Abstract
This study aimed to determine the relationship between the metabolic and endocrinological pathologies in polycystic ovary syndrome (PCOS) and the levels of arsenic, chromium, cadmium, lead, mercury, antimony, zinc, and copper to evaluate the relationship of these toxic metals with inflammatory/oxidative parameters. This study included a total of 154 patients (84 with PCOS, 70 healthy volunteers). Metabolic and endocrine parameters and arsenic, chromium, cadmium, lead, mercury, antimony, zinc, and copper serum levels of the patients were compared between the groups. Considering the action mechanism of toxic metals, serum malondialdehyde (MDA), superoxide dismutase (SOD), serum total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), tumor necrosis factor-alpha (TNFα), and high-sensitivity C-reactive protein (HsCRP) levels were determined. Serum TAS (p = 0.002), OSI (p = 0.006), SOD (p = 0.006), zinc (p = 0.010), and copper (p = 0.030) values were statistically lower whereas TOS (p = 0.008), MDA (p < 0.001), HsCRP (p < 0.001), TNFα (p < 0.001), antimony (p < 0.001), cadmium (p < 0.001), lead (p < 0.001), and mercury (p < 0.001) levels were significantly higher in the PCOS group than those determined in the control group. Antimony was positively correlated with fasting glucose (FG) and HOMA-IR while cadmium, in addition to FG and HOMA-IR, positively correlated with insulin and lead had a positive correlation only with FG (p < 0.05). Also, these three heavy metals correlated positively with some oxidative system and inflammatory parameters and negatively with the antioxidant system parameters (p < 0.05). In conclusion, heavy metal exposures in PCOS may be related to insulin resistance and hirsutism through oxidative and inflammatory mechanisms. This approach can be used to identify the risky patient group and to develop new treatment modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PCOS is a common and important endocrinological disease of which its metabolic effects can be progressive. International prevalence rate ranges from 5 to 21% [1, 2]. In a study conducted in Turkey, the prevalence has been reported as 19.9% [3]. It is characterized by insulin resistance (IR), irregular and anovulatory menstrual cycles, and clinical and/or biochemical hyperandrogenism. Insulin resistance is of importance regarding the development and persistence of this disease [4]. The cause of hyperinsulinemia, hyperandrogenemia, obesity, and the changing hormonal course in PCOS is still unknown. This metabolic course is associated with chronic low-dose inflammation and oxidative stress [5]. However, the factors triggering these damage mechanisms remain unknown. So, can these damage mechanisms in patients be triggered by toxic metal exposure?
The metabolic effects of metals were first introduced in the 1970s, and it was suggested that cadmium inhibited the secretion activity of pancreatic beta cells [6]. Over the years, numerous heavy metals have been shown to increase body mass index (BMI) and waist circumference and produce endocrine-disrupting effects [7]. Examining the literature, it was seen that these studies were carried out especially in patients having type 2 diabetes mellitus (type II DM). Also, some heavy metals were held responsible for the development of gestational diabetes (GDM) [8, 9]. Recent studies indicate that these metabolic effects are associated with oxidative damage mechanisms. Chen et al. have reported that heavy metals catalyzed oxidative stress reactions, leading to the formation of free oxygen radicals (ROS) and that ROS decreased insulin gene promoter activity by pancreatic β cells with insulin mRNA expression [10].
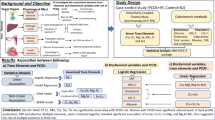
Today, PCOS is regarded as a complex, multifactorial disorder resulting from genetic, environmental, and life style interactions [11]. Toxic metal exposure can be inevitable in every period of life. The majority of heavy metals are toxic even at low concentrations and are capable of entering the food chain and inflict damage to living organisms in which they accumulate [12]. Also, the degree of toxicity in the individual depends on factors such as the route of exposure, the number of exposures, the duration of exposure, the type of metal ion, age, health status, nutrition, and the individual’s genetic characteristics [13]. The studies on these exposures suggest that mercury accumulation in the adrenal gland can lead to enzymatic defects that cause hyperandrogenemia [14], while arsenic, chromium, and zinc may impair insulin and glucose metabolism [10] and the effects of the accumulation of trace elements such as copper, zinc, iron, and oxidative are associated with stress and cardiovascular disease cytokine production, inflammation, and the immune system [15]. All these metabolic changes are similar to the metabolic dysfunction seen in PCOS. However, the number of studies evaluating these effects in PCOS is limited. The present study aimed to evaluate the relationship between endocrine and metabolic parameters in PCOS, considering the action mechanism of some heavy metals (arsenic, antimony, cadmium, lead, mercury, crom) and trace elements (zinc, copper).
Materials and Method
Study Population
This study was carried out prospectively between December 2019 - March 2020 at Bozok University Faculty of Medicine Obstetrics and Gynecology Department. The study was approved by the Yozgat Bozok University Clinical Research Ethics Committee (2017-KAEK-189_2019.12.11_15), and an informed consent was obtained from all participants.
According to the Rotterdam criteria, patients were diagnosed with PCOS in cases where at least two of the following criteria exist: oligo/amenorrhea, clinical or biochemical hyperandrogenism, and PCO in ultrasonography [16]. Clinical hyperandrogenism was defined as the presence of hirsutism (Ferriman-Galwey score [FGS ≥ 8]) and/or acne and/or alopecia. Menstrual cycle duration less than 24 days and longer than 35 days were regarded as abnormal. Cycles lasting more than 35 days were defined as oligomenorrhea while menstrual absence within 6 months was defined as amenorrhea. Anovulation was defined as the serum progesterone level < 3 ng/mL on days 21–24 of the menstrual cycle. From the patients who applied to gynecology outpatient clinics, those who met the criteria of Rotterdam diagnosis criteria constituted the study group. Patients who admitted for routine control, without oligo-amenorrhea and who did not meet the PCOS diagnostic criteria, were included in the control group. Clinical and ultrasonographic evaluations of the patients were carried out by two researchers appointed from the research team (DAK-EB). Information on age, menstrual cycle patterns, gravidity, parity status, personal and family medical history, and drug use status of all the patients were provided from the subjects. The height, weight, and waist and hip circumference measurements of the participants were carried out by the same researcher. Waist circumference was measured at the narrowest point between the costal margin and the iliac crest during normal breathing while the hip circumference was measured at the widest point on the hips. The waist-hip ratio (WHR) was calculated.
The patients with the following cases were excluded from the study, pregnancy; hypertension; smoking; drugs (such as fertility drugs, oral anti-diabetic agents, and oral contraceptive pills); and endocrinal disorders such as diabetes, hypo/hyperthyroidism, hyperprolactinemia, Cushing syndrome, androgen-secreting neoplasms, and other adrenal disorders. Also, patients with psychiatric disorders and those using antioxidants (vitamin C, etc.) and food supplements were excluded from the study.
Metabolic Analysis
Blood samples for metabolic analysis were taken between 8 and 9 AM following 12-h fasting. Fasting glucose (FG), fasting insulin, and lipid in serum measurements were carried out. Glucose and lipid profile assay, the levels of FG, total cholesterol (TC), HDL cholesterol (HDL-C), and triglyceride (TG) were analyzed using e assay kits (Abbott®, IL, USA) with an autoanalyzer (Aeroset®, Abbott®, IL, USA). LDL cholesterol (LDL-C) was calculated by the Friedewald formula. Insulin resistance was determined using the homeostatic model assessment insulin resistance index (HOMA-IR). HOMA-IR formula: fasting plasma glucose (mg/dL) × fasting serum insulin (mU/mL)/405 [17].
Hormonal Assay
Blood samples were collected on the 2nd or 3rd days of the menstrual cycle. Serum insulin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol (E2) were measured by the electrochemiluminescence immunoassay (ECLIA) using commercial kits (Abbott Laboratories) suitable for the autoanalyzer (Architect i2000; Abbott Laboratories, Abbott Park, IL, USA).
Analysis of Tumor Necrosis Factor-α and High-Sensitivity C-Reactive Protein
Elisa Kits were utilized to determine TNFα serum levels (Bioassay Technology, People’s Republic of China) and HsCRP (DRG International, Inc., USA). Measurements of ELISA kits were carried out using an ELISA microplate reader (Spectrostar Nano, BMG, Labtech, Allmendgrün, Ortenberg, Germany).
Evaluation of Inflammatory and Oxidant Parameters
Serum MDA level was determined according to Gocmen et al. [18]. Total superoxide dismutase (SOD) activity was examined utilizing the SOD Activity Assay kit (Rel Assay Diagnostics kit; Mega Tıp, Gaziantep, Turkey), according to the manufacturer's instructions. The TAS and TOS values were measured using commercial kits (Rel Assay Diagnostics kit; Mega Tıp, Gaziantep, Turkey) and calculated according to the method by Erel and OSI. The TOS:TAS ratio was used as the OSI and was calculated according to the following formula: OSI (arbitrary units) = [(TOS, μmol H2O2/L)/(TAS, mmol Trolox equiv./L)].
Toxicological Analysis
Blood samples were transferred to Yozgat Bozok University Science and Technology Application and Research Center (BİLTEM) under cold chain conditions. The arsenic (As), chromium (Cr), cadmium (Cd), antimony (Sb), mercury (Hg), lead (Pb), copper (Cu), and zinc (Zn) analyses were performed in the toxicology laboratory. All blood samples (by taking 1 mL blood) were treated with acid (5 mL nitric acid and 5 mL water) in the microwave incinerator (Milestone, Start D, USA) before analysis. Analyzes were performed on inductively coupled plasma mass spectrometry (ICPMS, ICAPQc Thermo Scientific, USA). An 11-point (0.1–500 ppb) calibration curve was formed for all trace elements and heavy metals. Method validations were performed with the Whole Blood Label-2 (Seronorm) certified standard material. A minimum r2 value of 0.9955 was calculated for all calibrations.
Statistical Analysis
The statistical package software SPSS 20 (IBM Corp. released 2011. IBM SPSS Statistics for Windows, version 20.0, Armonk, NY: IBM Corp.) was used to evaluate the data. Data was expressed as mean ± SD and in percentages. Continuous variables were investigated using analytical methods (Kolmogrov-Simirnov/Shapiro-Wilk’s test) to determine whether or not they are normally distributed. The Man-Whitney U test was utilized for the non-parametric numerical data while the Student t test was adopted for the parametric numerical data. Relationships between categorical variables were analyzed by the chi-square test. Bivariate correlations were investigated by the Spearman’s correlation analysis. p < 0.05 were accepted as statistically significant.
Results
A total of 154 participants who met the criteria of the study were included in the study. The control group consisted of 70 healthy (45.5%) patients while the study group consisted of 84 PCOS (54.5%) patients. Group properties are shown in Table 1.
Group Comparisons
There were statistically significant differences in BMI, WHR, gravidity, parity, FGS FG, FSH, E2, insulin, and HOMA-IR values between the groups. BMI, WHR, FGS, FG, insulin, HOMA-IR, and E2 values in the PCOS group were significantly higher than the control group (p < 0.05). Gravidity, parity, and FSH values were significantly lower in the PCOS group (p < 0.05). Among the groups, other demographic properties and metabolic values were similar (p > 0.05).
Markers
The values of oxidative, antioxidative, and inflammatory system markers between the groups are given in Table 2. The mean TAS, OSI, and SOD values showing the capacity of the antioxidant system were significantly lower in the PCOS group (p = 0.002, p = 0.006, p = 0.006, respectively). The median values of TOS and MDA showing oxidative system capacity were significantly higher in the PCOS group (p = 0.008, p < 0.001, respectively). Inflammatory markers (HsCRP and TNFα) were significantly higher in the PCOS group (p > 0.05).
Relationship of Heavy Metals with PCOS
In the present study, the relationship of heavy metals and trace elements with PCOS is given in Table 2. Plasma Sb, Cd, Pb, and Hg levels were found to be significantly higher in the PCOS group compared with those in the control (p < 0.05), and there were no significant relationships between the groups in terms of Cr and As (p > 0.05). Examining the relationship of these heavy metals with HOMA-IR, it was found that only Sb and Cd were positively correlated (r = 0.250 and p = 0.002, r = 0.363 and p < 0.001, respectively) and there was no significant relationship between As, Cr, Hg, and Pb (Table 3). Examining the levels of trace elements, Zn and Cu were found to be statistically significantly lower in the PCOS group compared with those in the control group (Table 2). There was a significant positive correlation between Cu and TAS (r = 0.230 and p = 0.004), whereas there was a significant negative correlation between Zn and MDA and TNFα (r = − 0.178 and p = 0.028, r = − 0.276 and p = 0.001, respectively). There were no significant relationships between Zn and Cu and other oxidation and inflammatory parameters (p > 0.05). Also, there was a negative correlation between serum Zn levels and FG (r = − 0.235 and p = 0.003) (Table 3). It was determined that Sb was positively correlated with MDA and TNFα (r = 0.199 and p = 0.013, r = 0.189 and p = 0.019, respectively) whereas it was negatively correlated with TAS, OSI, and SOD (r = − 0.255 and p = 0.001, r = − 0.284 and p < 0.001, r = − 0.284 and p < 0.001, respectively). It was determined that Cd was positively correlated with MDA and HsCRP (r = 0.262 and p = 0.001, r = 0.207 and p = 0.010, respectively) and negatively correlated with TAS (r = − 0.231 and p = 0.004). Also, there was a positive correlation between Cd and plasma FG and insulin levels (r = 0.293 and p < 0.001, r = 0.307 and p < 0.001, respectively). Sb had a positive correlation with FG (r = 0.58 and p < 0.001). A statistically significant positive correlation was found between Pb and FG (r = 0.189 and p = 0.019). Pb was positively correlated with MDA, TOS, and HsCRP (r = 0.299 and p < 0.001, r = 0.204 and p = 0.011, r = 0.292 and p < 0.001, respectively) whereas it was negatively correlated with TAS, OSI, and SOD (r = − 0.220 and p = 0.006, r = − 0.186 and p = 0.021, r = − 0.186 and p = 0.021, respectively) (Fig. 1). In the Spearman correlation analysis between heavy metals and hormone (FSH, LH, and E2) and lipid (LDL-C, HDL-C, TC and TG) levels, no significant correlations were found (p > 0.05). WHR was positively correlated with Cd, Hg, Pb, and Sb (r = 0.219 and p = 0.006, r = 0.249 and p = 0.002, r = 0.242 and p = 0.002, r = 0.222 and p = 0.006, respectively) while BMI was positively correlated only with Cd (r = 0.250 and p = 0.002) (Table 4). Also, Cd, Pb, TNFα, and HsCRP levels were significantly higher in patients with hirsutism compared those without (p < 0.05) (Fig. 2).
Discussion
In the present study, it was seen that inflammatory and oxidative damage in PCOS patients was significantly higher than that in the control group. In these patients, plasma Cd, Sb, Hg, and Pb levels were found to be significantly higher whereas Zn and Cu were significantly lower than those in the control group. It was found that Sb was positively correlated with FG and HOMA-IR while Cd, in addition to FG and HOMA-IR, was positively correlated with insulin, whereas Pb had a positive correlation only with FG (p < 0.05). Also, these three heavy metals were determined to be correlated positively with some oxidative system and inflammatory parameters whereas negatively with the antioxidant system parameters.
The endocrinological effects of metals are based on oxidative stress. Toxic metals cause the production of ROS and reactive nitrogen species. These radicals cause damage to membrane lipids, proteins, and DNA and activate apoptosis and tissue degradation [12]. Researches indicate the existence of similar processes in PCOS. However, it is still not clear whether this process is a cause or an effect. However, there has been an increase in studies in recent years reporting that IR and hyperandrogenemia occur due to oxidative damage in PCOS [19,20,21].
In 2005, Gonzales et al. have stated that, regardless of obesity, high blood glucose increased the release of TNFα and ROS from mononuclear cells in these patients. Also, in these patients, again, regardless of obesity, an increase in inflammatory parameters such as HsCRP was determined. However, there have been no studies examining the relationship between metabolic and endocrine problems in PCOS with heavy metals, oxidative, and inflammatory systems. In the present study, two basic parameters were used to show possible damage mechanisms. The first was the parameters associated with inflammation (TNFα and HsCRP), and the second were those associated with an oxidative pathway (MDA, SOD, TAS, TOS, OSI).
IR is an important “upstream” driver for the reproductive and metabolic abnormalities in numerous cases with PCOS predisposing the population to the development of glucose intolerance and ultimately type 2 DM [22]. In experimental studies, IR and diabetogenic effects of heavy metals were attributed to the damage they cause in pancreatic β cells [23]. Also, it has been stated that environmental estrogens or xenoestrogens may also play a role in type 2 DM etiology [24]. Antimony is a metal estrogen showing both mechanisms of action [23, 25]. This metal was thought to ultimately cause oxidative damage and reduce insulin transcription and secretion [ 23]. There are no studies evaluating Sb and related metabolic problems in PCOS patients. In the present study, Sb showed a positive correlation with HOMA-IR and FG. Also, the relationship of Sb with TAS, TOS, HsCRP, and TNFα suggests that it forms IR through oxidative and inflammatory pathways. Menke et al. have reported that there was a significant relationship between Sb and HOMA-IR in both diabetic and non-diabetic patients [26]. Zhang et al., in their study assessing 2093 pregnant women with GDM, have reported that there was a significant relationship between urinary Sb and GDM [27].
It was seen that studies on Sb were often focused on the evaluation of urinary levels. Although the urinary level of this heavy metal, which has a half-life of more than 30 days, does not reflect the present situation, the significant statistical data found in these studies may prove that antimony is indeed an effective endocrine disruptor. In the present study, serum levels of Sb and all other heavy metals were evaluated. Therefore, it was thought that these values, especially Sb, reflected the present levels more accurately.
Cd is not physiologically or biochemically required but its exposure level is high [10]. It cannot directly produce free radicals but indirectly contributes to the formation of ROS [28]. Cadmium-induced cellular toxicity was studied in various targets including metalloenzymes interference, thiol protein alterations, energy metabolism inhibition, DNA and membrane structure/function alterations, and excessive oxidative damage [10]. Numerous researches have reported that cadmium-induced hyperglycemia was related to increased lipid peroxidation, decreased insulin release, and increased activation of gluconeogenic enzymes and impaired insulin receptors [29,30,31]. Studies also report that there is a relationship between urinary Cd levels and type 2 DM [32, 33], as well as studies suggesting the opposite [34]. In a study evaluating Cd levels, no significant differences were found between the cases with PCOS and healthy volunteers [35]. However, in the study, it was seen that there were few patients and the parameters related to insulin resistance were not evaluated. A similar result was reported by Zheng et al [27]. The researchers did not evaluate the oxidative and inflammatory system markers. In the current study, in the PCOS group, Cd was correlated with FG and HOMA-IR. It was determined that this was also related to oxidative and inflammatory system parameters. Therefore, it can be suggested that Cd causes damage through these pathways and this damage is related to metabolic problems in PCOS, particularly IR.
There are studies in the literature reporting that Pb was associated with high blood glucose and impaired glucose tolerance [36]. In the present study, Pb was not associated with HOMA-IR; however, it was found to have a positive correlation with FG. Experimental studies have associated this effect of Pb with delta-aminolevulinic acid synthase and catalase activation by increasing MDA and oxidized glutathione and ROS levels, causing the reduction of plasma reduced glutathione levels [37]. In the present study, Pb was found to be correlated with the oxidative system and inflammatory system parameters.
Evaluating the studies on the Zn and Cu levels in PCOS patients, it was seen that the results showed variations. In the present study, these two trace element levels were found to be low in PCOS patients. As reported in a previous study, this was associated with increased chronic inflammation in PCOS and its increased use due to increased oxidative stress [38]. Cu and Zn are trace elements that contribute to many biological reactions. SOD is also a co-factor of catalase and cytochrome c oxidase enzymes. Zn also plays an active role in insulin release and modulation of the inflammatory system and reduces TNFα and IL-1 release by inhibiting the pro-inflammatory response. In the present study, a negative correlation was found between Zn and MDA and TNFα, and a positive correlation between Cu and TAS. Also, similar to our study, clinical and epidemiological studies showed that low Zn levels were associated with FG [39]. Zn maintains this effect by increasing antioxidant capacity, affecting the insulin synthesis and secretion [40]. An improvement was reported in insulin resistance in patients with PCOS after zinc supplementation [41, 42]. It has been reported that hirsutism also improved in PCOS patients with Zn replacement [43, 44]. These results and the results of the present study suggest that low Zn levels in PCOS patients may be associated with insulin resistance and hyperandrogenemia.
The idea that heavy metals may affect reproductive hormones was introduced in the 2000s. The studies showed that every 1-μg/L increase in Cd levels may be associated with a 21% increase in early follicular phase E2 levels, and, in women aged 35–60 years, serum FSH and LH concentrations increase as the blood Pb levels increase [45, 46]. In the present study, no relationship was found between hormone levels and heavy metals. This can be associated with the fact that the patients in the study were young. There are studies in the literature supporting this result. In a study in which premenopausal 252 women were evaluated, no relationship was found between heavy metals and ovulatory functions [47]. In another study, it was shown that chronic Cd exposure in women aged 42–60 may be associated with increased FSH levels [48]. If we could make a long-term follow-up of patients included in the present study, we would have possibly seen changes in hormone levels.
In the present study, hyperandrogenemia was evaluated clinically. In patients with hirsutism, TNFα and HsCRP and Cd and Pb were found to be significantly high. TNFα secretion may enhance serine phosphorylation to increase the activity of the 17,20-lyase arm of CYP17 [48]. TNFα also stimulates the proliferation of ovarian theca cells in PCOS [49, 50]. Also, insulin resistance is known to contribute to hyperandrogenemia [20]. All these data suggest that Cd and Pb, which have a high effect on glucose metabolism, may also contribute to hirsutism. Also, the effects of toxic metals appear as a separate research subject for the patient group who were followed up with idiopathic hirsutism diagnosis.
It has been stated that weight gain or weight loss, depending on the toxic metal, is usually seen at much lower levels of exposure of metals compared with that making animals or humans ill. The contribution of heavy metals to obesity was associated with the replacement of essential micronutrients with essential metals or their ability to induce oxidative stress [51]. In the present study, the BMI values of the PCOS group were significantly higher; however, not all of these patients were obese (26.2%). Evaluating the heavy metal relationships, there was a significant positive correlation between WHR and Cd, Hg, Pb, and Sb, while there was a relationship of BMI only with Cd. In a study based on the data of the National Health and Nutrition Examination Survey (NHANES) in which the relationship of the levels of some toxic metals with waist circumference and BMI were examined, it was found that molibden, antimony, and tungsten had no significant relationships with waist circumference and BMI. While barium and thallium were positively associated with BMI and waist circumference, cadmium, cobalt, cesium, and lead were found to have a negative correlation [51]. The positive correlation of some toxic metals (barium and thallium) with obesity can be explained with the fact that these toxic metals induce oxidative stress, increasing lipogenesis in a way that inhibits energy production [52]. However, even if it is known that metal-induced oxidative stress causes obesity as the major factor, the fact that how some metals, such as barium and thallium, positively associated with obesity, whereas other metals, such as lead, cadmium, and cesium, negatively associated with obesity, remains unknown [51]. Differently organized prospective studies are required to evaluate the relationship between heavy metals and obesity.
In the present study, the relationship between serum lipids and heavy metals and other parameters were not significant. The fact that all of the patients in the present study were not obese (17.5%) and were young may have affected the results. It is known that there is a relationship between inflammatory processes and dyslipidemia in PCOS. Studies are stating that lipid-induced inflammation may be a powerful stimulator of dyslipidemia in PCOS especially in the presence of obesity, with the combined effects of inflammation and derangements in lipids leading to the early onset of atherogenesis [20].
The most important limitation of the present study was that long-term monitoring of heavy metal exposures could not be carried out. Some metabolic problems, for which no relationships were determined, may show improvement after a certain exposure period, as indicated in the literature. Although serum levels provided an accurate assessment, determining the levels in other body components such as urine and hair could allow us to conduct a stronger analysis. However, since it was thought that the patients in the study had environmental exposures to metals possibly through inhalation and diet, their short-term biomarker levels may represent a steady state of exposure.
As a result, environmental heavy metal exposures, especially Sb, Cd, and Pb, may trigger metabolic problems associated with PCOS, which progress with oxidative damage and low-dose inflammation. The present study presented results regarding the multifactorial risk factors related to PCOS and may contribute to identifying cases with high-risk PCOS. In this manner, the study will shed light on the formation of better management and preventive strategies.
Change history
20 November 2020
The original version of this article unfortunately contained a mistake in the Study Population section under Materials and Method.
References
Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, Norman RJ, Costello MF, Groups GD (2011) Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust 195:S65–S112
Roe AH, Dokras A (2011) The diagnosis of polycystic ovary syndrome in adolescents. Rev Obstet Gynecol 4(2):45–51
Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H (2012) Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 27(10):3067–3073
Amato M, Vesco R, Vigneri E, Ciresi A, Giordano C (2015) Hyperinsulinism and polycystic ovary syndrome (PCOS): role of insulin clearance. J Endocrinol Investig 38(12):1319–1326
Yeon Lee J, Baw C-K, Gupta S, Aziz N, Agarwal A (2010) Role of oxidative stress in polycystic ovary syndrome. Curr Womens Health Rev 6(2):96–107
Ghafghazi T, Mennear JH (1975) The inhibitory effect of cadmium on the secretory activity of the isolated perfused rat pancreas. Toxicol Appl Pharmacol 31(1):134–142
Padilla MA, Elobeid M, Ruden DM, Allison DB (2010) An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int J Environ Res Public Health 7(9):3332–3347
Liu W, Zhang B, Huang Z, Pan X, Chen X, Hu C, Liu H, Jiang Y, Sun X, Peng Y (2018) Cadmium body burden and gestational diabetes mellitus: a prospective study. Environ Health Perspect 126(2):027006
Farzan SF, Gossai A, Chen Y, Chasan-Taber L, Baker E, Karagas M (2016) Maternal arsenic exposure and gestational diabetes and glucose intolerance in the new hampshire birth cohort study. Environ Health 15(1):106
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH (2009) Heavy metals, islet function and diabetes development. Islets 1(3):169–176
Insenser M, Montes-Nieto R, Murri M, Escobar-Morreale HF (2013) Proteomic and metabolomic approaches to the study of polycystic ovary syndrome. Mol Cell Endocrinol 370(1-2):65–77
Sanchez T (2018) Effects of mercury, lead, arsenic and zinc to human renal oxidative stress and functions: a review. Arch Med 4(1):2
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Runnebaum B, Rabe T (1997) Gynecological endocrinology and reproductive medicine, vol 1. Springer Verlag, Berlin Heidelberg
Ganz T, Nemeth E (2015) Iron homeostasis in host defence and inflammation. Nat Rev Immunol 15(8):500–510
Eshre R (2004) Asrm-sponsored pcos consensus workshop group revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Legro RS, Castracane VD, Kauffman RP (2004) Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59(2):141–154
Yesim Göçmen A, Gümüşü S, Semiz E (2004) Association between paraoxonase-1 activity and lipid peroxidation indicator levels in people living in the antalya region with angiographically documented coronary artery disease. Clin Cardiol 27(7):426–430
Coskun A, Arikan T, Kilinc M, Arikan DC, Ekerbicer HC (2013) Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 168(2):183–186
González F, Considine RV, Abdelhadi OA, Acton AJ (2020) Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in PCOS. J Clin Endocrinol Metab 105. https://doi.org/10.1210/clinem/dgaa108
Jeelani H, Ganie MA, Masood A, Amin S, Kawa IA, Fatima Q, Manzoor S, Parvez T, Naikoo NA, Rashid F (2019) Assessment of pon1 activity and circulating tf levels in relation to BMI, testosterone, HOMA-IR, HDL-C, LDL-C, CHO, SOD activity and tac in women with PCOS: an observational study. Diabetes Metab Syndr 13(5):2907–2915
Sattar N (2009) PCOS, insulin resistance and long-term risks for diabetes and vascular disease. Br J Diabetes Vasc Dis 9(1):15–18
Hectors T, Vanparys C, Van Der Ven K, Martens G, Jorens P, Van Gaal L, Covaci A, De Coen W, Blust R (2011) Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 54(6):1273–1290
Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A (2008) Pancreatic insulin content regulation by the estrogen receptor erα. PLoS One 3(4):e2069
Sundar S, Chakravarty J (2010) Antimony toxicity. Int J Environ Res Public Health 7(12):4267–4277
Menke A, Guallar E, Cowie CC (2016) Metals in urine and diabetes in us adults. Diabetes 65(1):164–171
Zheng G, Wang L, Guo Z, Sun L, Wang L, Wang C, Zuo Z, Qiu H (2015) Association of serum heavy metals and trace element concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Biol Trace Elem Res 167(1):1–10
Galán A, García-Bermejo L, Troyano A, Vilaboa NE, Fernández C, de Blas E, Aller P (2001) The role of intracellular oxidation in death induction (apoptosis and necrosis) in human promonocytic cells treated with stress inducers (cadmium, heat, x-rays). Eur J Cell Biol 80(4):312–320
Lei L, Jin T, Zhou Y (2006) The effects of cadmium on the levels of insulin in smelters. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 24(1):3–6
Świergosz-Kowalewska R (2001) Cadmium distribution and toxicity in tissues of small rodents. Microsc Res Tech 55(3):208–222
Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, Kim EK, Lachaal M, Jung CY, Lee W (2003) Cadmium induces impaired glucose tolerance in rat by down-regulating glut4 expression in adipocytes. Arch Biochem Biophys 413(2):213–220
Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S (2010) Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: a population study. Environ Res 110(6):612–616
Moon SS (2013) Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea national health and nutrition examination survey (KNHANES) 2009–2010. Diabet Med 30(4):e143–e148
Schwartz GG, Il’yasova D, Ivanova A (2003) Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 26(2):468–470
Kurdoglu Z, Kurdoglu M, Demir H, Sahin H (2012) Serum trace elements and heavy metals in polycystic ovary syndrome. Hum Exp Toxicol 31(5):452–456
Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, Guo H, He M, Zhang X, Yuan J (2015) Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in china. PLoS One 10(4):e0123742. https://doi.org/10.1371/journal.pone.0123742
Tandon S, Singh S, Prasad S, Srivastava S, Siddiqui M (2002) Reversal of lead-induced oxidative stress by chelating agent, antioxidant, or their combination in the rat. Environ Res 90(1):61–66
Nazem MR, Hedayati M, Asadi M, Emami A (2016) Mutual interaction between obesity and zinc deficiency. J Obesity 2(2):028
Valko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Guler I, Himmetoglu O, Turp A, Erdem A, Erdem M, Onan MA, Taskiran C, Taslipinar MY, Guner H (2014) Zinc and homocysteine levels in polycystic ovarian syndrome patients with insulin resistance. Biol Trace Elem Res 158(3):297–304
Taylor CG (2005) Zinc, the pancreas, and diabetes: Insights from rodent studies and future directions. Biometals 18(4):305–312
Foroozanfard F, Jamilian M, Jafari Z, Khassaf A, Hosseini A, Khorammian H, Asemi Z (2015) Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes 123(04):215–220
Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z (2016) Effects of zinc supplementation on endocrine outcomes in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 170(2):271–278
Spritzer PM, Lecke SB, Fabris VC, Ziegelmann PK, Amaral L (2017) Blood trace element concentrations in polycystic ovary syndrome: systematic review and meta-analysis. Biol Trace Elem Res 175(2):254–262
Jackson L, Howards P, Wactawski-Wende J, Schisterman E (2011) The association between cadmium, lead and mercury blood levels and reproductive hormones among healthy, premenopausal women. Hum Reprod 26(10):2887–2895
Krieg EF Jr (2007) The relationships between blood lead levels and serum follicle stimulating hormone and luteinizing hormone in the third national health and nutrition examination survey. Environ Res 104(3):374–382
Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, Wactawski-Wende J (2011) Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect 119(8):1156–1161
Gallagher CM, Moonga BS, Kovach JS (2010) Cadmium, follicle-stimulating hormone, and effects on bone in women age 42–60 years, NHANES iii. Environ Res 110(1):105–111
Spaczynski RZ, Arici A, Duleba AJ (1999) Tumor necrosis factor-α stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod 61(4):993–998
Zhang L-H, Rodriguez H, Ohno S, Miller WL (1995) Serine phosphorylation of human p450c17 increases 17, 20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci 92(23):10619–10623
Hatch EE, Nelson JW, Stahlhut RW, Webster TF (2010) Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int J Androl 33(2):324–332
Mailloux R, Lemire J, Appanna V (2007) Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: a link to obesity. Cell Physiol Biochem 20(5):627–638
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Yozgat Bozok University Clinical Research Ethics Committee (2017-KAEK-189_2019.12.11_15), and an informed consent was obtained from all participants.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article unfortunately contained a mistake in the Study Population section under Materials and Method. In the first sentence, January 2019 and February 2020 should be December 2019 - March 2020.
Rights and permissions
About this article
Cite this article
Kirmizi, D.A., Baser, E., Turksoy, V.A. et al. Are Heavy Metal Exposure and Trace Element Levels Related to Metabolic and Endocrine Problems in Polycystic Ovary Syndrome?. Biol Trace Elem Res 198, 77–86 (2020). https://doi.org/10.1007/s12011-020-02220-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02220-w