Abstract
Staphylococcus aureus causes a range of chronic infections in humans by exploiting its biofilm machinery and drug-tolerance property. Although several strategies have been proposed to eradicate biofilm-linked issues, here, we have explored whether piperine, a bioactive plant alkaloid, can disintegrate an already existing Staphylococcal biofilm. Towards this direction, the cells of S. aureus were allowed to develop biofilm first followed by treatment with the test concentrations (8 and 16 µg/mL) of piperine. In this connection, several assays such as total protein recovery assay, crystal violet assay, extracellular polymeric substances (EPS) measurement assay, fluorescein diacetate hydrolysis assay, and fluorescence microscopic image analysis confirmed the biofilm-disintegrating property of piperine against S. aureus. Piperine reduced the cellular auto-aggregation by decreasing the cell surface hydrophobicity. On further investigation, we observed that piperine could down regulate the dltA gene expression that might reduce the cell surface hydrophobicity of S. aureus. It was also observed that the piperine-induced accumulation of reactive oxygen species (ROS) could enhance biofilm disintegration by decreasing the cell surface hydrophobicity of the test organism. Together, all the observations suggested that piperine could be used as a potential molecule for the effective management of the pre-existing biofilm of S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biofilm-forming ability of Staphylococcus aureus, an opportunistic human pathogen, has been widely known to cause a plethora of pathogenic infections such as surgical site infections, catheter implant-related infections, central line-associated bloodstream infections, ventilator associated pneumonia, and endocarditis [1]. Biofilm mode of growth essentially provides a chronic reservoir of infections with an elevated resistivity to immune defenses and conventional antibiotics up to 10 to 1000 times than its planktonic counterpart [1]. Bacterial biofilm is defined as an aggregation of bacterial cells which are embedded within a complex hydrated matrix made up of self-produced extracellular polymeric substances (EPS) that are usually attached to a surface. In this connection, EPS acts as a protective shield for the matrix encased cells by intercepting the entry of harmful substances such as antibiotics into the bacterial community [2]. However, efforts have been put forward to explore compounds having efficient antibiofilm therapeutics against S. aureus [3]. In this regard, literature stated some effective strategies to control biofilm-linked challenges such as inhibition of microbial biofilm formation and disintegration of developed biofilm [4, 5]. Thus, we sought to explore natural molecules having either biofilm inhibiting activity or disintegrating activity or both. Towards this direction, piperine (a bioactive compound of pepper) has been selected for the present study due to its diverse biological activities (anti-parasitic, anticancer, antimicrobial, and other pharmaceutical effects) [6]. Besides, in our previous study, it was showed that piperine could efficiently inhibit microbial biofilm formation of S. aureus [7]. However, the disintegrating property of the same has not been tested on the pre-existing biofilm developed by S. aureus. Therefore, in the current study, we aimed to examine the disintegrating property of piperine against our test organism S. aureus. In this connection, a series of experiments have been conducted and the results of the same showed that piperine could disintegrate the developed biofilm of S. aureus efficiently.
Materials and Methods
Microbial Strain, Growth Media, and Culture Conditions
Staphylococcus aureus (MTCC 96) has been used as organism of interest for the present study. Luria–Bertani (LB) broth media (HiMedia, India) was used for cultivating the test organism. The organism was grown in sterile Luria–Bertani broth media at 37 °C for 24 h. In this study, piperine (purchased from Merck; SKU: P49007-5G) was dissolved into dimethyl sulfoxide (DMSO) for the preparation of the stock solution (10 mg/mL). The prepared stock solution was diluted further in the growth media as per requirements.
Measurement of Biofilm Development by S. aureus
Crystal Violet (CV) Assay
To determine the time kinetics of S. aureus biofilm formation, cells (1 × 105 CFU/mL) were inoculated in sterile LB media (5 mL). After that, sterile coverslips were also added to it. All the tubes were then incubated at 37 °C for various time periods (6 h, 12 h, 18 h, and 24 h). Post incubation, the degree of microbial biofilm development was measured by conducting CV assay [8]. In brief, planktonic cells were discarded from the growth media followed by washing the tubes with sterile double distilled water. After that, 0.4% of CV solution (5 mL) was added to each tube and incubated at room temperature for 30 min to stain the tube adhered biofilm mass (if any). After the incubation, the excess stain was washed and tubes were air-dried. Thereafter, 33% of glacial acetic acid (5 mL) was added to the respective tubes to dissolve the biofilm-bound CV, and the OD600 was measured to estimate the intensity of the dissolved CV in each tube.
Microscopic Image Analysis
The degree of biofilm formed by S. aureus with the progression of time was tested by microscopic image analysis [5]. To perform this experiment, the coverslips were recovered from the respective tubes at different time intervals (6 h, 12 h, 18 h, and 24 h) and stained with 0.4% CV solution for 30 min. Thereafter, these coverslips were washed, air-dried, and seen under a light microscope (OLYMPUS CX21i).
EPS Measurement Assay
The extent of EPS production has often been measured to understand the extent of microbial biofilm development over a surface [9]. To perform this experiment, cells (1 × 105 CFU/mL) were allowed to grow in sterile LB for various periods (6 h, 12 h, 18 h, and 24 h) of time as described above. Post incubation, planktonic cells were discarded from the respective tube and washed gently, followed by the addition of 5 mL of phosphate buffer saline (PBS) (pH 7.4). Then, each tube was vortexed adequately and suspension of biofilm was centrifuged for 20 min at 6000 rpm at 4 °C. Post centrifugation, the supernatant was separately collected. The cell pellet was suspended again in 10 mM EDTA, vortexed, and re-centrifuged for 10 min at 6000 rpm to extract the cell-bound EPS. The supernatant was collected and mixed with the earlier supernatant. Then, 2.2 volumes of chilled absolute ethanol were mixed with total pooled supernatant and incubated for 1 h at − 20 °C. Post incubation, it was centrifuged at 8000 rpm for 10 min to collect the pellet. The EPS pellet was dissolved in sterile PBS and was quantified by following the protocol of phenol–sulfuric acid method [10].
Estimation of Biofilm Disintegration
CV Assay
The biofilm-disintegrating property of piperine against S. aureus was determined by allowing the test organism to develop biofilm first and then the developed biofilm was either treated with piperine or left untreated (control set) [5]. Bacterial cells (1 × 105 CFU/mL) were inoculated in autoclaved LB broth for 24 h at 37 °C to develop biofilm over the glass surface. Post incubation, the developed biofilm was either treated with the selected piperine concentrations or left untreated for various time periods (2 h, 4 h, 6 h, and 8 h). Finally, the CV assay was conducted to determine the degree of biofilm-disintegrating property of piperine against S. aureus [5].
Total Biofilm Protein Measurement
Total recovered biofilm protein represents an indirect quantification of microbial association over the glass tube as the robust biofilm often exhibits a higher protein recovery and vice versa [11]. To quantify the degree of residual biofilm under the exposure of piperine, 24 h developed biofilm cells were either exposed to piperine of left unexposed for another 6 h at 37 °C. Post incubation, the planktonic cells were removed and biofilm cells were taken into consideration. Then, 5 mL of NaOH (0.3 N) was added to each tube followed by boiling at 100 °C for 10 min. Then, supernatant was collected after centrifugation and the protein content of the supernatant was assessed by following the Lowry method [12].
Estimation of EPS Matrix
To understand the effect of piperine on the extent of biofilm disintegration, cells were allowed to develop biofilm on the glass surface by following the methods described above. Thereafter, the developed biofilm was either treated with piperine or left untreated at 37 °C for 6 h. After the required incubation, non-biofilm cells were discarded and tubes were washed and dried adequately. Afterwards, the degree of biofilm remained on the tube was estimated by EPS measurement assay [10].
Measurement of Metabolic Activity
The metabolic activity of the developed biofilm of S. aureus under the exposure of piperine was evaluated by fluorescein diacetate (FDA) hydrolysis assay [13]. To do the test, the existing biofilm on the glass surface was either treated with piperine or kept untreated (control) for 6 h at 37 °C. Thereafter, the non-biofilm cells were discarded followed by gentle washing of the tubes with sterile distilled water. Then, phosphate buffer (5 mL) (pH 7.4) was added to each tube and vortexed to resuspend the tube adhered biofilm cells into the buffer. Afterwards, 500 µL of FDA (5 mg/mL) dissolved in dimethyl sulfoxide was transferred to each tube and kept aside in dark for 1 h at room temperature. The tubes were then centrifuged for 10 min at 10,000 rpm. Finally, the amount of fluorescein present in the supernatant was estimated by measuring the absorbance of fluorescein at 490 nm.
Fluorescence Microscopic Image Analysis
The biofilm-disintegrating property of piperine against S. aureus was analyzed by following the protocol of fluorescence microscopy [11]. Briefly, cells (1 × 105 CFU/mL) were allowed to develop biofilm on coverslips by following the methods described above. Then, the developed biofilm on the coverslips was stained with acridine orange (4 μg/mL) for 15 min. Post staining, the coverslips were rinsed with autoclaved distilled water, air-dried, and finally viewed under a fluorescence microscope (FITC filter) at an excitation and emission wavelengths of 491 nm and 516 nm, respectively.
Scanning Electron Microscopic Image Analysis
The disintegrating property of piperine against pre-existing biofilm of S. aureus was tested by performing scanning electron microscopic image analysis. Briefly, cells (1 × 105 CFU/mL) were allowed to develop biofilm over the coverslips for 24 h at 37 °C following either being treated with piperine or left untreated for another 6 h under similar conditions. Post treatment, the coverslips were washed with sterile phosphate buffer saline (pH 7.4) solution, and the biofilm over the coverslips (if any) was fixed with 2.5% glutaraldehyde for 1 h. Then, the coverslips were dried in a vacuum, coated with gold, and finally viewed under SEM.
Measurement of Non-biofilm Cells
Biofilm disintegration leads to an increase in the count of the non-biofilm cells. Hence, to understand the effect of piperine on the count of non-biofilm cells of S. aureus, the developed biofilm was treated with piperine (8 and 16 µg/mL) for 6 h. One similar set was also kept as a control wherein the existing biofilm was not exposed to piperine. After the desired period of incubation, the broth culture was collected from the experimental sets and the degree of non-biofilm cells present in it was measured by counting the colony-forming units (CFU) [5].
Effect of DNase I on Biofilm Disintegration
DNase I often cleaves the eDNA of biofilm matrix that results in the disintegration of the pre-existing biofilm structure efficiently [5]. In this connection, to understand the effect of DNase I on the developed biofilm of the test organism, the biofilm was treated with either 10 µL (1 mg/mL) of DNase I or left untreated for 6 h at 37 °C. Post incubation, the degree of biofilm cells remained on the surface was determined by CV assay as described previously.
Measurement of Extracellular DNA (eDNA)
To understand the effect of piperine on the eDNA of the developed biofilm of S. aureus, eDNA was extracted and measured from the biofilm that was either treated or untreated with piperine [5, 14]. To do the test, similar number (1 × 105 CFU/mL) of cells was inoculated into glass petri plates having 20 mL of sterile LB and incubated at 37 °C for 24 h. After the incubation, the developed biofilm was either treated (8 and 16 μg/mL) or untreated with piperine for 6 h at 37 °C. Post incubation, the extents of the biofilm cells remained on the Petri dish under different conditions were scrapped and centrifuged at 8000 rpm for 8 min. Post centrifugation, supernatant was collected as it carries the EPS part. To extract the cell-bound EPS, cell pellets were collected, suspended in 500 μL of EDTA (10 mM), and centrifuged again at 8000 rpm for 8 min. Then, the supernatant was collected and pooled with the previous supernatant. The pooled fraction was mixed with 2.2 volumes of chilled absolute ethanol and incubated further for 1 h at 8 °C. Then, the suspension was centrifuged at 8000 rpm for 8 min. After that, the pellet was collected and suspended in 500 μL of Tris–EDTA buffer and 150 μL of ice-cold isopropanol. Afterwards, the suspension was incubated at 4 °C for 3 h followed by centrifuging the same at 12,000 rpm for 15 min. Then, the pellet was collected, mixed with Tris–EDTA buffer (500 μL), treated with 10 μL of Proteinase K (10 mg/mL), and incubated for another 1 h at 37 °C to degrade the contaminated protein (if any). After the incubation, 150 μL ice-cold isopropanol was added into it. Then, the suspension was centrifuged at 8000 rpm for 8 min. Finally, the pellet was collected and suspended again in 50 μL of Tris-buffer. The concentrations of the extracted eDNA were subsequently analyzed through spectrophotometric analysis and agarose gel electrophoresis.
Analysis of the Effect of Piperine on Cell Auto-aggregation
Microbial auto-aggregation plays a vital role in the assembly as well as colonization to a surface [15]. Thus, efforts were given to understand the influence of piperine on the auto-aggregation profile of the already existing biofilm cells of S. aureus. To understand the same, an equal number of cells (1 × 105 CFU/mL) were grown in sterile LB media for 24 h at 37 °C. Post incubation, biofilm cells was either treated with the selected concentrations (8 and 16 µg/mL) of piperine or left untreated (as control). All the tubes were incubated at 37 °C for 6 h. After the incubation, an equal number of biofilm cells under different conditions were suspended in phosphate buffer saline (PBS) (pH 7.4) and incubated at 37 °C for 20 h. After the incubation, the upper portion of the suspension was collected and OD600 was measured with the perception that higher OD would indicate an enhanced auto-aggregation of bacterial cells and vice versa [15].
Measurement of Cell Surface Hydrophobicity
Cell surface hydrophobicity of the biofilm cells was measured under the presence and absence of piperine by following the bacterial adherence to hydrocarbon (BATH) assay [16]. To perform this experiment, the developed biofilm cells were either treated with the selected doses (8 and 16 µg/mL) of piperine or left untreated (control) for another 6 h at 37 °C. Post treatment, the tubes were washed with sterile double distilled water. Then, 5 mL of phosphate buffer saline (pH 7.4) was added to each tube and vortexed vigorously such that the glass adhered biofilm cells (if any) got suspended into the PBS. Thereafter, the PBS suspended cells were centrifuged at 6000 rpm for 10 min followed by suspending the cell pellet in 3.4 mL of sterile double distilled water. The OD of each suspension was measured at 420 nm which would be considered initial OD. Thereafter, 0.6 mL chloroform was mixed with the collected suspension and vortexed adequately. Then, all the tubes were kept standing for 30 min at room temperature for the separation of aqueous and organic phase. Finally, the upper phase (aqueous) was collected from each tube and the OD of the same was measured again that would be considered the final OD.
The cell surface hydrophobicity of the test organism was calculated by adhering to the following formula:
Real‐Time PCR Analysis
The dltA gene expression of the biofilm cells of S. aureus was measured under the presence and absence of piperine by real-time PCR. To do the test, the pre-existing biofilm cells were either exposed to varying concentrations (8 and 16 μg/mL) of piperine or left untreated for 6 h at 37 °C. Post incubation, a similar number of biofilm cells were collected from each set and Trizol reagent (Invitrogen) was used to extract the mRNA of the test organism. Spectrophotometric analysis was conducted to determine the purity and yield of the extracted RNA. After that, the first-strand cDNA was synthesized from the mRNA using reverse transcriptase M-MLV (Takara). One milligram of cDNA was collected for further real-time amplification by SYBR Premix kit (Applied Biosystems). A negative control reaction mixture was also taken into consideration where cDNA was absent. The dltA gene expression level was calculated by comparative threshold cycle method (2−ΔΔCt) with 16S rRNA as the control gene. Primer sequences of the mentioned genes are mentioned in Table 1.
Measurement of Intracellular ROS Accumulation
To measure the intracellular ROS generation under the presence of piperine, the DCFDA (2′,7′-dichlorofluorescein diacetate)-dependent ROS measurement assay was performed [5]. Microbial esterase converts DCFDA (a fluorogenic probe) into a non-fluorescent counterpart (2′,7′-dichlorofluorescein [DCF]) [5]. The intracellular ROS (if any) can oxidize this non-fluorogenic DCF to a fluorogenic DCF which can be further estimated by a fluorescence spectrophotometer. To determine the effect of piperine on the cellular accumulation of ROS in the biofilm cells, the biofilm cells of S. aureus were either treated with piperine or kept untreated for 6 h under similar conditions. Post incubation, both piperine-treated and piperine-untreated biofilm cells (1 × 108 CFU/mL) were further incubated with DCFDA at 37 °C for 30 min. To validate our observations, a similar set of experiment was also conducted where biofilm cells were exposed to an antioxidant (ascorbic acid) along with the piperine. A control experiment was also performed in which the biofilm cells were neither exposed to piperine nor ascorbic acid. Finally, the amount of fluorogenic DCF was measured by a fluorescence spectrophotometer at the excitation and emission wavelength of 488 nm and 535 nm, respectively. [17].
Analysis of the Effect of Piperine on Cell Autolysis
To characterize the effect of piperine on the autolysis (induced) of the pre-existing biofilm cells of S. aureus, an equal number of cells (1 × 105 CFU/mL) were inoculated into sterile tubes containing sterile LB broth (supplemented with 1 M NaCl) for 24 h at 37 °C. Post incubation, planktonic cells were discarded and the adhered biofilm cells were taken into consideration. In this regard, 5 mL of LB media (supplemented with 1 M NaCl) was added in each tube and further treated with either selected concentrations (8 and 16 µg/mL) of piperine or left untreated (control). Then, all the tubes were incubated for 6 h at 37 °C. After 6 h of treatment, cells (if any) were centrifuged at 6000 rpm for 10 min. The cell pellets were collected and an equal number of cells were suspended in autolysis buffer (0.1% Triton X-100 and 50 mM Tris–HCl). The induced autolysis was estimated by measuring OD at 580 nm in every 1-h interval over a period of 3 h [15].
Cell Membrane Permeability Assay (EtBr Influx Assay)
Ethidium bromide (EtBr) influx method was followed to measure the cell membrane permeability under different treatment conditions [18]. In this connection, the developed biofilm cells were challenged with either piperine or left untreated for 6 h at 37 °C. Post incubation, biofilm cells were collected by scrapping and subsequently centrifuged at 8000 rpm for 8 min. Thereafter, an equal number of biofilm cells were dissolved in 1 mL of sterile phosphate buffer. Then, EtBr was added into it to attain a final concentration of 0.5 µg/mL. After an incubation of 30 min, the amount of fluorescence developed under different conditions was determined by a fluorimeter at an excitation wavelength of 520 nm and emission wavelength of 590 nm [19].
Analysis of the Effect of Piperine on the Catheter-Associated Biofilm
To test the effect of piperine on the developed biofilm of S. aureus on catheter, the biofilm was first developed on catheter and further challenged with piperine. Catheter pieces (width 0.3 cm and length 1 cm) were sterilized by washing them with 70% ethanol. In short, an equal number of cells (1 × 105 CFU/mL) were allowed to grow in tubes containing sterile LB. After that, catheter piece was added to each tube under aseptic condition. Afterwards, all the tubes were incubated at 37 °C for 24 h to develop biofilm on the catheter surface. Post incubation, catheters were recovered from the tube and the adhered biofilm on the catheter was either challenged with piperine or left untreated for 6 h under similar condition. After 6 h of exposure, catheter was recovered from each tube under aseptic conditions. In this connection, CV assay, total protein recovery assay, EPS measurement assay, and FDA hydrolysis assay were conducted to confirm the biofilm-disintegrating property of piperine against the developed biofilm on catheter.
Statistical Analysis
To achieve statistical confidence, each experiment was repeated thrice. The significance test was performed by one-way analysis of variance (ANOVA). Here, the significance level was represented as p value < 0.05 (*), p value < 0.01 (**), and p value < 0.001 (***) in comparison with control. The p values > 0.05 indicated no significant difference, thereby presented as N.S. (no statistical difference).
Results and Discussion
S. aureus Developed Substantial Biofilm with the Advancement of Time
S. aureus, being an opportunistic human pathogen, can cause several infections by involving biofilm [20]. Towards an effective management of biofilm threats, piperine has been reported as a promising molecule against S. aureus [7]. However, the disintegrating property of piperine against the existing biofilm of S. aureus has not been tested yet. In this connection, the time-dependent biofilm formation of the test organism was determined by growing cells in sterile LB media for different lengths of time (up to 30 h). Several experiments (CV assay, light microscopy, and measurement of EPS) were conducted to understand the degree of biofilm formation of the test organism with the progression of time. Post incubation, the degree of microbial biofilm development at respective time points was estimated by performing CV assay. The result of CV assay revealed that with the advancement of incubation period, the degree of microbial biofilm formation got increased consistently (Fig. 1A). The result also suggested that the maximum biofilm formation took place at 24 h of incubation (Fig. 1A). Beyond this time, no significant increase in biofilm development was noticed (Fig. 1A). To confirm this observation, light microscopic image analysis was carried out in which we observed that the highest level of microbial colonization occurred when the cells were grown for 24 h in sterile LB (Fig. 1B). The result showed that with the increasing time of incubation of the test organism, the extents of microbial clusters were found to increase considerably (Fig. 1B). To gain further confidence, we measured the EPS of Staphylococcal biofilm at different time points as it was already reported that 85% of the biofilm mass are composed of EPS matrices [21]. The result suggested that the amount of EPS was found to increase with the progression of incubation (Fig. 1C). As expected, the amount of EPS production was found to be the highest when the cells were grown for 24 h (Fig. 1C). Therefore, all these experiments demonstrated that the extent of biofilm formation of the test organism got increased considerably with the progression of time.
S. aureus efficiently developed biofilm with the progression of time. An equal number of cells were grown in sterile LB media at 37 °C for varying periods of time. To it, several coverslips were also incorporated to allow the formation of biofilm over it. Post incubation, planktonic cells were discarded and biofilm cells were taken into consideration. A CV assay profile. The degree of biofilm formation at different time intervals was quantified by performing CV assay. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values < 0.05 were marked with (*), p values < 0.01 were marked with (**), and p values < 0.001 were marked with (***) to show the statistical difference among the observations. B Light microscopic image analysis. Respective coverslips were recovered from each experimental set followed by staining with CV (0.4%) and viewed under a light microscope. The figure is a representative image of 20 different fields of three independent experiments. C EPS profile. The amount of EPS formation at different time intervals was measured by following phenol–sulfuric acid method. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values < 0.01 were marked with (**), and p values < 0.001 were marked with (***) to show the statistical difference among the observations
Piperine Could Disintegrate the Developed Biofilm of S. aureus
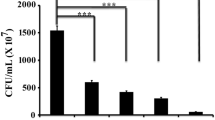
To test whether piperine could disintegrate the already existing biofilm of S. aureus, the developed biofilm was challenged with the different concentrations (0, 8, 16, and 24 µg/mL) of piperine for a period of 6 h at 37 °C. Post incubation, the extent of biofilm disintegration under different concentrations of piperine was measured by following CV assay. The result of the CV assay revealed that with the increase of piperine concentrations, the extent of biofilm disintegration got increased considerably (Supplementary Fig. 1A and 1B). However, we had noticed no significant difference in the extent of biofilm disintegration between the piperine concentration of 16 µg/mL and 24 µg/mL (Supplementary Fig. 1A and 1B). Besides, we had also observed that the biofilm disintegration was found to be the highest when the developed biofilm was incubated with piperine (16 µg/mL) for a period of 6 h (Fig. 2A and B). Furthermore, no significant changes in biofilm disintegration were observed beyond 6 h of incubation period (Fig. 2A and B). Therefore, in the current study, piperine was incubated with the pre-existing biofilm for a period of 6 h. Since piperine was dissolved in DMSO, the effect of DMSO on S. aureus was also taken into account. In this study, the antibacterial and antibiofilm activities of the working concentrations of DMSO (0.08 and 0.16% [v/v]) were used in which we observed that the mentioned concentrations of DMSO did not exhibit any antibacterial as well as antibiofilm activity against S. aureus (Supplementary Fig. 2A and 2B). Thus, the result suggested that piperine could be the causative agent behind biofilm disintegration. To study further, we had measured the protein content of the already existing biofilm under the presence and absence of piperine as the higher protein recovery indicates the formation of a matured biofilm and vice versa. The result revealed that the recovery of protein got reduced considerably when the pre-existing biofilm was treated with rising concentrations of piperine (Fig. 2C). EPS happens to be the major functional and structural components of biofilm and could be considered as an indicator of the extent of biofilm formation [22]. Thus, we had determined the EPS profile of the test organism and noticed that the amount of EPS got reduced considerably when the developed biofilm was challenged with the increasing concentrations of piperine (Fig. 2C). Hence, the result of CV assay, recovery of total biofilm protein, and EPS measurement assay indicated the considerable disintegration of the already existing biofilm under the presence of piperine. Then, we measured the metabolic activity of the already existing biofilm cells under the presence and absence of piperine through FDA hydrolysis test. The result of the FDA hydrolysis assay revealed that the extent of the metabolic activity was found to decrease when the pre-existing biofilm was incubated with the selected doses (8 and 16 µg/mL) of piperine (Fig. 2C). Thus, all the results indicated the biofilm-disintegrating property of piperine against the pre-existing biofilm of S. aureus. Besides, it was noticed from the fluorescence microscopic image that the maximum degree of microbial clusters was observed when the developed biofilm was not exposed to piperine (Fig. 2D). However, the degree of such clusters was reduced noticeably when the developed biofilm cells were exposed to the rising concentrations of piperine (Fig. 2D). Further confirmation was done by SEM image analysis. The result revealed that maximum microbial cells adhered over the coverslip when the pre-existing biofilm of S. aureus was not exposed to piperine (Fig. 2E). However, the extent of adhered biofilm cells got reduced substantially when the pre-existing biofilm was incubated with piperine (Fig. 2E). Biofilm disintegration reveals the dissociation of cells from the pre-existing biofilm that increases the count of non-biofilm cells [5]. A schematic diagram has been presented to address the non-biofilm cells in a microbial population (Fig. 2F). In this context, the non-biofilm cells were measured under the presence and absence of piperine by determining the viable microbial counts. The result showed that the number of non-biofilm cells was found to be the lowest in the control set in which the pre-existing biofilm was incubated without piperine (Fig. 2G). However, the count of non-biofilm cells was increased significantly when the developed biofilm was challenged with piperine (Fig. 2G). Therefore, the result suggested that piperine could promote the disintegration of the pre-existing biofilm that resulted in the increase of the count of non-biofilm cells (Fig. 2G). Thus, all the results demonstrated the biofilm-disintegrating property of piperine against the pre-existing biofilm of S. aureus. In the present study, attempts have been taken to test the antibiofilm activity of piperine against a drug-resistant strain of S. aureus (methicillin-resistant Staphylococcus aureus [MRSA]) as MRSA could cause several acute infections involving biofilm. The result showed that piperine could efficiently disintegrate the pre-existing biofilm of MRSA (Supplementary Fig. 3A and 3B). Taken together, the result suggested that piperine exhibited substantial biofilm disintegration against S. aureus as well as MRSA.
Piperine exhibited efficient disintegration of the pre-existing biofilm of S. aureus. Equal numbers of cells were allowed to develop microbial biofilm for 24 h. Then, the developed biofilm was either treated with piperine for different time periods or left untreated. Post incubation, planktonic cells were discarded and the degree of residual biofilm was analyzed. A CV staining profile. The degree of residual biofilm adhered to glass tube was analyzed by CV staining at different time points. B CV assay profile. The extent of biofilm disintegration of S. aureus under the influence of piperine was estimated by measuring the OD of CV-stained acetic acid solution at 630 nm. C Measurement of biofilm disintegration. The extent of biofilm disintegration of S. aureus under the exposure of piperine was determined by total biofilm protein recovery assay, EPS measurement assay, and FDA hydrolysis assay. Each experiment was repeated three times. Error bars represented the standard error of the mean. The p values < 0.05 were marked with (*), p values < 0.01 were marked with (**), and p values < 0.001 were marked with (***) to show the statistical difference among the observations. D Fluorescence microscopic image analysis. The extent of the disintegration of the pre-formed biofilm of the test organism under the exposure of piperine was observed under a fluorescence microscope. The figure is a representative image of 20 different fields of three independent experiments. E Scanning electron microscopy. The degree of disintegration of the pre-formed biofilm of S. aureus was observed under a scanning electron microscope. The figure is a representative image of 20 different fields of three independent experiments. F Non-biofilm cells profile. A schematic diagram of the accumulation of non-biofilm cells under piperine exposure. Planktonic as well as piperine-induced biofilm-disintegrated cells were together considered non-biofilm cells. G Estimation of non-biofilm cells. The pre-formed biofilm cells were challenged with piperine for a period of 6 h followed by measuring the abundance of non-biofilm cells by CFU method. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values < 0.05 were marked with (*) and p values < 0.01 were marked with (**) to show the statistical difference among the observations
Degradation of Extracellular DNA (eDNA) Could Promote the Disintegration of the Developed Biofilm of S. aureus
Extracellular DNA (eDNA), an important component of EPS, plays a key role in maintaining the structural integrity of biofilm network [23]. Literature report suggested that eDNA could stabilize the ionic interactions in assembling the biofilm network [24]. Therefore, in this study, the possible role of eDNA in holding the structural integrity of the existing biofilm of S. aureus was explored. To this end, 24 h matured biofilm of the test organism was either treated with DNase I or left untreated for another 6 h at 37 °C. Post incubation, CV assay was performed to assess the degree of residual biofilm in each tube. The result showed that the degree of biofilm disintegration got enhanced by ~ 39% under the influence of DNase I (Fig. 3A). Thus, it could be stated that DNase I could degrade the eDNA content of the pre-existing biofilm matrix, thereby disintegrating the same efficiently. Furthermore, the existing biofilm of S. aureus was either treated with piperine or kept untreated under similar condition for 6 h. Post incubation, the eDNA was extracted from both piperine-treated and piperine-untreated sets of experiment and quantified by spectrophotometer. The result showed that eDNA content reduced by ~ 64% under piperine exposure (16 µg/mL) (Fig. 3B). For reassurance, the extracted eDNA was run through an agarose gel electrophoresis. The result revealed that the band intensity of extracted eDNA from piperine-treated biofilm was much dimmer than that of eDNA from piperine-untreated biofilm (Fig. 3C). Therefore, the result revealed that the extent of eDNA recovery got decreased with the increasing concentrations of piperine. Thus, the results indicated that the selected concentrations (8 and 16 µg/mL) of piperine could disintegrate the existing biofilm by decreasing the amount of eDNA.
The amount of eDNA associated with biofilm got reduced during biofilm disintegration. A Role of eDNA in holding the integrity of biofilm structure. The developed biofilm was either treated with DNase I or left untreated followed by incubating the same for 6 h. Post incubation, the degree of residual biofilm under the exposure of DNase I was estimated by CV assay. B Quantification of eDNA content. Both piperine-treated and piperine-untreated biofilm were taken into consideration for eDNA extraction. The concentration of extracted eDNA was measured by recording the absorbance at 260 nm. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values < 0.001 were marked with (***) to show the statistical difference among the observations. C Agarose gel electrophoresis analysis of the extracted eDNA. The qualitative analysis of the extracted eDNA of both piperine-treated and piperine-untreated biofilm was done by agarose gel electrophoresis. The figure is a representative image of three independent experiments
Piperine Promotes Biofilm Disintegration by Inhibiting Microbial Auto-aggregation and Cell Surface Hydrophobicity
Auto-aggregation happens to be a well-known phenomenon which leads to microbial biofilm formation that enables the cells to establish an effective communication [25]. Besides, it has also been reported that the microbial auto-aggregation often helps to develop biofilm by strengthening the colonization process [26, 27]. In this connection, we had measured the degree of microbial auto-aggregation of the biofilm cells both in the presence and absence of piperine. The result showed that the auto-aggregation of microbial biofilm cells got reduced by ~ 69% while treated with desired concentration of piperine, i.e., 16 µg/mL (Fig. 4A). Thus, the result indicated that the reduced microbial auto-aggregation profile did not allow the cells to withstand in the biofilm network. In this context, literature report indicated that the cell surface hydrophobicity could play an important role in the microbial auto-aggregation part [28]. An increase or decrease in the cell surface hydrophobicity could enhance or decrease the microbial auto-aggregation part, respectively [29]. Therefore, cell surface hydrophobicity of both piperine-treated and piperine-untreated biofilm cells were estimated where the maximum cell surface hydrophobicity was observed in piperine-untreated biofilm cells (Fig. 4B). However, significant reduction of the cell surface hydrophobicity was found to take place with the rising concentrations of piperine (Fig. 4B). The maximum reduction in cell surface hydrophobicity took place when the biofilm cells were treated with the piperine at a concentration of 16 µg/mL (Fig. 4B). Therefore, the experimental result indicated that piperine could reduce the cell surface hydrophobicity of the biofilm cells that resulted in the inhibition of microbial auto-aggregation which could promote the disintegration of the developed biofilm. Furthermore, the literature survey revealed that the d-alanination of teichoic acid was found to enhance the cell surface hydrophobicity of S. aureus [5]. It was reported that DltA could function as a d-alanine: d-alanyl carrier protein ligase that resulted in the increase of cell surface hydrophobicity of the bacteria by introducing more d-alanine in the teichoic acid [30]. Hence, we had measured the expression of dltA gene of the biofilm cells of S. aureus under the presence and absence of piperine. The result revealed that the expression level of dltA gene got reduced in the biofilm cells with the increasing concentrations of piperine (Fig. 4C). Thus, the observation indicated that the treatment of piperine was found to minimize the expression of dltA gene that resulted in the decrease of cell surface hydrophobicity of the bacteria. Taken together, all observations suggested that piperine treatment could reduce the cell surface hydrophobicity of the test bacteria that could lead to the disintegration of biofilm by inhibiting microbial auto-aggregation.
Microbial auto-aggregation of the biofilm cells got reduced under the influence of piperine. A Microbial auto-aggregation profile. The percentage of auto-aggregation of the microbial biofilm under the exposure of piperine was evaluated by recording the absorbance of the aggregated biofilm cells. B Cell surface hydrophobicity profile. The cell surface hydrophobicity of biofilm cells under the exposure of piperine was measured by performing BATH assay. C Expression profile of dltA gene. The dltA gene expression (responsible for cell surface hydrophobicity of S. aureus) under the influence of piperine was quantified by conducting RT-PCR analysis. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values > 0.05 were marked as N.S. (no statistical difference), p values < 0.05 were marked with (*), p values < 0.01 were marked with (**), and p values < 0.001 were marked with (***) to show the statistical difference among the observations
Piperine-Induced ROS Accumulation Could Promote the Disintegration of the Pre‐existing Biofilm
A previous report suggests that accumulated ROS could be a causative agent for the disintegration of the existing biofilm [5]. In this context, we tested whether the ROS profile of the biofilm cells got changed under the influence of the piperine. Towards this direction, the developed biofilm cells were either incubated with piperine or left untreated at 37 °C for 6 h. Post incubation, DCFDA method was considered to measure the ROS profile of the biofilm cells. As a result, the lowest ROS accumulation was found in case of control wherein the highest ROS accumulation was found in piperine-treated set (~ 5-folds higher than control) (Fig. 5A). However, being an antioxidant, ascorbic acid scavenged the piperine-induced ROS accumulation in the cells (Fig. 5A). To further understand the effect of ROS accumulation on the extent of biofilm disintegration, the CV assay was followed to measure the amount of biofilm remained under the exposure of piperine and ascorbic acid. The result revealed that the biofilm disintegration and ROS accumulation shared a directly proportional relationship (Fig. 5B). The result further showed that the piperine-induced biofilm disintegration got reduced under the influence of ascorbic acid (Fig. 5B). Thus, the result suggested that the piperine-mediated biofilm disintegration could be attributed to the cellular accumulation of ROS. Since the cell surface hydrophobicity could play a vital role in biofilm assembly, we assessed whether the cell surface hydrophobicity of microbial biofilm cells got changed under the influence of piperine and ascorbic acid. To analyze the effect of ROS accumulation on the cell surface hydrophobicity of the biofilm cells, a similar set of experiment was taken into consideration wherein the developed biofilm cells were treated with piperine and ascorbic acid for 6 h. After the incubation, the similar numbers of cells were collected from each condition and BATH assay was conducted to determine the cell surface hydrophobicity of the biofilm cells. The result showed that the cell surface hydrophobicity of the biofilm cells got decreased by ~ 75% when the biofilm cells were treated with piperine only (Fig. 5C). However, the extent of the cell surface hydrophobicity got increased considerably when the biofilm cells were treated with both piperine and ascorbic acid (Fig. 5C). To further validate the observation, a contour plot was constructed to understand the relationship among three variables, namely accumulation of ROS, cell surface hydrophobicity, and degree of biofilm disintegration under the presence and absence of piperine. The result revealed that with the enhancement of ROS accumulation, the cell surface hydrophobicity of the biofilm cells got decreased that resulted in the promotion of biofilm disintegration (Fig. 5D). Taken together, the results suggested that the exposure of piperine was found to generate ROS which could reduce the cell surface hydrophobicity, thereby causing the disintegration of the developed biofilm of the test organism.
Piperine exhibited considerable accumulation of ROS in the test organism that resulted in the disintegration of the pre-existing biofilm. A ROS profile. The developed biofilm cells were either challenged with piperine or left untreated for 6 h followed by measuring the intracellular ROS in the test organism by DCFDA method. B Effect of ROS on biofilm disintegration. The effect of ROS accumulation on the disintegration of the pre-formed biofilm was evaluated by performing CV assay. C Effect of ROS on cell surface hydrophobicity. The effect of ROS on the cell surface hydrophobicity of biofilm cells was estimated by BATH assay. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values > 0.05 were marked as N.S. (no statistical difference), p values < 0.05 were marked with (*), p values < 0.01 were marked with (**), and p values < 0.001 were marked with (***) to show the statistical difference among the observations. D Analysis of surface plot. A surface plot was constructed to understand the relation among accumulated ROS, biofilm disintegration, and cell surface hydrophobicity of the biofilm cells of S. aureus
Piperine-Treated Biofilm-Disintegrated Cells Showed Increased Susceptibility to Induced Autolysis
Biofilm offers considerable protection against external agents and hence is able to develop a robust microbial community structure on a given surface [31]. Therefore, we wanted to examine the extent of induced autolysis of the piperine-treated and piperine-untreated biofilm cells under external stress, namely Triton X. In this connection, the result showed that the biofilm cells which were not treated with piperine could show higher microbial growth under the exposure of Triton X (Fig. 6A). However, the result indicated that with the increase of piperine concentrations, the biofilm cells could show reduced microbial growth under the exposure of Triton X (Fig. 6A). Thus, the result revealed that the piperine-treated cells showed increased susceptibility under the influence of Triton X. The result suggested that piperine exposure was able to disintegrate the pre-existing biofilm matrix and hence the disintegrated biofilm cells could show increased susceptibility against Triton X. To further understand the mechanism, we had measured the membrane permeability of the cells under the presence and absence of piperine as membrane permeability often influences the diffusion of molecules into the cells [19]. Therefore, we allowed the cells to develop biofilm, and subsequently, the biofilm cells were either treated or untreated with piperine for 6 h. After the incubation, the cells were collected from either source and membrane permeability was measured by following ethidium bromide influx assay. The result showed that the treatment of piperine was able to increase the membrane permeability of S. aureus (Fig. 6B). The result indicated that the increased membrane permeability might allow the compound (piperine) to enter into the cells, thereby showing increased potential towards biofilm disintegration. In this regard, further investigation is required to unveil the underlying mechanism.
Piperine treatment made the biofilm-disintegrated cells more susceptible towards induced autolysis and increased the membrane permeability considerably. The developed biofilm cells were treated under the presence and absence of piperine for 6 h. A Induced autolysis profile. An equal number of piperine-treated and piperine-untreated biofilm cells were further challenged with autolysis buffer for 3 h. The microbial growth in the presence of autolysis buffer was estimated by measuring the OD at 600 nm. B Membrane permeability profile. The piperine-treated and piperine-untreated biofilm cells were centrifuged followed by EtBr influx assay. Each experiment was repeated three times. Error bars represented the standard error of the mean. The p values < 0.05 were marked with (*), and p values < 0.01 were marked with (**) to show the statistical difference among the observations
Piperine Could Disintegrate the Microbial Biofilm Developed on Catheter
Catheter-associated infections are increasingly gaining attention as it is one of the most widespread causes of biofilm-mediated nosocomial contagion [32, 33]. Therefore, management of catheter-mediated infections has become the imperative need of the hour [34]. Furthermore, several studies have implicated S. aureus as one of the major microbial pathogens responsible for catheter-associated infections [35, 36]. Thus, in this connection, an in vitro investigation was employed wherein the pre-existing biofilms of S. aureus on catheter tubes were challenged with the selected concentrations (0 and 16 µg/mL) of piperine. The results of CV staining assay clearly indicated that there was considerable disintegration of S. aureus biofilms in the presence of the test concentrations of piperine (Fig. 7A, B, and C). Furthermore, the result revealed that the protein recovery from the biofilm cells got reduced by ~ 54% under the presence of piperine at a concentration of 16 µg/mL (Fig. 7B and C). Furthermore, the EPS content of biofilms cells got reduced by ~ 40% when treated with piperine at a concentration of 16 µg/mL (Fig. 7B and C). A disruption of EPS matrix could lead to reduced metabolic activity of the microbial cells. Hence, we had measured the FDA hydrolysis activity of the biofilm cells under the presence and absence of piperine. In this regard, we observed that the metabolic activity of the biofilm cells got reduced significantly when the cells were challenged with piperine (Fig. 7B and C). Taken together, the results of this smart in vitro investigation demonstrably established that piperine treatment could significantly disintegrate the pre-existing biofilm of S. aureus on the catheter tubes. Therefore, the findings of this study (under laboratory conditions) may provide vital insights into the management of catheter-associated nosocomial biofilm-mediated infections of S. aureus.
Piperine efficiently disintegrated the pre-formed biofilm of S. aureus from the catheter surface. An equal number of cells was allowed to develop biofilm on the catheter surface followed by treating the same with either piperine or left untreated. Post treatment, catheter tubes were recovered and the degree of biofilm disintegration from the catheter surface was analyzed. A CV staining profile. The residual biofilm adhered to the catheter tube was taken into consideration for CV staining. B Residual biofilm profile. The extent of residual biofilm present on the catheter tube under the presence and absence of piperine was carried out by performing CV staining, Lowry method, FDA hydrolysis, and EPS extraction method. C Quantitative measurement of biofilm disintegration. The degree of piperine-induced disintegration of the pre-existing biofilm from the catheter surface was measured by recording the absorbance at 630 nm, 720 nm, and 490 nm, respectively. Each experiment was repeated thrice. Error bars represented the standard error of the mean. The p values < 0.001 were marked with (***) to show the statistical difference among the observations
Conclusion
An alarming rise of biofilm-induced drug resistance often exhibits serious threat to public healthcare, and hence, it demands an optimistic solution for the effective management of the same. In this connection, piperine, a natural compound, has disintegrated the pre-existing biofilm of opportunistic human pathogen, S. aureus. Therefore, on further exploration, piperine could be used as an efficacious molecule to curb such biofilm-associated diseases.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Software, namely Minitab 19 (trial version) and NCSS (trial version), were used for the current study. And we were allowed to access the same for a period of 30 days.
References
Dauros-Singorenko, P., Wiles, S., & Swift, S. (2020). Staphylococcus aureus biofilms and their response to a relevant in vivo iron source. Frontiers in Microbiology, 11, 509525.
Tokam Kuaté, C. R., Bisso Ndezo, B., & Dzoyem, J. P. (2021). Synergistic antibiofilm effect of thymol and piperine in combination with aminoglycosides antibiotics against four Salmonella enterica serovars. Evidence-Based Complementary and Alternative Medicine, 2021, 1–9.
Archer, N. K., Mazaitis, M. J., Costerton, J. W., Leid, J. G., Powers, M. E., & Shirtliff, M. E. (2011). Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence, 2(5), 445–459.
Gupta, P., Sarkar, S., Das, B., Bhattacharjee, S., & Tribedi, P. (2016). Biofilm, pathogenesis and prevention-a journey to break the wall: A review. Archives of Microbiology, 198(1), 1–15.
Paul, P., Das, S., Chatterjee, S., Shukla, A., Chakraborty, P., Sarkar, S., & Tribedi, P. (2021). 1, 4-Naphthoquinone disintegrates the pre-existing biofilm of Staphylococcus aureus by accumulating reactive oxygen species. Archives of Microbiology, 203(8), 4981–4992.
Quijia, C. R., & Chorilli, M. (2020). Characteristics, biological properties and analytical methods of piperine: A review. Critical Reviews in Analytical Chemistry, 50(1), 62–77.
Das, S., Paul, P., Chatterjee, S., Chakraborty, P., Sarker, R. K., Das, A., & Tribedi, P. (2022). Piperine exhibits promising antibiofilm activity against Staphylococcus aureus by accumulating reactive oxygen species (ROS). Archives of Microbiology, 204(1), 1–11.
Mukherjee, K., Tribedi, P., Mukhopadhyay, B., & Sil, A. K. (2013). Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiology Letters, 338(2), 177–183.
Tribedi, P., & Sil, A. K. (2013). Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environmental Science and Pollution Research, 20(6), 4146–4153.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
Tribedi, P., Gupta, A. D., & Sil, A. K. (2015). Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: An effective strategy for efficient survival and polymer degradation. Bioresources and Bioprocessing, 2(1), 1–10.
Lowry, O. H. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Chakraborty, P., & Tribedi, P. (2019). Functional diversity performs a key role in the isolation of nitrogen-fixing and phosphate-solubilizing bacteria from soil. Folia Microbiologica, 64(3), 461–470.
Sahu, P. K., Iyer, P. S., Oak, A. M., Pardesi, K. R., & Chopade, B. A. (2012). Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. The Scientific World Journal, 2012, 1–10.
Askoura, M., Yousef, N., Mansour, B., & Yehia, F. A. Z. A. (2022). Antibiofilm and staphyloxanthin inhibitory potential of terbinafine against Staphylococcus aureus: In vitro and in vivo studies. Annals of Clinical Microbiology and Antimicrobials, 21(1), 1–17.
Rosenberg, M., Perry, A., Bayer, E. A., Gutnick, D. L., Rosenberg, E., & Ofek, I. (1981). Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infection and Immunity, 33(1), 29–33.
Dwivedi, S., Wahab, R., Khan, F., Mishra, Y. K., Musarrat, J., & Al-Khedhairy, A. A. (2014). Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE, 9(11), e111289.
Miki, T., & Hardt, W. D. (2013). Outer membrane permeabilization is an essential step in the killing of gram-negative bacteria by the lectin Reg IIIβ. PLoS One, 8(7), e69901.
Chatterjee, S., Das, S., Paul, P., Chakraborty, P., Sarkar, S., Das, A., & Tribedi, P. (2022). Synergistic interaction of cuminaldehyde and tobramycin: A potential strategy for the efficient management of biofilm caused by Pseudomonas aeruginosa. Folia Microbiologica 68(1), 1–13.
Melo, T. A., Dos Santos, T. F., de Almeida, M. E., Junior, L. A. G. F., Andrade, E. F., Rezende, R. P., & Romano, C. C. (2016). Inhibition of Staphylococcus aureus biofilm by Lactobacillus isolated from fine cocoa. BMC Microbiology, 16(1), 1–9.
Pinto, R. M., Soares, F. A., Reis, S., Nunes, C., & Van Dijck, P. (2020). Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Frontiers in Microbiology, 11, 952.
Jachlewski, S., Jachlewski, W. D., Linne, U., Bräsen, C., Wingender, J., & Siebers, B. (2015). Isolation of extracellular polymeric substances from biofilms of the thermoacidophilic archaeon Sulfolobus acidocaldarius. Frontiers in Bioengineering and Biotechnology, 3, 123.
Devaraj, A., Buzzo, J. R., Mashburn-Warren, L., Gloag, E. S., Novotny, L. A., Stoodley, P., & Goodman, S. D. (2019). The extracellular DNA lattice of bacterial biofilms is structurally related to Holliday junction recombination intermediates. Proceedings of the National Academy of Sciences, 116(50), 25068–25077.
Campoccia, D., Montanaro, L., & Arciola, C. R. (2021). Tracing the origins of extracellular DNA in bacterial biofilms: Story of death and predation to community benefit. Biofouling, 37(9–10), 1022–1039.
Trunk, T., Khalil, H. S., & Leo, J. C. (2018). Bacterial autoaggregation. AIMS Microbiology, 4(1), 140.
Nwoko, E. S. Q., & Okeke, I. N. (2021). Bacteria autoaggregation: How and why bacteria stick together. Biochemical Society Transactions, 49(3), 1147–1157.
Isenring, J., Geirnaert, A., Lacroix, C., & Stevens, M. J. (2021). Bistable auto-aggregation phenotype in Lactiplantibacillus plantarum emerges after cultivation in in vitro colonic microbiota. BMC Microbiology, 21(1), 1–13.
Burel, C., Dreyfus, R., & Purevdorj-Gage, L. (2021). Physical mechanisms driving the reversible aggregation of Staphylococcus aureus and response to antimicrobials. Scientific Reports, 11(1), 1–9.
Rahman, M., Kim, W. S., Kumura, H., & Shimazaki, K. I. (2008). Autoaggregation and surface hydrophobicity of bifidobacteria. World Journal of Microbiology and Biotechnology, 24(8), 1593–1598.
Gross, M., Cramton, S. E., Götz, F., & Peschel, A. (2001). Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infection and Immunity, 69(5), 3423–3426.
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P., & Hall-Stoodley, L. (2017). Targeting microbial biofilms: Current and prospective therapeutic strategies. Nature Reviews Microbiology, 15(12), 740–755.
Pelling, H., Nzakizwanayo, J., Milo, S., Denham, E. L., MacFarlane, W. M., Bock, L. J., & Jones, B. V. (2019). Bacterial biofilm formation on indwelling urethral catheters. Letters in Applied Microbiology, 68(4), 277–293.
Murugan, K., Selvanayaki, K., & Al-Sohaibani, S. (2016). Urinary catheter indwelling clinical pathogen biofilm formation, exopolysaccharide characterization and their growth influencing parameters. Saudi Journal of Biological Sciences, 23(1), 150–159.
Trautner, B. W., & Darouiche, R. O. (2004). Catheter-associated infections: Pathogenesis affects prevention. Archives of Internal Medicine, 164(8), 842–850.
Pascual, A. (2002). Pathogenesis of catheter-related infections: Lessons for new designs. Clinical Microbiology and Infection, 8(5), 256–264.
Alby-Laurent, F., Lambe, C., Ferroni, A., Salvi, N., Lebeaux, D., Le Gouëz, M., & Toubiana, J. (2019). Salvage strategy for long-term central venous catheter-associated Staphylococcus aureus infections in children. Frontiers in Pediatrics, 6, 427.
Funding
The authors would like to thank The Neotia University for providing the financial assistance through sanctioning minor grant (R&D/2020/F2) in carrying out the shared research activity.
Author information
Authors and Affiliations
Contributions
SD, RR, PP, PC, SC, MM, and SS performed the experiments and analyzed the results. ADG analyzed the results and helped in writing the manuscript. DM and PT conceived the idea, designed the experiments, analyzed the results, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Roy, R., Paul, P. et al. Piperine, a Plant Alkaloid, Exhibits Efficient Disintegration of the Pre-existing Biofilm of Staphylococcus aureus: a Step Towards Effective Management of Biofilm Threats. Appl Biochem Biotechnol 196, 1272–1291 (2024). https://doi.org/10.1007/s12010-023-04610-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04610-x