Abstract
Microorganisms embedded within an extracellular polymeric matrix are known as biofilm. The extensive use of antibiotics to overcome the biofilm-linked challenges has led to the emergence of multidrug-resistant strains. Staphylococcus aureus is one such nosocomial pathogen that is known to cause biofilm-linked infections. Thus, novel strategies have been adopted in this study to inhibit the biofilm formation of S. aureus. Two natural compounds, namely, 1,4-naphthoquinone (a quinone derivative) and tryptophan (aromatic amino acid), have been chosen as they could independently show efficient antibiofilm activity. To enhance the antibiofilm potential, the two compounds were combined and tested against the same organism. Several experiments like crystal violet (CV) assay, protein estimation, extracellular polymeric substance (EPS) extraction, and estimation of metabolic activity confirmed that the combination of the two compounds could significantly inhibit the biofilm formation of S. aureus. To comprehend the underlying mechanism, efforts were further directed to understand whether the two compounds could inhibit biofilm formation by compromising the cell surface hydrophobicity of the bacteria. The results revealed that the cell surface hydrophobicity got reduced by ~ 49% when the compounds were applied together. Thus, the combinations could show enhanced antibiofilm activity by attenuating cell surface hydrophobicity. Further studies revealed that the selected concentrations of the compounds could disintegrate (~ 70%) the pre-existing biofilm of the test bacteria without showing any antimicrobial activity. Hence, the combined application of tryptophan and 1,4-naphthoquinone could be used to inhibit the biofilm threats of S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biofilm mode of existence is the most predominant form of bacterial existence in nature (Gebreyohannes et al. 2019). Moreover, the growing resistance of microbial biofilm is the leading cause of nosocomial infections in the healthcare units (Sharma et al. 2014; Pachori et al. 2019). The biofilm aggregates could show antibiotic resistance up to 1000 times higher than their planktonic (free-living) counterpart (Gebreyohannes et al. 2019). The drug-resistant property of the biofilm could be attributed to the production of the extracellular polymeric substances (EPS). The EPS matrix has been reported to impede the diffusion of several antibiotics, thereby strategically helping the bacterial biofilm to survive against different antibiotics (Di Martino 2018). Among the biofilm-forming organisms, S. aureus (a Gram-positive round-shaped bacterium) happens to be an opportunistic pathogen that can develop biofilm efficiently on human hosts (Azmi et al. 2019). Literature survey revealed that the biofilm of S. aureus could give rise to various chronic infections like osteomyelitis, endocarditis, and cystic fibrosis (Paharik and Horswill 2016). Thus, to deal with such challenges, the use of natural molecules has attracted considerable attention in recent times. Moreover, natural molecules are cost-effective and less toxic and can prove to be advantageous in this direction (Mishra et al. 2020). The quinone group of compounds have shown diverse biological functions including antibiofilm activity (Martínez and Benito 2005; Paul et al. 2021a). 1,4-Naphthoquinone, one of the most significant and largely distributed quinones, has revealed a remarkable variety of therapeutic activities including antibacterial, antiviral, antifungal, and anticancer properties (Tandon et al. 2006; Ibis et al. 2011; Mallavadhani et al. 2014; Wang et al. 2022). Additionally, in our previous study, we showed that the mentioned compound exhibited efficient antibiofilm activity against the biofilm formation of S. aureus (Paul et al. 2021a, b). However, to further increase the antibiofilm efficacy of this compound, combinatorial approaches have been followed as literature survey suggested that the combinatorial process work more efficiently than individual ones (Gupta et al. 2017). In this connection, it was reported that the combination of three compounds, namely, thymoquinone, tetrazine-capped silver nanoparticle, and tryptophan, exhibited increased antibiofilm activity against Pseudomonas aeruginosa than their individual effects (Chakraborty et al. 2021). Moreover, the use of several natural antibiofilm agents antagonizing bacterial resistance happens to be a promising approach for the management of biofilm threats (Abreu et al. 2016). The combinations of vitexin along with two antibiotics, namely, azithromycin and gentamicin, was found to exhibit remarkable antibiofilm characteristics against P. aeruginosa (Das et al. 2016). Although 1,4-naphthoquinone and tryptophan were separately reported to show considerable antibiofilm activity, their combined action is yet to be tested on the test organism. The results showed that the combination of the two compounds, namely, 1,4-naphthoquinone and tryptophan worked more efficiently than their individual application towards the biofilm management of S. aureus.
Methods and materials
Microbial strain, chemicals, and culture conditions
A Gram-positive bacterium, S. aureus (accession number MTCC 96), was selected as the test organism for the present study. Luria Broth (LB) (HiMedia, India) was used for the optimum growth of the bacteria. The bacterium was cultivated at 37 °C for 24 h. 1,4-Naphthoquinone (purity ≥ 98%) and tryptophan were purchased from Sigma Aldrich and SRL, India, respectively.
Determination of microbial biofilm formation by crystal violet assay
Crystal violet (CV) assay is a widely used assay to determine the biofilm formation of S. aureus under different conditions (Xu et al. 2016). Hence, in this study, CV assay was followed to examine the combined effect of the compounds (1,4-naphthoquinone and tryptophan) on the biofilm profile of S. aureus. Firstly, S. aureus (1 × 105 CFU/mL) were inoculated in several test tubes containing 5 mL of sterile LB media. Thereafter, the selected concentrations (10, 20, and 30 μg/mL) of tryptophan and 1,4-naphthoquinone (10 μg/mL) were added to the growth media either separately or in combination. Alongside, a control set was also maintained wherein an equal number of organisms were grown without being exposed to any of the test concentrations of the mentioned molecules. Thereafter, all the growth media including control were incubated at 37 °C for 24 h. Post incubation, the planktonic cells were removed from the respective test tubes and washed twice with autoclaved double-distilled water. The washed tubes were further air-dried and incubated for 15 min with CV (0.4%) solution. After the incubation, the CV solution was carefully discarded from the respective tubes and gently rinsed with sterile double-distilled water. The CV-stained tubes were further dissolved in (33%) glacial acetic acid and the optical density (OD) of the same was determined at 630 nm.
Estimation of total biofilm protein
Total biofilm protein could be determined to understand the degree of biofilm formed by a microorganism under a given condition (Chen and Stewart 2000). In this direction, the total biofilm protein of S. aureus was determined under the different combinations of 1,4-naphthoquinone and tryptophan. To do the test, a similar number of microbial cells (1 × 105 CFU/mL) were allowed to grow in different tubes in the absence and presence of the mentioned compounds (1,4-naphthoquinone and tryptophan). All the tubes were incubated for 24 h at 37 °C. After the incubation, the planktonic cells were discarded from the tubes. The tubes were then rinsed with sterile double-distilled water and further mixed with 5 mL of NaOH (0.3 N). After that, the tubes were boiled in the water bath for 30 min at 100 °C. Post incubation, the boiled suspensions were centrifuged for 10 min at 3000 × g. The supernatant was collected, and the protein content of the supernatant was determined by following the Lowry method (Lowry et al. 1951).
Fluorescence microscopic image analysis
Fluorescence microscopic analysis was carried out to understand the combined effect of the compounds on the biofilm-forming ability of the microorganism under different conditions. To test the same, the similar number (1 × 105 CFU/mL) of cells were inoculated in sterile LB media challenged with different combinations of 1,4-naphthoquinone and tryptophan. Sterile cover slips were added to all the tubes to allow the formation of microbial biofilm over it. A control set was prepared where the cover slips were added to the growth media in which the cells were not exposed to any concentration of the test compound. All the experimental sets were incubated for 24 h at 37 °C. After the incubation, the cover slips were meticulously recovered from each tube and the adhered microbial population over it was stained with acridine orange (4 μg/mL) for 15 min (Tribedi et al. 2015). Thereafter, the stained cover slips were rinsed with sterile double-distilled water, dried, and observed under a fluorescence microscope (FITC filter) at an excitation and emission wavelength of 491 nm and 516 nm, respectively (Chakraborty et al. 2020).
Measurement of EPS matrix
The production of EPS by S. aureus under different combinations of 1,4-naphthoquinone and tryptophan was determined by following the phenol sulfuric acid method (Dubois et al. 1956). To carry out the experiment, an equal number (1 × 105 CFU/mL) of the bacteria was grown in different petri plates having 20-mL sterile LB challenged with either 1,4-naphthoquinone or tryptophan or both. A control set was also prepared in which the cells were not treated with either 1,4-naphthoquinone or tryptophan or both. All the plates were then incubated at 37 °C for 24 h. Post incubation, the biofilm cells were collected from the plates as described above. The microbial suspensions were then centrifuged at 2250 × g for 10 min. Post centrifugation, the pellet was collected, exposed to 10 mM EDTA, vortexed for 10 min, and re-centrifuged at 2250 × g for 10 min. The supernatant obtained from the second round of centrifugation was pooled with the supernatant collected from the first phase of centrifugation. Finally, the pooled supernatant was treated with 2.2 volumes of chilled absolute ethanol and incubated at − 20 °C for 1 h. Then, the recovered samples were centrifuged again at 4 °C for 20 min at 2250 × g. The final pellets obtained were dissolved in sterile double distilled water. Thereafter, 2 mL of each of the samples were mixed with 1 mL of phenol and 5 mL of concentrated sulfuric acid. The suspensions were further boiled for 10 min and its absorbance was recorded at 490 nm (Dubois et al. 1956).
Determination of cell surface hydrophobicity
Bacterial adhesion to hydrocarbon (BATH) assay was followed to estimate the cell surface hydrophobicity of the test organism under different conditions (Rosenberg et al. 1981). To understand the effect of the compounds alone as well as in combination on the cell surface hydrophobicity of the test organism, cells were grown under varying concentrations of 1,4-naphthoquinone and tryptophan for 24 h at 37 °C. A control set was prepared wherein the test organism was grown in the absence of the compounds. After the incubation, equal number of cells were collected from each growth media, washed with sterile double-distilled water and re-suspended in phosphate urea magnesium (PUM) buffer [K2HPO4 (17 gL−1), KH2PO4 (726 gL−1), urea (18 gL−1) and MgSO4,7H2O (2 gL −1)] such that the OD400 could reach to 1–1.2. An aliquot of this suspension was added equally to different tubes in which increasing volumes (ranging from 0 to 0.2 mL) of organic solvent such as n-hexadecane was added. Then, the tubes were shaken for 10 min and allowed to stand for another 15 min to complete the phase separation. The final OD of the aqueous suspensions was again estimated at 400 nm. The formula which was used for measuring cell surface hydrophobicity is as follows:
Cell surface hydrophobicity (in %) = 100 × {(initial OD-final OD) ∕ initial OD}.
Determination of the metabolic activity
The metabolic activity of the test organism under the presence and absence of the test compounds was determined by following the protocol of fluorescein diacetate (FDA) hydrolysis assay (Paul et al. 2021a). To do the test, equal number (1 × 105 CFU/mL) of microorganisms either unchallenged or challenged with different combinations of compounds were grown in sterile LB for 24 h at 37 °C. After the incubation, the planktonic cells were removed from each experimental set. The biofilm cells of both treated and untreated cells were exposed to fluorescein diacetate (FDA) (10 µg/mL) in 5 mL of phosphate buffer solution (60 mM sodium phosphate buffer, pH 7.6) and kept under incubation at 37 °C for 2 h. After the incubation, the tubes were vortexed gently and centrifuged at 3000 × g for 10 min. The supernatant was collected and the absorbance of the same was measured at 494 nm (Adam and Duncan 2001).
Analysis of biofilm disintegration study
To carry out the biofilm disintegration of the test organism under exposure to the test compounds, the cells were allowed to form biofilm over the glass surface by growing them in sterile LB for 24 h at 37 °C. Thereafter, the developed biofilm was challenged with either 1,4-naphthoquinone or tryptophan or a combination of both. A control set was also maintained where the developed biofilm was neither treated with 1,4-naphthoquinone nor tryptophan. Later, all the experimental sets were incubated at 37 °C for 6 h. Post incubation, the amount of biofilm that remained on the glass surface under different combinations was determined by performing a series of experiments like CV assay, estimation of total biofilm protein, analysis of fluorescence microscopic observation as explained previously.
Quantification of eDNA
The concentration of eDNA could be measured to understand the effect of any compound on the microbial biofilm profile (Sahu et al. 2012). To understand the effect of the compounds (either alone or in combination) on the eDNA profile of the test organism, equal number of cells (1 × 105 CFU/mL) were first inoculated into several petri plates containing sterile LB (20 mL) media. All the petri plates were then incubated for 24 h at 37 °C. After the incubation, the adhered biofilm cells were recovered from each plate through scrapping. These cells were further treated with either tryptophan or 1,4-naphthoquinone or a combination of both for 6 h at 37 °C. A control set was also prepared where the microbial biofilm population was neither treated with 1,4-naphthoquinone nor tryptophan. Post incubation, the biofilm cells treated or untreated with the compounds was subjected to centrifugation at 3000 × g for 8 min. The cell pellet obtained was further re-suspended in 10 mM EDTA and centrifuged again at 3000 × g for 8 min. The two sets of supernatants collected from each centrifugation was pooled and treated with 2.2 volume of chilled absolute ethanol and incubated for 1 h at 8 °C and re-centrifuged at 6000 × g for 8 min. The obtained pellet was suspended in Tris–EDTA buffer (500 µL) and ice-cold isopropanol (150 µL). Thereafter, the suspension was incubated at 4 °C for 3 h and re-subjected to centrifugation at 4500 × g for 15 min. The obtained pellet was further suspended in Tris–EDTA buffer (500 µL) with 10 µL Proteinase K (10 mg/mL) and incubated for another 1 h at 37 °C. Post incubation, ice-cold isopropanol (150 μL) was added and centrifuged at 3000 × g for 8 min. The pellet finally obtained was collected and re-suspended in 50 µL of Tris–EDTA buffer (10 mM). A densitometric analysis of the DNA bands of agarose gel electrophoresis was carried out to understand the amount of eDNA produced by microbial biofilm under different conditions.
Analysis of the antimicrobial assay
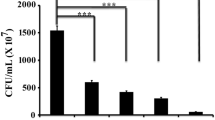
The antimicrobial activity of the tested concentrations of tryptophan and 1,4-naphthoquinone was determined against S. aureus by measuring the microbial viability (Zhou et al. 2012). To do the test, an equal number (1 × 105 CFU/mL) of microorganisms were allowed to grow in sterile LB supplemented with varying concentrations of tryptophan and 1,4-naphthoquinone for 24 h at 37 °C. Post incubation, the microbial viability in each experimental set was determined by following the colony forming unit (CFU) assay (Zhou et al. 2012).
Statistical analysis
Each experiment was repeated three times to achieve statistical confidence. One-way analysis of variance (ANOVA) was used to carry out the significance test. The degree of significance was incorporated as p value < 0.05 (*), p value < 0.01 (**), and p value < 0.001 (***) in contrast to control. The p values more than 0.05 indicated that there was no significant difference and hence presented as N.S. (no statistical difference).
Results
Tryptophan and 1,4-naphthoquinone showed efficient antibiofilm activity against S. aureus
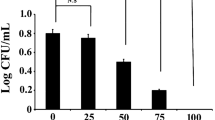
To test the individual effect of 1,4-naphthoquinone and tryptophan on the biofilm inhibition of S. aureus, the cells were separately grown in sterile LB challenged with the mentioned compounds. A control set was also taken wherein the bacterial cells were neither incubated with 1,4-naphthoquinone nor tryptophan. The effect of the mentioned compounds towards biofilm inhibition was examined by performing CV assay. The result of CV assay showed that tryptophan could independently inhibit the biofilm formation of S. aureus (Fig. 1A). The maximum biofilm inhibition (~ 39%) was observed when the cells were treated with tryptophan at a concentration of 30 μg/mL (Fig. 1A). The results further demonstrated that beyond this concentration (30 μg/mL), no further change in biofilm inhibition was observed (Fig. 1A). Besides tryptophan, the results also revealed that 1,4-naphthoquinone could inhibit the biofilm formation of S. aureus. The highest biofilm inhibition (~ 50%) was seen to take place when the test organism was treated with 1,4-naphthoquinone at a concentration of 10 μg/mL (Fig. 1B). Therefore, the results indicated that the compounds, namely, tryptophan and 1,4-naphthoquinone could show significant biofilm inhibition against S. aureus. Hence, tryptophan at a concentration of 30 μg/mL and 1,4-naphthoquinone at a concentration of 10 μg/mL were selected for further investigations.
Antibiofilm effect of the test compounds on S. aureus. Equal number of cells was added to several tubes containing sterile LB media. To it, several concentrations of tryptophan and 1,4-naphthoqionone was separately added. Alongside, a control set was also prepared where the test bacteria were not exposed to tryptophan or 1,4-naphthoquinone. Post incubation, the adhered biofilm cells were quantified by CV assay. A Biofilm profile of the cells under the influence of tryptophan. B Biofilm profile of the cells under the influence of 1,4-naphthoquinone. Error bars demonstrated the mean ± standard deviation. The significant difference in the results with the control was illustrated by calculating the respective p values. The p values less than 0.05, 0.01, and 0.001 were marked with (*), (**), and (***), respectively
Combinatorial application of the test compounds showed increased antibiofilm efficacy
To test whether the combination of the two compounds could increase the antibiofilm efficacy than their individual effect, the cells were allowed to grow in the presence of either tryptophan or 1,4-naphthoquinone or both. It was observed that the highest (~ 57%) biofilm inhibition took place when the cells were treated with tryptophan (30 µg/mL) and 1,4-naphthoquinone (10 µg/mL) together (Fig. 2A). Though the said compounds could inhibit biofilm formation individually, the degree of biofilm inhibition got enhanced significantly when they were applied together (Fig. 2A). To further validate the findings, the protein content of biofilm in each set was estimated. The results showed that highest protein recovery was observed when the cells were not exposed to either 1,4-naphthoquinone or tryptophan (Fig. 2B). However, the amount of protein recovery decreased significantly when the cells were exposed to both tryptophan (30 µg/mL) and 1,4-naphthoquinone (10 µg/mL) (Fig. 2B). Consistent with the findings of the CV assay experiments, biofilm protein estimation assay also demonstrated that maximum biofilm inhibition took place when the cells were exposed to both tryptophan and 1,4-naphthoquinone (Fig. 2A and B). Furthermore, maximum amount of EPS was produced by the microorganisms that were not treated with either tryptophan or 1,4-naphthoquinone (Fig. 2C). However, the secretion of EPS decreased by ~ 28% when the cells were exposed to only tryptophan (Fig. 2C). The treatment of 1,4-naphthoquinone was found to reduce the secretion of EPS by ~ 21% (Fig. 2C). The amount of EPS secretion got decreased by ~ 48% when the cells were exposed to both tryptophan and 1,4-naphthoquinone (Fig. 2C). To measure the extent of microbial metabolic activity under different conditions, we performed FDA assay. The result indicated that the metabolic activity of the biofilm was found to be the maximum when the biofilm was not exposed to the compounds (Fig. 2D). However, the same got reduced significantly when the cells were treated with either tryptophan or 1,4-naphthoquinone (Fig. 2D). As expected, the highest reduction in metabolic activity was noticed when the cells were treated with both the compounds (Fig. 2D).
Combinatorial application of antibiofilm molecules significantly inhibited the biofilm formed by S. aureus. An equal number of the test bacteria was inoculated into freshly prepared LB media under the influence of different combinations of tryptophan (Trp) and 1,4-naphthoquinone (1,4-NQ). All the samples were incubated at 37 °C for 24 h. Post incubation, planktonic cells were discarded and the amount of biofilm cells were subjected to different experiments. A The extent of biofilm cells under different conditions was measured by performing CV assay. B The amount of total biofilm protein under different conditions was measured by Lowry assay. C The amount of EPS recovered from biofilm under different conditions was measured by phenol–sulfuric acid method. D The metabolic activity of biofilm under different conditions was measured by FDA assay. Error bars demonstrated mean ± standard deviation. The significant difference in the results with respect to control was illustrated by calculating the respective p values. The p values less than 0.05, 0.01, and 0.001 were marked with (*), (**), and, (***), respectively
Combinatorial application of the test compounds significantly inhibited the cell surface hydrophobicity of the test organism
To understand whether the cell surface hydrophobicity could influence the formation of biofilm of S. aureus, cells were grown under different combinations of the mentioned compounds. The highest level of cell surface hydrophobicity was observed in the cells that were not exposed to the test compounds (Fig. 3A). However, the results revealed that the cell surface hydrophobicity diminished significantly when exposed to the compounds either individually or in combination (Fig. 3A). The cell surface hydrophobicity was reduced by ~ 24% and ~ 14% when exposed to 1,4-naphthoquinone and tryptophan, respectively (Fig. 3A). The result also revealed the maximum (~ 49%) reduction in cell surface hydrophobicity when both the compounds were applied on the test organism (Fig. 3A). Thus, the result indicated that the test compounds were found to reduce the cell surface hydrophobicity of S. aureus substantially. To understand further, a fluorescence microscopic analysis was carried out in which we observed that the microbial colonization was found to be the maximum when the cells were not treated with any compounds (Fig. 3B). In this condition, several microbial clusters were noticed over the surface indicating the substantial development of biofilm. However, the extent of microbial clusters was reduced considerably when the cells were exposed to either tryptophan or 1,4-naphthoquinone or both (tryptophan and 1,4-naphthoquinone) (Fig. 3B). The maximum reduction in microbial colonization occurred when the cells were exposed to both tryptophan and 1,4-naphthoquinone (Fig. 3B). Thus, the observations so far revealed that the tested concentrations of the compound effectively inhibited the biofilm formation of S. aureus by attenuating the cell surface hydrophobicity.
Combinatorial application of the antibiofilm molecules inhibited the cell surface hydrophobicity of the bacteria that resulted in decreased microbial attachment. A Cell surface hydrophobicity measurement. An equal number of the test bacteria were inoculated into freshly prepared LB media under different combinations of tryptophan (Trp) and 1,4-naphthoquinone (1,4-NQ). Post incubation, BATH assay was followed to estimate the cell surface hydrophobicity of cells under different conditions. Error bars demonstrated the mean ± standard deviation. The significant difference in the results with the control set was illustrated by calculating the respective p values. The p values less than 0.05 and 0.01 were marked with (*) and (**), respectively. B Fluorescence microscopic image analysis. The microbial colonization of the test bacteria under the influence of different combinations of the antibiofilm molecules was analyzed by observing the acridine orange-stained cells under a fluorescence microscope
Combinations of tryptophan and 1,4-naphthoquinone showed efficient disintegration against the pre-existing biofilm of S. aureus
In this study, attempts have been made to show whether the combination of the compounds (tryptophan and 1,4-naphthoquinone) could effectively disintegrate the pre-existing biofilm of S. aureus. Thus, the pre-existing biofilm of S. aureus was formed as mentioned in the Methods and materials section. Thereafter, the pre-formed biofilm was exposed to different concentrations of 1,4-naphthoquinone and tryptophan individually as well as in combination and incubated for another 6 h. Post incubation, it was observed that ~ 60% of the biofilm got disintegrated when the pre-existing biofilm cells were exposed to 1,4-naphthoquinone alone (Fig. 4A). It was also noticed that ~ 50% biofilm disintegration took place when the pre-existing biofilm cells were exposed to tryptophan alone (Fig. 4A). However, the maximum biofilm disintegration (~ 70%) was observed when the two compounds were applied in combination on the pre-existing biofilm cells (Fig. 4A). To further validate the observations, total biofilm protein was measured under different conditions as it reflects the microbial load of the biofilm in a given population. We observed that tryptophan and 1,4-naphthoquinone showed biofilm disintegration independently as the protein recovery got decreased significantly under the exposure of the two compounds (Fig. 4B). However, the lowest recovery of protein was observed when the pre-existing biofilm was exposed to both tryptophan and 1,4-naphthoquinone (Fig. 4B). The maximum protein was recovered from the biofilm when it was not exposed to either tryptophan or 1,4-naphthoquinone (Fig. 4B). To further confirm this result, fluorescence microscopic image analysis was carried out, wherein the pre-existing biofilm was exposed to the different concentrations of the test compounds (individually as well as in combination). The fluorescence microscopic observation revealed that the cells which were not exposed to any of the test compounds exhibited the formation of substantial biofilm clusters (Fig. 4C). The biofilm clusters were seen to disperse efficiently in the presence of the different combinations of the compounds (Fig. 4C). Furthermore, the amount of eDNA associated with biofilm was measured under the presence and absence of the compounds. The results indicated that the least amount of eDNA was extracted from the pre-existing biofilm which was exposed to both the compounds (Fig. 4D). However, maximum amount of eDNA was extracted from the pre-existing biofilm which was not exposed to any compound (Fig. 4D). Thus, it could be suggested that the exposure of 1,4-naphthoquinone and tryptophan was found to reduce the amount of eDNA significantly that resulted in the disintegration of the pre-existing biofilm.
The combination of the antibiofilm molecules exhibited efficient disintegration of the pre-existing biofilm of S. aureus. The test bacteria were grown in sterile LB media at 37 °C for 24 h to develop biofilm over the surface. The developed biofilm was then exposed to the test compounds (tryptophan and 1,4-naphthoquinone) individually as well as in combination and further incubated at 37 °C for 6 h. After the incubation, the planktonic cells were discarded, washed with sterile double-distilled water, and adhered biofilm cells were dried effectively. A CV assay. The degree of the pre-existing biofilm of the test bacteria under the individual as well as combined form of the antibiofilm molecules (Trp and 1,4-NQ) were stained with CV solution. B Protein estimation. The total protein of the pre-existing biofilm of the test bacteria under different conditions was estimated by following the protocol of Lowry assay. C Fluorescence microscopic image analysis. The pre-existing biofilm of S. aureus was treated with either tryptophan (Trp) or 1,4-naphthoquinone (1,4-NQ) or both. A control set was also prepared in which the pre-exiting biofilm was not exposed to any test molecule. Thereafter, the difference in the biofilm aggregates under different conditions were analyzed under a fluorescence microscope. D eDNA estimation profile. The pre-existing biofilm on petri dishes was challenged with either Trp, 1,4-NQ or both for 6 h at 37 °C. A control set was also kept in which the pre-existing biofilm was not exposed to the test compounds. Thereafter, the eDNA was collected from the pre-existing biofilm under different conditions by abiding to the protocol mentioned in “Methods and materials” section. Error bars demonstrated the mean ± standard deviation. The significant difference in the results with the control set was illustrated by calculating the respective p values. The p values less than 0.05, 0.01, and 0.001 were marked with (*), (**), and, (***), respectively
The combinatorial study did not show any considerable antimicrobial activity against S. aureus
The antimicrobial activity of the tested concentrations of tryptophan and 1,4-naphthoquinone alone or in combination was determined against S. aureus by following the microbial viability. In this regard, S. aureus cells were inoculated in sterile LB supplemented with tryptophan (30 µg/mL) or 1,4-naphthoquinone (10 µg/mL) or both and incubated at 37 °C for 24 h. The result showed that the microbial viability remained similar for the cells that were either treated with tryptophan or 1,4-naphthoquinone or both (1,4-naphthoquinone and tryptophan) (Fig. 5). The result indicated that the combination of the two natural compounds, 1,4-naphthoquinone and tryptophan, could effectively inhibit as well as disintegrate the biofilm without affecting the growth profile of the test organism.
The tested concentrations of the antibiofilm molecules did not show antimicrobial activity. A similar number of cells of S. aureus were inoculated in several test tubes containing sterile LB media supplemented with either tryptophan (Trp) or 1,4-naphthoquinone (1,4-NQ) or both. A control set was also prepared where the cells were not exposed to any test compounds. After an incubation of 24 h at 37 °C, the microbial viability of the cells under different condition was determined. Error bars demonstrated the mean ± standard deviation. The significant difference in the results with the control set was illustrated by calculating the respective p values. The p value calculations revealed no contrasting difference between the treated and untreated samples hence marked with N.S. (non-significant)
Discussion
Drug resistance amongst pathogenic microorganisms has become a widespread phenomenon that might explain the persistence of bacterial infections even after the application of appropriate antimicrobial therapy (Balaban et al. 2019). In view of this, biofilm associated bacterial infections are becoming a major problem in the expanding population (Kiedrowski and Horswill 2011; Bjarnsholt 2013). S. aureus is one such Gram-positive bacterium that has been reported to significantly increase the morbidity and mortality, particularly when associated with biofilm infections (Moormeier and Bayles 2017). Therefore, novel approaches need to be adopted to combat such challenges. Two natural compounds, namely, 1,4-naphthoquinone and tryptophan, have been selected for this study and were tested against the biofilm profile of S. aureus. The results revealed that both the test compounds could exhibit efficient antibiofilm characteristics against S. aureus. However, to further increase the efficacy of the compounds, a combinatorial study was undertaken as literature revealed that combinatorial applications could enhance antibiofilm activities compared to their single application (Zhou et al. 2018). In this regard, a series of experiments were performed to test the efficacy of the two compounds in combination towards biofilm inhibition. Crystal violet (CV) assay has been found to be a well-reported assay which can be used to measure the extent of biofilm inhibition (Mukherjee et al. 2013) as well as the disintegration of pre-existing biofilm (Paul et al. 2021b) under different conditions. Thus, to test the extent of biofilm inhibition of S. aureus under the presence and absence of the compounds (tryptophan and 1,4-naphthoquinone), CV assay was undertaken as CV could bind to the biofilm cells as well as the EPS of biofilm. The result revealed that the individual effect of the compounds showed efficient antibiofilm activity. However, when the two compounds were combined together, the efficacy of the compounds towards biofilm inhibition got increased significantly. Furthermore, estimation of the amount of protein under the presence and absence of the compounds could also help us to understand the extent of microbial biofilm formed on a surface under different conditions (Kroukamp et al. 2010). Increased protein recovery is considered to be an indicator of robust biofilm formation, whilst a reduced protein recovery indicates poor biofilm formation (Kroukamp et al. 2010). We observed that the least amount of protein was recovered from the set which was treated with both tryptophan and 1,4-naphthoquinone. The maximum amount of biofilm was formed when S. aureus cells were not exposed to any concentrations of the test compounds. Thus, the CV assay and protein assay suggested that the mentioned compounds in combination could show the highest biofilm inhibition against S. aureus. Furthermore, extracellular polymeric substance (EPS) was reported to play a key role in holding the biofilm integrity (Flemming et al. 2007). Therefore, the amount of EPS was measured under the presence and absence of the mentioned compounds as it happens to be a major indicator of biofilm (Gupta et al. 2016). The least amount of EPS was recovered when the organism was exposed to both the compounds (tryptophan and 1,4-naphthoquinone). The maximum amount of EPS was recovered from the cells that were not exposed to any concentrations of the test compounds. Moreover, the increased secretion of EPS marks the formation of a matured biofilm (Flemming et al. 2007). In addition, biofilm oftentimes facilitates the discharge of different virulence factors including proteases while evading the host tissue (Granato et al. 2018). Microbial protease could hydrolyze the protein layer enabling the cells to enter the host tissue which resulted in the spreading of pathogenicity (Koo et al. 2017). Fluorescein diacetate is a broad spectrum substrate for a plethora of proteases, esterase, and lipase enzymes (Alef and Nannipieri 1995). Thus, in this study, to understand the role of the test compounds on the secretion of enzyme produced by biofilm, we performed the FDA assay of the microorganisms growing under different conditions. The result indicated that the combinations of the compounds at the selected concentrations might weaken the pathogenicity by inhibiting the secretion of different hydrolytic enzymes including protease from the test organism. Thus, it can be stated that the combination of the two compounds significantly increased the efficacy of biofilm inhibition of S. aureus.
Cell surface hydrophobicity constitutes one of the most important key factors of microbial cell surface colonization (Pompilio et al. 2008; Tribedi and Sil 2014). In this connection, cells with higher cell surface hydrophobicity showed better colonization and cells with lower cell surface hydrophobicity showed compromised microbial colonization (Sarker et al. 2020). Thus, to elucidate the mechanism behind the biofilm inhibition of S. aureus, the cell surface hydrophobicity was measured under the presence and absence of the test compounds. The result revealed that when the compounds (tryptophan and 1,4-naphthoquinone) were individually added to S. aureus cells, the cell surface hydrophobicity of the bacteria was decreased. However, when the two compounds were applied in combination, the reduction in cell surface hydrophobicity was found to be even greater. This result was further validated by fluorescence microscopic analysis. Fluorescence microscopic study helps in visualization of bacterial adherence on any given surface (Hannig et al. 2007). Thus, with the help of fluorescence microscopic analysis, several biofilm clusters were observed when S. aureus cells were not exposed to any test compounds. However, when the same set was exposed to the test compounds in combination, the biofilm clusters were decreased substantially. Thus, the reduced cell surface hydrophobicity of S. aureus under the combined effect of the two compounds could inhibit the development of biofilm. In this study, attempts have also been made to show whether the combination of the compounds (tryptophan and 1,4-naphthoquinone) could effectively disintegrate the pre-existing biofilm of S. aureus. In this regard, it was observed that the combination of the two compounds could effectively disintegrate the pre-existing biofilm of S. aureus. Furthermore, literature report revealed that extracellular DNA (eDNA), which is a major component of the EPS matrix, could play a very crucial role in keeping the biofilm architecture (Das et al. 2013). The reduction in the amount of eDNA was found to exhibit effective disintegration of the pre-existing biofilm (Okshevsky and Meyer 2015). The results indicated that the least amount of eDNA was extracted from the pre-existing biofilm which was exposed to both the compounds. However, the maximum amount of eDNA was extracted from the pre-existing biofilm which was not exposed to any compound. Taken together, it could be stated that the combination of the compounds not only inhibited the biofilm formation of S. aureus but also efficiently disintegrated the pre-existing biofilm considerably.
An important characteristic of a promising antibiofilm agent is to show efficient antibiofilm activity at a concentration or concentrations which does not show any harm to the concerned microorganism (Das et al. 2016). The antimicrobial activity of the tested concentrations of tryptophan and 1,4-naphthoquinone alone or in combination was determined against S. aureus by following the microbial viability assay. It was observed that the tested concentrations of the compounds individually as well as in combination did not exhibit any antimicrobial activities. Thus, these compounds in combination may be recommended to manage the biofilm-linked threats of S. aureus.
Conclusion
This study demonstrably establishes the combination of bioactive compounds, namely, 1,4-naphthoquinone and tryptophan as an effective means to limit biofilm formation as well as induce disintegration of pre-existing biofilms. Since biofilm formation constitutes an important strategy for mediating microbial pathogenesis as well as a vital mechanism for antibiotic resistance amongst pathogenic microorganisms including S aureus, effective management and premature disintegration of microbial biofilm is the imperative need of the hour in the context of public health management. Furthermore, this strategy can be used in conjunction with application of conventional antibiotics to develop a comprehensive integrated approach for smart management of biofilm mediated microbial pathogenesis.
Availability of data and material
The datasets produced during the present study are available from the corresponding author based on the logical request.
Code availability
Minitab 19 (trial version) was used to analyze the statistical observations.
References
Abreu AC, Saavedra MJ, Simões LC, Simões M (2016) Combinatorial approaches with selected phytochemicals to increase antibiotic efficacy against Staphylococcus aureus biofilms. Biofouling 32:1103–1114
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soc Sci Med 33:943–951
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London 1–576
Azmi K, Qrei W, Abdeen Z (2019) Screening of genes encoding adhesion factors and biofilm production in methicillin resistant strains of Staphylococcus aureus isolated from Palestinian patients. BMC Genom 20:1–12
Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C (2019) Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS 121:1–58
Chakraborty P, Dastidar DG, Paul P, Dutta S, Basu D, Sharma SR, Basu S, Sarker RK, Sen A, Sarkar A, Tribedi P (2020) Inhibition of biofilm formation of Pseudomonas aeruginosa by caffeine: a potential approach for sustainable management of biofilm. Arch Microbiol 202:623–635
Chakraborty P, Paul P, Kumari M, Bhattacharjee S, Singh M, Maiti D, Dastidar DG, Akhter Y, Kundu T, Das A, Tribedi P (2021) Attenuation of Pseudomonas aeruginosa biofilm by thymoquinone: an individual and combinatorial study with tetrazine-capped silver nanoparticles and tryptophan. Folia Microbiol 66:255–271
Chen X, Stewart PS (2000) Biofilm removal caused by chemical treatments. Water Res 34:4229–4233
Das T, Sehar S, Manefield M (2013) The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ Microbiol Rep 5:778–786
Das MC, Sandhu P, Gupta P, Rudrapaul P, De UC, Tribedi P, Akhter Y, Bhattacharjee S (2016) Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: a combinatorial study with azithromycin and gentamicin. Sci Rep 6:23347
Di Martino P (2018) Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol 4:274–288
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells.” J Bacteriol 189:7945–7947
Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 5:e02192
Granato ET, Ziegenhain C, Marvig RL, Kümmerli R (2018) Low spatial structure and selection against secreted virulence factors attenuates pathogenicity in Pseudomonas aeruginosa. ISME J 12:2907–2918
Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P (2016) Biofilm, pathogenesis and prevention-a journey to break the wall: a review. Arch Microbiol 198:1–15
Gupta P, Sarkar A, Sandhu P, Daware A, Das MC, Akhter Y, Bhattacharjee S (2017) Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. J Appl Microbiol 123:246
Hannig C, Hannig M, Rehmer O, Braun G, Hellwig E, Al-Ahmad A (2007) Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch Oral Biol 52:1048–1056
Ibis C, Tuyun AF, Ozsoy-Gunes Z, Bahar H, Stasevych MV, Musyanovych RY, Komarovska-Porokhnyavets O, Novikov V (2011) Synthesis and biological evaluation of novel nitrogen- and sulfur-containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur J Med Chem 46:5861–5867
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15:740–755
Kroukamp O, Dumitrache RG, Wolfaardt GM (2010) Pronounced effect of the nature of the inoculum on biofilm development in flow systems. Appl Environ Microbiol 76:6025–6031
Kiedrowski MR, Horswill AR (2011) New approaches for treating Staphylococcal biofilm infections. Ann NY Acad Sci 1241:104–121
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martínez MJA, Benito PB (2005) Biological activity of quinones. Stud Nat Prod Chem 30:303–366
Mishra R, Panda AK, De Mandal S, Shakeel M, Bisht SS, Khan J (2020) Natural antibiofilm agents: strategies to control biofilm-forming pathogens. Front Microbiol 11:566325
Moormeier DE, Bayles KW (2017) Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol 104:365–376
Mukherjee K, Tribedi P, Mukhopadhyay B, Sil AK (2013) Antibacterial activity of long-chain fatty alcohols against Mycobacteria. FEMS Microbiol Lett 338:177–183
Mallavadhani UV, Prasad CV, Shrivastava S, Naidu VG (2014) Synthesis and anticancer activity of some novel 5, 6-fused hybrids of juglone based 1,4-naphthoquinones. Eur J Med Chem 83:84–91
Okshevsky M, Meyer RL (2015) The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol 41:341–352
Pachori P, Gothalwal R, Gandhi P (2019) Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis 6:109–119
Paharik AE, Horswill AR (2016) The Staphylococcal biofilm: adhesins, regulation, and host response. Microbiol Spectr 4:529–566
Paul P, Chakraborty P, Chatterjee A, Sarker RK, Dastidar DG, Kundu T, Sarkar N, Das A, Tribedi P (2021a) 1,4-Naphthoquinone accumulates reactive oxygen species in Staphylococcus aureus: a promising approach towards effective management of biofilm threat. Arch Microbiol 203:1183–1193
Paul P, Das S, Chatterjee S, Shukla A, Chakraborty P, Sarkar S, Maiti D, Das A, Tribedi P (2021b) 1, 4-Naphthoquinone disintegrates the pre-existing biofilm of Staphylococcus aureus by accumulating reactive oxygen species. Arch Microbiol 203:4981–4992
Pompilio A, Piccolomini R, Picciani C, D’Antonio D, Savini V, Di Bonaventura G (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287:41–47
Rosenberg M, Perry A, Bayer EA, Gutnick DL, Rosenberg E, Ofek I (1981) Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect Immun 33:29–33
Sarker RK, Chakraborty P, Paul P, Chatterjee A, Tribedi P (2020) Degradation of low-density poly ethylene (LDPE) by Enterobacter cloacae AKS7: a potential step towards sustainable environmental remediation. Arch Microbiol 202:2117–2125
Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R (2014) Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals 42:1–7
Sahu PK, Iyer PS, Oak AM, Pardesi KR, Chopade BA (2012) Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. Sci World J 2012:1–10
Tribedi P, Sil AK (2014) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol 116:295–303
Tribedi P, Gupta AD, Sil AK (2015) Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresour Bioprocess 2:1–10
Tandon VK, Maurya HK, Yadav DB, Tripathi A, Kumar M, Shukla PK (2006) Naphtho [2,3-b][1,4]-thiazine-5,10-diones and 3-substituted-1,4-dioxo-1,4-dihydronaphthalen-2-yl-thioalkanoate derivatives: synthesis and biological evaluation as potential antibacterial and antifungal agents. Bioorg Med Chem Lett 16:5883–5887
Wang W, Cheng CWT, Zhang Q (2022) 1, 4-Naphthoquinone Analogs and Their Application as Antibacterial Agents. Chemistry Select 7:e202203330
Xu Z, Liang Y, Lin S, Chen D, Li B, Li L, Deng Y (2016) Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr Microbiol 73:474–482
Zhou Y, Kong Y, Kundu S, Liang CJD, H, (2012) Antibacterial activities of gold and silver nanoparticles against Escherichia coli and Bacillus Calmette-Guerin. J Nano Biotechnol 10:1–9
Zhou JW, Hou B, Liu GY, Jiang H, Sun B, Wang ZN, Shi RF, Xu Y, Wang R, Jia AQ (2018) Attenuation of Pseudomonas aeruginosa biofilm by hordenine: a combinatorial study with aminoglycoside antibiotics. Appl Microbiol Biotechnol 102:9745–9758
Acknowledgements
The authors would like to express their sincere regards to Dr. Amlan Das (Research and Development Project Manager, National Institute of Biomedical Genomics, Kalyani, WB India), for sharing 1,4-naphthoquinone with us to perform the required research work. The authors would also express their sincere thanks to Dr. Anirban Das Gupta (Assistant Professor, Department of Biotechnology, The Neotia University) for correcting the language of the manuscript.
Author information
Authors and Affiliations
Contributions
PP, RR, SD, SS, SC, MM, AS, and PC performed the experiments, analyzed the results, and wrote the manuscript. PT conceived the idea, designed the experiments, analyzed the results, and rearranged the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paul, P., Roy, R., Das, S. et al. The combinatorial applications of 1,4-naphthoquinone and tryptophan inhibit the biofilm formation of Staphylococcus aureus. Folia Microbiol 68, 801–811 (2023). https://doi.org/10.1007/s12223-023-01054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-023-01054-y