertension or a chromium-deficient state can switch on the insulin axis promoting the disease of hyperinsulinemia.
References
World Health Organization and Food and Agriculture organization of the United Nations (2004) Vitamin and mineral requirements in human nutrition, vol 17, 2nd ed. Sun Fung, Bangkok, pp 328–329
Özkaya MO, Naziroglu M (2010) Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil Steril 94:2465–2466
Özkaya MO, Naziroglu M, Barak C, Berkkanoglu M (2011) Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol Trace Elem Res 139:1–9
Ehrmann DA (2005) Polycystic ovary syndrome. N Engl J Med 352:1223–1236
Sattar N (2009) PCOS, insulin resistance and long-term risks for diabetes and vascular disease. Br J Diab & Vas Disease 9:15–18
Muneyvirci-Delale O, Nacharaju VL, Altura BM, Altura BT (1998) Sex steroid hormones modulate serum ionized magnesium and Ca levels throughout the menstrual cycle in women. Fertil Steril 69:958–962
Rumawas ME, McKeown NM, Rogers G, Meigs JB, Wilson PWF, Jacques PF (2006) Magnesium intake is related to improved insulin homeostasis in the Framingham offspring cohort. J Am Coll Nutr 25:486–492
Blostein-Fujii A, DiSilvestro RA, Frid D, Katz C, Malarkey W (1997) Short-term Zn supplementation in women with non-insulin dependent diabetes mellitus: effects on serum 5'-nucleotidase activities, insulin-like growth factor I concentrations, and lipoprotein oxidation rates in vitro. Am J Clin Nutr 66:639–642
Cunningham JJ, Fu A, Mearkle PL, Brown RG (1994) Hyperzinuria in individuals with insulin-dependent diabetes mellitus: concurrent zinc status and the effect of high-dose zinc supplementation. Metab 43:1558–1562
Shroff R, Kirschner A, Maifeld M, Van Beek JRE, Jagasia D, Dokras AD (2007) Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab 92:4609–4614
Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF II, Fitzpatrick LA (2003) Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2562–2268
Pasquali R, Cantobelli S, Vicennati V et al (1995) Nitrendipine treatment in women with polycystic ovarian syndrome: evidence for a lack of effects of Ca channel blockers on insulin, androgens, and sex hormone-binding globulin. J Clin Endocrinol Metab 80:3346–3350
Beer NA, Jakubowicz DJ, Beer RM, Nestler JE (1993) The Ca channel blocker amlodipine raises serum dehydroepiandrosterone sulfate and androstenedione, but lowers serum cortisol, in insulin resistant obese and hypertensive men. J Clin Endocrinol Metab 76:1464–1469
Wang ZQ, Cefalu WT (2010) Current concepts about Cr supplementation in type 2 diabetes and insulin resistance. Curr Diab Rep 10:145–151
Diamanti-Kandarakis E, Bourguignon J-P, Guidice LC et al (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30:293–342
González F, Rote NS, Minium J, Kirwan JP (2006) Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 91:336–340
Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JMC, Sattar N (2001) Low grade chronic inflammation in women with polycystic ovary syndrome. J Clin Endocrinol Metab 86:2453–2455
Zidenberg-Cherr S, Keen CL (1991) Essential trace elements in antioxidant processes. In: Dreosti IE (ed) Trace elements, micronutrients and free radicals. Humana Press, New Jersey, pp 107–127
Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and log-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47
Deepa R, Shanthirani CS, Premalatha G, Sastry NG, Mohan V (2002) Prevalence of insulin resistance syndrome in a selected south Indian population—the Chennai urban population study-7 [CUPS-7]. Indian J Med Res 115:118–127
Chu CJ, Lee SD, Hung TH et al (2009) Insulin resistance is a major determinant of sustained virologic response in genotype 1 chronic hepatitis C patients receiving preginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther 29:46–54
Panidis D, Koliakos G, Kourtis A, Farmakiotis D, Mouslech T, Rousso D (2004) Serum resistin levels in women with PCOS. Fertil Steril 81:361–366
Schwarz K, Mertz W (1959) Cr (III) and the glucose tolerance factor. Arch Biochem Biophys 85:292–295
Kurdoglu Z, Kurdoglu M, Demir H, Sahin HG (2012) Serum trace elements and heavy metals in polycystic ovary syndrome. Hum Exp Toxicol 31:452–456
Sharifi F, Mazloomi S, Hajihosseini R, Mazloomzadeh S (2012) Serum magnesium concentrations in polycystic ovary syndrome and its association with insulin resistance. Gynecol Endocrinol 28:7–11
Kauffman RP, Tullar PE, Nipp RD, Castracane VD (2011) Serum magnesium concentrations and metabolic variables in polycystic ovary syndrome. Acta Obstet Gynecol Scand 90:452–458
Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P (2003) Hierarchy of the β-cell signals controlling insulin secretion. Eur J Clin Invest 33:742–750
Jing X, Li DQ, Olofsson CS et al (2004) CaV2.3 Ca channels control second-phase insulin release. J Clin Invest 115:146–154
Zhang A, Cheng TP, Altura BM (1992) Magnesium regulates intracellular free ionized Ca concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta 1134:25–29
Naziroglu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca2+ signalling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37:2065–2075
Jang YJ, Ryu HJ, Choi YO et al (2003) Effects of an intracellular Ca2+ chelator on insulin resistance and hypertension in high-fat-fed rats and spontaneously hypertensive rats. Metab 53:269–272
Aoki K, Miyagawa K (1990) Correlation of increased serum Ca with elevated pressure and vascular resistance during Ca infusion in normotensive man. J Hypertens 8:579–583
Wang H, Kruszewski A, Brautigan DL (2005) Cellular Cr enhances activation of insulin receptor kinase. Biochem 44:8167–8175
Walter RM Jr, Uriu-Hare JY, Olin KL et al (1991) Copper, Zn, manganese, and magnesium status and complications of diabetes mellitus. Diab Care 14:1050–1056
Stamatiadis D, Bulteau-Portois MC, Mowszowicz I (1998) Inhibition of 5 alpha-reductase activity in human skin by Zn and azelaic acid. Br J Dermatol 119:627–632
Naziriglu M (2012) Molecular role of catalase on oxidative stress-induced (Ca+2) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Naziriglu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Speisky H, Go’mez M, Burgos-Bravo F et al (2009) Generation of superoxide radicals by copper-glutathione complexes: redox consequences associated with their interaction with reduced glutathione. Bioorg Med Chem 17:1803–1810
Zhang D, Luo WY, Liao H, Wang CF, Sun Y (2008) The effects of oxidative stress to PCOS. Sichuan Da Xue Xue Bao Yi Xue Ban 39:421–423
Metwally M, Saravelos SH, Ledger WL, Li TC (2010) Body mass index and risk of miscarriage in women with recurrent miscarriage. Fertil Steril 94:290–295
Acknowledgment
The authors sincerely acknowledge the scientific and technical help and advice of Dr. Ratna Chattopadhyay, Sr. Embryologist IVF Unit, Institute of Reproductive Medicine, Kolkata.
Author information
Authors and Affiliations
Corresponding author
Additional information
Precis: Polycystic ovary syndrome in conjunction with insulin resistance demonstrates a significant alterations in mineral status
Rights and permissions
About this article
Cite this article
Chakraborty, P., Ghosh, S., Goswami, S. et al. Altered Trace Mineral Milieu Might Play An Aetiological Role in the Pathogenesis of Polycystic Ovary Syndrome. Biol Trace Elem Res 152, 9–15 (2013). https://doi.org/10.1007/s12011-012-9592-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9592-5