Abstract
Yarrowia lipolytica lipase obtained by solid-state fermentation was characterized and applied in the synthesis of esters with commercial value in the food industry. The effect of different conditions on the hydrolysis activity of this biocatalyst was evaluated in the presence of metal ions, solvents, detergents, several pH and temperature parameters, and different substrates. Storage stability was also studied. The solid biocatalyst produced in soybean meal was used in synthesis reactions aiming to produce short-, medium-, and long-chain esters. Results showed that the best fermentation condition to produce the biocatalyst was using soybean oil (3% w/w), moisture content (55% w/v), and inoculum of 2.1 mgdry biomass/gsoybean meal at 28 °C for 14 h. High substrate conversion for ethyl octanoate, cetyl stearate, and stearyl palmitate synthesis was achieved in the presence of non-polar solvents in less than 6 h using a substrate molar ratio of 1:1 at 38 °C with 10–15% (w/v) of biocatalyst. This work showed the high potential of Y. lipolytica lipase to be used in the synthesis of different esters. Also, that it can be considered an attractive and economical process alternative to obtain high-added value products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several types of esters are employed daily in different food industry sectors, being directly used in food or beverage formulations or even indirectly as adjuvants in food-packaging materials. Short and medium alkyl esters present high relevance as flavoring and aroma enhancers once they confer fruity or herbal flavor to food products and can be applied to formulate confectionery and dairy products, ice creams, cakes, alcoholic, and soft drinks, for example. These molecules are usually synthesized by acids and alcohols with two to eight carbon atoms [1, 2].
The global market of flavors and fragrances is constantly growing, being valued in 2015 at US$26.0 billion with perspectives to reach US$37.0 billion in 2021, at a compound annual growth rate of 6.4% for 2016–2021. Moreover, it has been pointed out that the beverage sector was the largest global end-use market for flavors in 2015 with a share of 34%, followed by the dairy industry with 13% [3, 4]. Considering the high demand for these compounds, a search for eco-friendly production might aid to decrease the carbon footprint in the synthesis process.
Currently, most synthesized flavor esters are obtained by a chemical pathway that uses toxic solvents and catalysts and presents lack of substrate selectivity with formation of by-products that must be removed, increasing downstream steps. These flavors are also labeled as “artificial esters” [5, 6]. The extraction of flavor esters from vegetable sources is another option, but it needs a high amount of raw materials, which depends on seasonal and climatic conditions, making it expensive for industrial applications on a large scale [7, 8]. As defined by US and European legislation, a natural flavor can be considered “natural” if obtained from a natural source using a physical process or by microbial transformation or enzymatic synthesis employing precursors isolated from nature [9, 10].
Wax esters are constituted by long-chain fatty acids and alcohols linked by an ester bond and are found in natural sources such as beeswax, sperm oil, jojoba oil, carnauba wax, and sheep wool, for example [11]. These compounds are composed of unsaturated or saturated wax esters that are highly hydrophobic and neutral lipids [12, 13]. They have high economical relevance since they can be applied in several fields of industry including cosmetics, printing inks, lubricants, and food.
Concerning the use of wax esters in the food industry, specific ones (stearyl palmitate and cetyl stearate [14]) can be used as plasticizers or lubricants and adjuvants in food-packaging materials and their use is regulated by the Food and Drug Administration (FDA) and according to the Code of Federal Regulations (CFR), title 21, part 178, §178.3450.14. Furthermore, wax esters are mainly obtained by chemical synthesis and extraction from natural sources, sharing the same disadvantages and barriers found in the production of flavor esters by these traditional ways. As an alternative, enzymatic synthesis could be an interesting option to lead natural wax ester production [15, 16]. Moreover, lubricants derived from renewable sources present rapid biodegradability, low eco-toxicity, and can be labeled as biolubricants, increasing consequently the added value [17, 18].
The enzymatic synthesis of flavor and wax esters is accelerated by lipases (triacylglycerol hydrolase, E.C. 3.1.1.3). This biocatalyst is largely employed in esterification reactions in non-aqueous medium presenting high specificity and selectivity for the substrate [19]. Several works about the production of these molecules have been reported in literature. Garlapati and Banerjee [20] showed the production of flavor esters using lipase from Rhizopus oryzae NRRL 3562 covalently immobilized on activated silica. Ghamgui et al. [21] studied the production of isoamyl acetate (banana flavor) using lipase from Staphylococcus simulans in a solvent-free system. Another work carried out by Grosso et al. [22] showed the high catalytic activity of lipase from R. oryzae and immobilized in polyurethane foams.
Other studies also demonstrated the production of wax esters using lipase as a biocatalyst. Li et al. [23] used lipase from Candida sp. 99–125 on cetyl oleate synthesis in a solvent-free medium which shared similar physical and chemical characteristics with jojoba oil, an important substitute of spermaceti oil once it presents similar properties. Cetyl octanoate, a 24-carbon wax ester, was synthesized using Novozyme 435 from Candida antarctica in supercritical carbon dioxide achieving a yield of 99.5% [24]. In a work developed by Guncheva et al. [25], stearyl stearate was produced by an immobilized lipase from Bacillus stearothermophilus MC7, being one of the few works reporting a wax ester possible to be used in the food industry as an adjuvant with the purpose of lubrication or plasticizing allowed by FDA.
Most studies on the synthesis of flavor and wax esters have been performed using extracellular lipases due to their easy recovery after production and because they are more extensively studied. However, the use of whole cells displaying lipases on their surface presents advantages since they are naturally immobilized and steps of isolation and purification are not necessary, reducing production costs [26, 27]. Several studies have used whole cells as biocatalysts for ester production, demonstrating their efficiency [6, 28, 29].
Lipases can be obtained by submerged fermentation (SmF) or solid-state fermentation (SSF) and one of the advantages of SSF in lipase production is the possibility to use agro-industrial wastes and co-products as a support and a substrate for microbial growth. Since this process uses low-cost materials and is conducted in the absence or low content of free water, its cost is lower than that of SmF [30]. Several studies have reported lipase production by SSF using agro-industrial feedstocks by different microorganisms such as Aspergillus ibericus [31], Yarrowia lipolytica [32], Penicillium simplicissimum [33], Burkholderia cenocepacia [34], and Penicillium camemberti [35]. However, few works were on ester flavor and wax ester synthesis, most of them focused biodiesel production. Boratyński et al. [36] also showed the application of SSF using several lipase-producing filamentous fungi aiming at the production of enantiomerically enriched and pure aroma compounds used in the food industry based on kinetic resolution. The microbial kinetic resolution of racemic lactones and a volatile racemic ester (1-phenylethyl acetate) in the SSF medium was obtained, showing the potential of SSF to be applied in biotransformation processes using alternative feedstocks.
In the present study, a lipase from Y. lipolytica produced by SSF using soybean meal was applied to perform the synthesis of several flavor esters and biolubricant wax esters such as stearyl palmitate and cetyl stearate. Y. lipolytica is a non-conventional yeast with generally regarded as safe (GRAS) status and intense secretory activity, where lipase is one of most important products [37, 38]. To our knowledge, there are no reported works using lipase of Y. lipolytica produced by SSF aimed at the production of these kinds of esters. Furthermore, an extensive biochemical characterization of the biocatalyst was carried out for a better comprehension of its activity.
Materials and Methods
Microorganism and Inoculum Preparation
The Y. lipolytica IMUFRJ50682 wild strain used in this work was previously isolated from an estuary in Guanabara Bay, Rio de Janeiro, Brazil [39]. It was stored at 4 °C on YPD (w/v: yeast extract 1%; peptone, 2%; glucose, 2%) agar medium. For the pre-inoculum, cells were cultivated at 28 °C in a rotary shaker at 160 rpm for 72 h, in 500 mL flasks containing 200 mL YPD medium.
Production of a Solid Biocatalyst by Y. lipolytica in Soybean Meal
The soybean meal used in the solid-state fermentation was obtained from soybean oil extraction with hexane. This feedstock was initially grounded in a laboratory mill and then separated in a sieve where only particles presenting size ≤ 1.18 mm were recovered. Cylindrical polypropylene bioreactors containing 10 g of soybean meal covered with hydrophobic fabric were used to carry out fermentation. Bioreactors were sterilized at 120 °C for 20 min and then moisture was adjusted (w/v). In the present work, the fermentation process was performed as follows: 14 h of fermentation, 55% of moisture (w/v), 1.5% soybean oil (w/w), and inoculum concentration of 0.7 mgdry biomass/gsoybean meal. All fermentations were performed in an incubator chamber with saturated humidity at 28 °C [32]. After the fermentation process, the fermented solids were submitted to a lyophilization process for 72 h to remove all water content from the feedstock, thus obtaining the solid biocatalyst. In this work, all assays of biochemical characterization and ester syntheses were carried out using the dry solid fermented soybean meal rich in Y. lipolytica lipases.
Lipase Activity Determination
Lipase activity was determined according to the method proposed by Freire et al. [40]. Olive oil was used as substrate (5% w/v) and was emulsified with Arabic gum (5% w/v) in phosphate buffer (100 mM, pH 7.0). The lipase solid biocatalyst (0.5 g) was added to 19 mL of emulsion and incubated for 20 min and 200 rpm in a rotatory shaker at 37 °C. Reactions were stopped using 20 mL of acetone-ethanol solution (1:1 v/v) and 0.04 mol/L NaOH was applied to titrate the free fatty acids released in an automatic titrator (Metrohm 916 Ti-Touch) up to a final pH value of 11.0. The enzymatic activity was defined as the amount of enzyme that produces 1 μmol of fatty acid per minute, under the assay conditions. Enzyme activity was expressed as units per gram of initial dry weight of soybean meal. Control assays (blanks) were carried out adding the acetone-ethanol solution (1:1 v/v) prior to the enzyme extract for its inactivation. The following equation (Eq. 1) was used to calculate relative lipase activity (%) of the solid biocatalyst:
Effect of Metal Ions and Inhibitors
The influence of cations (Na+, Ca2+, Mn2+, Hg2+, Mg2+, and Fe3+) and inhibitors such as phenylmethylsulfonyl fluoride (PMSF), ethylenediamine tetraacetic acid (EDTA), and β-mercaptoethanol (β-met) were investigated on the lipase activity of the solid biocatalyst in different concentrations. All these additives were added during the hydrolysis reaction described in the “Lipase Activity Determination” section to measure lipase activity. A control without these additives was carried out and defined as 100%.
Effect of Organic Solvents and Detergents
Various organic solvents (9% v/v) were tested to measure the lipase activity of the solid biocatalyst. Detergents in three different concentrations were also used to study their influence on solid biocatalyst hydrolysis reaction. All organic solvents and detergents were added during the hydrolysis reaction described in the “Lipase Activity Determination” section to measure lipase activity. A control without organic solvents was carried out and defined as 100%.
Effect of Temperature and pH
Lipase activity of the solid biocatalyst was evaluated in different conditions of temperature (25, 37, and 50 °C) and pH. Acetate buffer (pH 3), potassium phosphate buffer (pH 4 to 8), and glycine buffer (pH 9) were used at a concentration of 100 mM. Olive oil was used as a substrate and the hydrolysis reaction was performed as described in the “Lipase Activity Determination” section to measure lipase activity.
Biocatalyst Thermostability
Solid biocatalyst thermostability was determined by incubating this biocatalyst in the presence of 100 mM potassium phosphate buffer and ethanol for up to 48 h at 37, 50, and 70 °C. After incubation time, lipase activity was measured as described in the “Lipase Activity Determination” section. Control was performed by mixing the biocatalyst with ethanol or phosphate buffer and without submitting to the incubation time evaluated. It was defined as 100%.
Solid Biocatalyst Storage Stability
Storage stability of the solid biocatalyst was determined at three different temperatures (− 10, 4, and 28 °C), for a period of 330 days. Samples were evaluated for their lipase activity in different periods of time. Olive oil was used as a substrate and the hydrolysis reaction was performed as described in “Lipase Activity Determination” section to measure lipase activity. Lipase activity of the biocatalyst before the first day of storage was defined as 100%.
Substrate Specificity Using Different Vegetable Oils, Animal and Vegetable Fat, and Synthetic Triacylglycerols
A wide range of different substrates was evaluated to study the enzyme activity of Y. lipolytica lipase. Natural sources of triacylglycerols (TAGs) (vegetable oils and lard 5% w/v) were evaluated. Olive oil was used as control and defined as 100%. Tributyrin (C4:0), tricaproin (C6:0), tricaprylin (C8:0), tripalmitin (C16:0), tristearin (C18:0), and triolein (C18:1) (Sigma-Aldrich) at 5.6 mM in potassium phosphate buffer (100 mM) were used as synthetic substrates [41]. Tricaprylin (C8:0) was used as control and defined as 100%. Lipase activity was measured according to the method described in the “Lipase Activity Determination” section.
Ester Synthesis
Ester syntheses were performed in sealed bioreactors with a temperature control water jacket attached to a water bath set at 38 °C. Substrate molar ratio in the presence of organic solvents was 1:1 at a molar concentration of 500 mM for flavor ester and 375 mM for wax ester reactions at a final volume of 15 and 20 mL, respectively. Solid biocatalyst amounts of 15% and 10% (w/v) were employed in the synthesis of flavor and lubricant esters, respectively. Moreover, synthesis reactions of wax esters were also evaluated in the presence of heptane and tert-butanol. Periodically, samples (100 μL) in triplicate were withdrawn and diluted in ethanol to study the progress of esterification reactions by acid consumption measurement by titration using 0.02 M NaOH in an automatic titrator (Metrohm 916 Ti-Touch). The percent of acid conversion (%) was measured using a relation between the number of moles of acid reacted (consumed) and the initial number of moles of acid in the reaction.
The titration method accuracy was tested and confirmed by the determination of ester conversion on gas chromatograph (Shimadzu, gas chromatograph mass spectrometer (GCMS)-QP2010 Ultra) for the chosen esters studied in the preliminary analyses and for the best reaction conditions found. Furthermore, synthesis reactions in the absence of organic solvents were evaluated for the selected esters at a molar ratio of 1:1 at 38 °C for ethyl octanoate and 55 °C for stearyl palmitate and cetyl stearate.
Improvement in Solid Biocatalyst Production Aiming to Optimize Ester Synthesis
Aiming to reduce the time of ester synthesis and maintaining high yield esterification, five different approaches were evaluated to produce the solid biocatalyst as evidenced in Table 1. All assays were initially tested under cetyl stearate production. Each assay had the objective to observe in which conditions high amount of lipases could be produced with the promotion of a rapid ester synthesis.
Assays 1 and 4 tested different solid biocatalysts produced with an exogenous cell load from SSF and SmF, respectively. The solid biocatalyst produced in Assay 1 received, after its fermentation, a load of Y. lipolytica cells (5 g/L) previously grown in SSF carried out in the same conditions described in control. These cells were extracted using potassium phosphate buffer (50 mM) and concentrated until reaching a suspension of 5 g/L. Then, this cell suspension was added with the fermented soybean meal produced as described in Assay 1 and homogenized. Thereafter, the homogenized material was lyophilized for 72 h.
In Assay 4, Y. lipolytica cell suspension (10 g/L) was obtained after recovering cells from YPD medium (w/v: yeast extract 1%; peptone, 2%; glucose, 2%). Cells were grown in YPD medium for 72 h at 28 °C in a rotary shaker at 160 rpm in 500 mL flasks. These cells were obtained by centrifugation (3000 rpm for 3 min) and concentrated until reaching a suspension of 10 g/L. Posteriorly, cell suspension was mixed to the fermented soybean meal and lyophilized for 72 h for solid biocatalyst production.
Assays 2 and 3 were performed varying the inoculum and soybean oil concentration of SSF using soybean meal. Fermentation control assay was prepared as described in the “Production of a Solid Biocatalyst by Y. lipolytica in Soybean Meal” section. All fermentations performed in this work were carried out using 55% of moisture (m/v) and temperature of 28 °C.
Reutilization of Biocatalyst in Ester Synthesis
In the best reaction conditions found, the solid biocatalyst was evaluated considering its consecutive reutilization in ethyl octanoate, stearyl palmitate, and cetyl stearate synthesis. The reutilization was conducted by direct removal of the reaction medium from the bioreactor and thereafter a new load of substrate at the same molar ratio (1:1) was employed to perform a new reaction. All reactions were conducted using hexane.
Gas Chromatography/Mass Spectrometry
A Shimadzu (GCMS-QP2010 Ultra) gas chromatograph equipped with a hydrogen flame ionization detector (FID) and a non-polar Restek RTX-1 silica capillary column (internal diameter, 0.25 mm; length, 30 m) was used to evaluate ester synthesis reactions.
The synthesis of ethyl octanoate was monitored analyzing the amount of octanoic acid consumed during the reaction time. Ethyl decanoate was used as internal standard to calculate the concentrations of the consumed octanoic acid. Temperature of injector and detector was set at 250 °C, and a split ratio of 1:20 was applied. Initially, the oven temperature was set at 50 °C and then increased up to 180 °C at a rate of 15 °C/min, held for 5 min, and then increased to 250 °C at a rate of 20 °C/min. The carrier gas was helium. Retention times of octanoic acid and ethyl octanoate were 8.4 and 8.8 min, respectively. All analyses were performed in triplicate.
To evaluate the synthesis of stearyl palmitate and cetyl stearate, the amount of alcohol consumed during the reaction time was considered. Ethyl decanoate was used as internal standard to calculate the concentration of the alcohol consumed. Temperatures of injector and detector were set at 300 and 320 °C, respectively. Splitless mode was employed. Initially, the oven temperature was set at 120 °C, then increased up to 280 °C at a rate of 15 °C/min and held for 5 min, and then increased to 295 °C at a rate of 2 °C/min and held for 60 min. The carrier gas was helium. Retention times of cetyl alcohol and stearyl alcohol were 10.2 and 11.9 min, respectively, and for stearyl palmitate and cetyl stearate, they were 65.5 and 65.7 min, respectively. All analyses were performed in triplicate.
Product formation from esterification reactions (ethyl octanoate, stearyl palmitate, and cetyl stearate) was confirmed by mass spectrometry analysis in a Shimadzu (GCMS-QP2010 Ultra). Compound identification was performed comparing their mass spectra with those of NIST library. Mass spectrometer ion source was set at a voltage of 70 eV and mass spectral ionization was set at 300 °C. The other analysis parameters were the same as described above.
Native-PAGE and Zymogram
Native-PAGE and zymogram were performed using the lipase crude extract obtained from the fermented soybean meal according to the method used by Souza et al. [32]. Polyacrylamide gel electrophoresis was conducted with known protein concentrations previously determined by Bradford [42] protein assay in a non-denaturing gel according to Laemmli [43] using a 12% separating gel and a 4% stacking gel. Zymogram was carried out using as substrate α-naphthyl acetate, a non-specific substrate able to be hydrolyzed by esterases and lipases. After native-PAGE, electrophoresis gels were incubated in a solution (100 mM phosphate buffer) of α-naphthyl acetate and Fast Blue RR, where naphthol compound released from enzymatically hydrolyzed substrate formed a complex with Fast Blue RR revealing brown bands in the gel. After this step, the gel was stained with Coomassie Blue R-250 to reveal other protein bands.
Scanning Electron Microscopy
The fermented soybean meal in different times and conditions and the non-fermented soybean meal were evaluated for fungal growth using a scanning electron microscope (Quanta FEG 250) at 15 kV. Samples were previously lyophilized for 72 h to remove all water content. After this process, they were placed on a double-sided conducting adhesive tape, fixed in aluminum stubs, and metalized with platinum for 180 s in high vacuum. Scanning electron microscopy was conducted in the Laboratory of Scanning and Transmission Electron Microscopy from the Military Engineering Institute (Rio de Janeiro, Brazil).
Results and Discussion
Influence of Metal Ions and Inhibitors on Solid Biocatalyst Activity
The lipolytic activity of the solid biocatalyst was investigated in the presence of several metal ions since it is known that they might influence the hydrolysis ability of several lipases and also esterases. According to Table 2, the solid biocatalyst was not affected in the presence of Mg2+ and NaCl in none of the concentrations tested. On the other hand, it was highly affected by1 mM Hg2+. Fe3+ (1 and 10 mM) also negatively affected lipase activity. Similar characteristics have been reported in other studies where Rhizopus chinensis lipase presented total inhibition by 10 mM Hg+2 and was strongly affected by Fe2+ and Penicillium sp. DS-39 lipase was significantly affected by 1 mM Hg2+ with relative activity of 26.8%, similar to reported in the current work, and it can be attributed to the reduction of key cysteine residues that are strongly oxidized by metal ions such as Fe2+/3+, Zn2+, Hg2+, Cd2+, and Cu2+ which could lead to an alteration of enzyme conformation [44,45,46].
In contrast, Sheng et al. [47] showed a psychrotrophic Y. lipolytica lipase which conserved 74% of activity in the presence of 5 mM Hg2+ and had its activity enhanced by 5 mM Fe3+. In the same way, Pseudomonas aeruginosa PseA lipase also showed 32% higher relative activity compared to control when exposed to 1 mM of Fe3+ [48]. Mn2+ and Ca2+ at 10 and 100 mM concentrations showed a reduction of lipase activity. However, 1 mM Ca2+, among all metal ions tested, provided a slight lipase activity increase of solid biocatalyst. Some authors have reported the activation of Y. lipolytica lipases in the presence of Ca2+. It has been shown that many lipases have a Ca2+ binding motif around the catalytic site and distinct role can be attributed, as a complex formation of enzymes-calcium ion, leading to more rigid and stable structure changes in reaction equilibrium by interacting with the organic acids [49, 50].
The inhibitors tested, PMSF and β-mercaptoethanol, did not affect lipase activity or slightly affected it. Lipases produced by Y. lipolytica present a serine residue at the active site. However, PMSF, a serine inhibitor, did not affect the solid biocatalyst activity. Some authors attribute this characteristic to the lid present in lipases, a hydrophobic domain that covers the active site and protects it from PMSF inhibitory effect [51]. Nevertheless, there are reports of negative influence of PMSF on lipase activity of Thermosyntropha lipolytica [52] and Y. lipolytica MSR80 (LIP9), indicating the bound of this inhibitor to serine residue in the catalytic site of these enzymes which consequently leads to its inactivation [53].
Y. lipolytica lipases are composed of cysteine residues that maintain their tridimensional structure, according to Fickers et al. [54]. LIP2, LIP7, and LIP8 proteins contain eight conserved cysteine residues, which may form disulfide bridges, but β-mercaptoethanol was unable to interfere on solid biocatalyst activity. This lipase activity could be preserved because of the environmental protection provided by soybean meal, since it was produced by SSF, or because disulfide bonds would be inaccessible to the reducing agent. Chelating agent EDTA at 1 mM did not affect lipase activity but at 10 mM reduced it to 41.2 ± 4.4 of relative activity (%). Since the solid biocatalyst is formed by a pool of lipases secreted and attached to the cell wall, it is possible to have metalloenzymes and other non-metal-dependent enzymes. Syal and Gupta [55] demonstrated that Y. lipolytica LIP7, LIP13, and LIP15 showed activity reduced by the chelating agent, whereas LIP4 and LIP5 were stable. However, further studies should be carried out to identify which lipases are being expressed by Y. lipolytica IMUFRJ 50682 when it is grown in SSF medium.
Effect of Detergents on Solid Biocatalyst Activity
In the presence of detergents, lipase solid biocatalyst showed slight activation by Tween 80 and Tween 20 at 0.001%, but when 0.01% of Triton X-100 was used, similar activity was found compared to the control (Table 2). Since this value represents 0.17 mM of Triton X-100, it is possible to note it is slightly below the critical micellar concentration (CMC) value of this detergent (CMC: Triton X-100 = 0.22–0.24 mM) contributing to lipase stability. Some authors have reported lipase stability in the presence of Triton X-100 as showed by Yu et al. [56] using Lip2 from Y. lipolytica which presented stability when 0.1% of this detergent was evaluated. Furthermore, in previous works, Triton X-100 was able to enhance lipase activity as demonstrated by Glogauer et al. [57] using 0.1% of this detergent reaching relative lipase activity 14.1% higher than control.
The solid biocatalyst lipase activity was drastically affected by the higher concentration of detergents assayed in this work. It should be attributed to detergent concentration above of its CMC (CMC: SDS = 8.2 mM, Tween 80 = 0.01 mM, and Tween 20 = 0.06 mM). In the same way of the results presented in this work, Aloulou et al. [58] showed for Y. lipolytica and Thermomyces lanuginosus lipases a decrease or inactivation of their hydrolytic activity in the presence of Triton X-100 and SDS when using detergent concentration above its CMC. It could be related to bind preventing between the lipase and the lipid substrate interface where micelle formation could avoid the complex enzyme-substrate formation. Yu et al. [56] showed a decrease of approximately 50% of relative activity in the presence of 1 mM SDS.
Glogauer et al. [57] also presented lipase activity inhibition by Triton X-100 at 1% and Tween 20, 40, and 60 at 0.1%. Golaki et al. [59] showed that Triton X-100 and Tween 20, 40, 65, and 80 at 1% were able to decrease the activity of a thermophilic lipase produced by Cohnella sp. A01 and suggested that probably the long acyl chains of these detergents could be acting as competitive inhibitors of lipase substrate.
Effect of Organic Solvents on Solid Biocatalyst Activity
The effect of organic solvents on solid biocatalyst activity is presented in Table 3. The biocatalyst showed promising results in the presence of the solvents tested, since most of them were able to activate lipase activity, mainly non-polar such as hexane and heptane and also polar solvents such as acetonitrile, dimethyl sulfoxide (DMSO), and acetone. On the other hand, lipase activity was negatively influenced in the presence of alcohols with more than two atoms in the carbon chain and chloroform. Some authors reported that solvent polarity can directly influence the relative activity of lipase, since the thin layer of water molecules necessary in the catalytic site can be stripped by polar solvents [45, 60]. However, in the present study, it was not possible to observe a direct relation between the organic solvent polarity and lipase activity of the biocatalyst in the concentrations tested, since aprotic polar solvents and hydrophobic alkane solvents were able to increase lipase activity.
Substrate Specificity
The ability of hydrolysis of several substrates (Table 4) demonstrated high preference for coconut oil and palm kernel oil achieving 164 ± 1.7 and 173.3 ± 1.1 of relative lipase activity, respectively. It is known that these oils have high content of medium-chain fatty acids in their composition. High lipase activity towards coconut oil was also reported by Syal and Gupta [55] using several lipases from Y. lipolytica. Furthermore, the solid biocatalyst showed high lipase activity for all vegetable oils tested, but its activity was reduced to approximately 30% when lard was used. Other works have demonstrated the ability of several Y. lipolytica lipases for vegetable oil hydrolysis. Kumari and Gupta [61] using three different lipases (LIP8, LIP14, and LIP18) showed high activity on ground nut oil mainly by LIP18. Yu et al. [56] using LIP2 demonstrated the ability to hydrolyze olive oil, soybean oil, and others. These results corroborated the production of true lipases by Y. lipolytica by SSF using soybean meal, but it is important to mention that this work did not explore which lipases were being expressed and produced by Y. lipolytica IMUFRJ 50682 since the biocatalyst used in hydrolysis reactions was composed of a pool of enzymes. When synthetic triglycerides were used (Table 4), the solid biocatalyst showed similar hydrolysis for C4:0, C6:0, and C8:0 and lower activity for the long-chain synthetic triglycerides.
Thermostability and Storage Stability
Thermostability is a desirable characteristic to be found in biocatalysts since several industrial applications could be carried out at high temperatures. In this work, two different environments were evaluated to observe the effect of aqueous medium (phosphate buffer) and organic medium (ethanol) on solid biocatalyst thermostability at three different temperatures (Fig. 1). Stable lipases at high temperatures in the presence of organic solvents are promising biocatalysts to be employed in esterification reactions. At 37 °C, the highest relative activity was reached for both biocatalysts incubated in phosphate buffer and ethanol, and for all periods tested, relative activity was kept above 50% of its original value. After 48 h, the solid biocatalyst showed 58.2 ± 3 and 79.5 ± 3% of relative activity when incubated in the presence of phosphate buffer and ethanol, respectively. However, at 50 °C, the solid biocatalyst in the presence of phosphate buffer reduced substantially its relative activity, presenting values of 35.4 ± 0.6 and 14.5 ± 0.2% after 3 and 48 h, respectively, and at 70 °C, lipase activity achieved even lower values, when the biocatalyst incubated in phosphate buffer was totally inactivated after 15 h. An interesting fact was that for all temperatures evaluated, the biocatalyst incubated in ethanol showed higher values of lipase activity than when incubated in phosphate buffer. Probably, ethanol helped to keep the conformational lipase structure, preserving its active site. Therefore, this characteristic indicates a better performance at high temperatures for synthesis reactions, for example, employing organic solvents.
Some authors have previously reported that Y. lipolytica lipase stability decreases at high temperatures, since its optimal temperature was found in the range of 30 to 37 °C, with stability decreasing at 40 °C [60, 62]. Yu et al. [56] using an extracellular LIP2 from Y. lipolytica reported a rapid decrease of lipase activity at 45 and 40 °C achieving 5 and 32% of residual activity after 4 h, respectively. In the same way, Kamoun et al. [63] showed thermostability of LIP8 after 1 h reporting residual activity (%) of 54.9 ± 0.8, 34.8 ± 0.3, and 10.2 ± 1.4 and total inactivation at 37, 40, 45, and 50 °C, respectively. In another study performed by Yu et al. [64], using LIP2 only 20% of residual activity was found after 30 min of incubation at 45 °C. According to these results, the biocatalyst produced through SSF using soybean meal can be considered more thermostable than other lipases reported in literature which could be attributed to its production at SSF, thereby achieving greater stability.
The storage stability of the solid biocatalyst was evaluated for a period of 300 days at different temperatures. According to Fig. 2, the solid biocatalyst showed high stability at all temperatures tested reaching after 300 days 79.9 ± 0.5, 82.8 ± 3.2, and 93 ± 0.1% of relative activity at 28, 4, and − 10 °C, respectively.
These results demonstrate that the solid biocatalyst has capacity to be used for long periods of times, even if stored at room temperature. No additives or post-treatment after the lyophilization process of solid biocatalyst production was carried out, reducing the production cost of this biocatalyst. However, the use of additives could improve lipase stability, as demonstrated by Darvishi et al. [65] using freeze-dried Y. lipolytica lipase supplemented with maltodextrin (0.5%), conserving up to 96% of its original activity after a period of 46 weeks at 4 and 25 °C.
Influence of pH and Temperature on Biocatalyst Lipase Activity
The optimum temperature and pH for lipase activity using the solid biocatalyst were investigated in a range of pH 3.0 to pH 9.0 at 25, 37, and 50 °C (Fig. 3). Maximum lipase activity was reached at 37 °C in pH 8.0 (106.7 ± 3.7 U/g). At 50 and 25 °C, lipase activity achieved higher values in pH 7.0 and 8.0, respectively. For all values of temperature and pH, the biocatalyst was active. Nevertheless, in neutral to basic pH values, solid biocatalyst lipase activity was higher than in acid values, except at 50 °C. The solid biocatalyst therefore showed to be sensitive to acid values of pH. In literature, several works have reported different conditions of pH and temperature of lipases produced by Y. lipolytica. The results found in the present work are in agreement with or are similar to reports from other authors.
Sun et al. [66] using an immobilized lipase of Y. lipolytica achieved maximum activity at 40 °C in pH 9. Kumari and Gupta [61], using LIP8, LIP14, and LIP18 of Y. lipolytica MSR80, showed optimum temperature of 40 °C and pH 7.0. In another study performed by Kumari et al. [67], equivalent results were presented using LIP2, LIP11, and LIP12, but the authors described low activity in acid values of pH. Yu et al. [64] using LIP2 of Y. lipolytica reached results very similar to those found in this work since optimum temperature and pH values of lipase were 40 and 8.0, respectively. However, it is not possible to state that the solid biocatalyst used in this work is composed only of LIP2 since the identification of which lipases are being expressed by Y. lipolytica wild strain was not performed. Syal and Gupta [55] showed that LIP4, LIP5, LIP7, LIP13, and LIP15 presented optimum activity at pH 7.0–8.0, although LIP 5, LIP7, and LIP13 presented low activity in acid pH values, and both LIP4 and LIP15 showed high activity in this condition.
All lipase activities were drastically affected by pH higher than 8.0. The effect of temperature was also evaluated and optimum conditions were found in the range from 30 to 50 °C. In contrast to these results, some authors reported optimum pH for LIP2 as slightly acid or at acid conditions with a significant decrease at pH above 8.0 [54] and optimum temperature at 55 °C [68]. A cold active lipase from Y. lipolytica NCIM 3639 reached maximum activity at pH 5.0 at 25 °C which decreased at pH 6.5 or when submitted to temperatures above 30 °C [69]. These results therefore show the great diversity of lipases produced by Y. lipolytica that can be influenced by many factors such as the microorganism strain, the cultivation medium, and the fermentation conditions, for example.
Preliminary Synthesis Reactions of Wax and Medium- and Short-Chain Flavor Esters
The use of organic solvents in enzymatic esterification reactions is widely applied since several benefits can be achieved in the synthesis processes. The non-aqueous environment surrounding the enzyme contributes to the catalytic behavior, where the reaction thermodynamic equilibrium is shifted to esterification. Furthermore, organic solvents are able to improve the solubility of substrates and products of reaction which can favor reaction conversion and lipase stability, activity, and selectivity are also influenced by the nature of the solvent, once various physicochemical effects on enzyme molecules can be produced [8, 70].
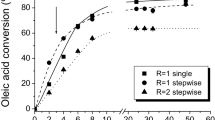
This study initially evaluated the production of cetyl stearate and stearyl palmitate in different periods of time using three solvents: hexane, heptane, and tert-butanol (Fig. 4). High substrate conversion into cetyl stearate and stearyl palmitate was found after 8 h of reaction when hexane and heptane were used. Conversion into cetyl stearate reached 94.5 ± 1.5 and 95.5 ± 1.1% in hexane and heptane, respectively, while into stearyl palmitate, it reached 95.7 ± 1.0 and 95.4 ± 1.0% in the same order. However, the reaction synthesis evaluated in the presence of tert-butanol did not have the same efficiency, since conversion to cetyl stearate and stearyl palmitate only achieved 18.7 ± 1.6 and 27.6 ± 1.9% after 24 h, respectively, demonstrating that tert-butanol can lead to lipase inactivation. The effect of tert-butanol on solid biocatalyst hydrolysis activity is demonstrated in Table 3 and at a concentration of 9% (v/v), relative activity of 85.6 ± 8.8 was found, already indicating a slight inactivation of the biocatalyst, whereas hexane and heptane enhanced its relative activity by more than 20%.
Time-course of the conversion of different substrates used in esterification reactions to synthesize stearyl palmitate and cetyl stearate in the presence of different solvents using the solid biocatalyst. Hexane, circles; heptane, triangles; tert-butanol, squares; dark symbols, stearyl palmitate; gray symbols, cetyl stearate. Reactions were performed in 1:1 substrate molar ratio, using 10% (w/v) of solid biocatalyst at 38 °C
It was previously reported that solvent polarity can affect lipase activity, and in general, the most suitable are the hydrophobic over hydrophilic solvents. Hydrophilic ones are able to interfere on the enzyme structure by removing the essential layer of water bound to the biocatalyst surface. The results found in this work are in agreement with these characteristics since tert-butanol showed negative effect in esterification reactions. Other studies have demonstrated the deleterious effect of hydrophilic organic solvents on lipase synthesis reactions. Yan et al. [71] studying the synthesis of short-chain flavor esters using a whole-cell lipase from Aspergillus oryzae demonstrated lower substrate conversion (less than 10%) in the presence of acetonitrile, acetone, and other solvents with LogP inferior to 1. In the same way, Xu et al. [72] using a whole-cell lipase from R. chinensis for ethyl hexanoate synthesis reported negative effect on molar conversion (%) in the presence of ethanol, acetone, tetrahydrofuran, and pentanol presenting LogP values in a range of − 0.24 to 1.30. On the other hand, both studies showed high conversion values in the presence of hydrophobic solvents such as hexane and heptane. Therefore, in order to reduce operational costs, since hexane and heptane presented very similar results, hexane was chosen to carry out all other synthesis reactions because of its lower cost when compared to heptane.
The syntheses of medium and short-chain flavor esters were evaluated (Fig. 5) using hexane. A molar ratio of 1:1 (acid/alcohol) at 500 mM was used. The esterification reaction was carried out at 38 °C using 15% (w/v) of solid biocatalyst for a period of 8 h. Ethyl butyrate, isoamyl propionate, butyl hexanoate, and isoamyl acetate presented substrate conversion into ester lower than 40%, Cinnamyl alcohol and acetic acid presented conversion of 61.4 ± 2.5% into cinnamyl acetate, ethanol and octanoic acid, and ethanol and decanoic acid reached the best conversion results, achieving 89.9 ± 0.8 and 79.9 ± 1.6%, respectively, into ethyl octanoate and ethyl decanoate.
The low conversion into some esters studied could be associated with the deactivation of the biocatalyst by the acyl donor (acids) in high concentrations. A future alternative to solve this problem would be to employ vinyl esters, for example, as the acyl donor rather than acids, performing a transesterification reaction with the acyl acceptor (alcohols) [8]. This strategy was employed by Lozano et al. [73] using immobilized C. antarctica lipase B in solvent-free synthesis of citronellyl esters using citronellol and alkyl vinyl ester as substrates. Several studies have reported the synthesis of flavor esters using commercial lipases or lipases obtained by SmF using fungi and in few reports by bacteria strains [5, 7, 74]. However, to our knowledge, the use of Y. lipolytica lipase produced by SSF to perform these reactions has not been reported yet.
Since esterification reactions to produce ethyl octanoate and ethyl decanoate showed the best conversions, a new kinetic (Fig. 6) was performed to evaluate after which time of reaction the highest conversion would be reached. The esterification reactions were performed up to 24 h and the best result showed conversion of 88 ± 0.8 and 77.4 ± 1.6% after 8 h, for ethanol with octanoic acid and ethanol with decanoic acid, respectively. The reaction using ethanol and octanoic acid showed the highest conversion among all substrates tested to produce flavor esters.
Evaluation of Solid Biocatalyst Production Improvement Aiming to Optimize Ester Synthesis
Since Y. lipolytica is dimorphic yeast, different lipases and amounts of these biocatalysts could be produced according to its morphology and growing conditions. Furthermore, Y. lipolytica is known to be a great lipase producer, possessing 16 paralogous genes coding for lipases, and several of these enzymes have been studied. Lip2 is a well-known extracellular lipase, LIP1, LIP2, and LIP6 are intracellular, whereas LIP7 and LIP8 are cell-bound lipases [38, 54, 63]. Thereby, different assays were carried out, as described in the “Improvement in Solid Biocatalyst Production Aiming to Optimize Ester Synthesis” section, aiming to increase lipase production and obtain a solid biocatalyst able to perform ester synthesis in a short period of time.
Assay 1 showed the highest substrate conversion (90.8 ± 0.2%) after 4 h of reaction, while Assay 4 was able to reach similar result (90.2 ± 1.3%) only after 8 h (Table 5). This could indicate that cells produced in SSF would carry lipases attached to their cell wall with an important role on the ester synthesis performed. Furthermore, these results could also corroborate the hypothesis of a pool of lipases with synthetic characteristics being produced under SSF.
The results obtained by Assay 2 verified that changes in fermentation conditions such as inoculum and inductor (soybean oil) concentration would improve solid biocatalyst performance in ester synthesis. It is possible to observe in Assay 2 that when high amounts of both soybean oil and inoculum were used the highest substrate conversion (92.9 ± 0.1%) was achieved after 6 h, demonstrating a synergistic effect between these fermentation conditions. It could lead to high development of Y. lipolytica cells during SSF increasing lipase production and therefore achieving high ester production in a shorter period of time when compared to control.
The fermentation control reached conversion similar to Assays 2 and 3 after 8 h of synthesis reaction. However, after 4 h, it achieved higher conversion than Assay 3, which could demonstrate that when only a high amount of inoculum is used, the conversion of cetyl alcohol with stearic acid into cetyl stearate could decrease in the first hours of reaction.
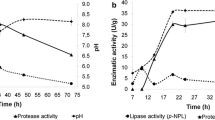
Although most assays performed have shown after 8 h of reaction a similar conversion about 90%, Assay 5 did not present satisfactory results. The fermentation process used to obtain this solid biocatalyst was conducted for a period of 18 h and achieved conversion of 46.1 ± 2.3% after 8 h of synthesis reaction. It could demonstrate that after 14 h of fermentation, there was a decrease of lipase content with important role in ester synthesis, which could be attributed to a decrease in lipase production by Y. lipolytica or also to an increase of protease production as was evidenced by Souza et al. [32].
The solid biocatalyst produced under the conditions described in Assay 2 (Table 5) was chosen to perform all other synthesis reactions in this work, since it presented a high conversion (%), similar to that found in Control assay, but in a shorter period of time (6 h). Although Assay 1 presented rapid substrate conversion into cetyl stearate, it was not chosen due to the high amount of fermented soybean meal used to obtain a cell suspension of 5 g/L, which consequently would increase the cost of the ester synthesis.
Synthesis of stearyl palmitate was also evaluated and 90% conversion for the combination of palmitic acid and stearyl alcohol was achieved after 6 h of reaction. In order to confirm product formation by the esterification reactions realized to obtain the wax esters cetyl stearate and stearyl palmitate, analysis by mass spectrometry in Shimadzu (GCMS-QP2010 Ultra) was performed and the production of these esters was confirmed.
Cetyl stearate and stearyl palmitate are wax esters that can be applied as plasticizers or lubricants and adjuvants in food-packaging materials in the food industry. It is worth noticing that the current work is the first to approach the synthesis of these wax esters using a green biocatalyst obtained by SSF. Guncheva et al. [25] demonstrated the production of stearyl stearate. In that study, esterification was carried out at 65 °C in the presence of 3-methyl-1-octyl imidazolium chloride which led to a higher reaction rate, achieving 95% conversion of the substrates after 5 h using a lipase from Bacillus stearothermophilus MC7 stabilized via immobilization on nanostructured tin dioxide. However, in solvent-free reaction, conversion was negatively affected. The syntheses of 12 wax esters, including cetyl stearate and stearyl palmitate, were shown using an immobilized commercial lipase from Candida rugosa lipase (CRL) in a non-solvent reaction. Results close to 100% of yield were achieved for all wax esters tested in reaction conditions of 50 °C for 10 h using a PEG2000-activated C. rugosa lipase [75].
Some studies have focused on the synthesis of cetyl oleate and oleyl oleate, once they are synthetic analogues of jojoba oil. Li et al. [23] using a mixture of cetyl alcohol and oleic acid (acid/alcohol molar ratio 1) reached a conversion rate of 98% after 8 h using a commercial lipase from Candida sp. 99–125 in a solvent-free system, while Bi et al. [76] showed a percentage yield of 92.6% for oleyl oleate under optimal conditions after 12 h in a solvent-free system. The solid biocatalyst can be considered a promising catalyst for wax ester synthesis, once it reached similar values of conversion (around 90%) in a similar or lower period of reaction time than the cited studies.
Evaluation of Ethyl Octanoate Production Using the Optimized Solid Biocatalyst
After determining the best conditions for solid biocatalyst production, a new kinetic of production (data not shown) for ethyl octanoate was performed, since this flavor ester presented the best results among others flavors esters tested. Using the biocatalyst obtained according to Assay 2 (Table 5) to produce ethyl octanoate, the highest substrate conversion (87.3 ± 0.4%) was achieved after a reaction carried out for 4 h, 4 h less than the result achieved using the solid biocatalyst control. Ethyl octanoate production was also confirmed by mass spectrometry in Shimadzu (GCMS-QP2010 Ultra).
Ethyl octanoate has a fruity flowery fragrance and is used as an apple-like (sour apple) flavor to the bouquet of certain drinks together with other medium chain ethyl esters such as ethyl hexanoate and ethyl decanoate. Ahmed et al. [77] using an alkaline lipase from Acinetobacter sp. EH28 in the synthesis of ethyl octanoate reached the highest fatty acid conversion (90%) after 6 days, using a molar ratio of 0.13:0.1 of octanoic acid and ethanol, respectively, in the presence of cyclohexane at 40 °C. Patel et al. [70] obtained esterification yield of 85% using a 0.15:0.1 M ratio (octanoic acid/ethanol) in cycle-octane reaction medium at 40 °C within 48 h of reaction. Esterification results were similar to those found in the current work. However, the solid biocatalyst performed the synthesis in a shorter period of time.
Gel Electrophoresis and Zymogram
The comparison of the lipase profile produced in control and Assay 2 fermentation conditions (Table 5) was performed and presented in Fig. 7. The zymogram and native-PAGE analysis evidenced two lipase/esterase bands in control fermentation conditions and three bands in Assay 2 fermentation conditions, where in the latter, two bands showed high intensity, indicating a higher enzyme production than in control conditions. The bands revealed in both gels presented a molecular weight of ~ 37 kDa in line 1. In line 5, high intensity bands showed molecular weight of ~ 40 and 37 kDa and weaker band ~ 30 kDa. It is interesting to note that lipases/esterases produced in SSF and revealed by α-naphthyl acetate (Fig. 7) in zymogram presented that same molecular weight range (30–45 kDa) than other lipases reported in literature [53].
Zymogram gel (gray gel) using α-naphthyl acetate as a substrate of lipases and esterases produced by Y. lipolytica in SSF using soybean meal. The gel was stained using Coomassie blue after zymographic analysis (blue gel). Line 1: lipase crude extract produced according to control fermentation conditions (Table 5). Line 2: molecular weight markers (kDa). Line 3: crude extract of non-fermented soybean meal. Line 4: molecular weight markers (kDa). Line 5: lipase crude extract produced according to Assay 2 fermentation conditions (Table 5). Black arrows indicate hydrolysis bands
Scanning electron micrographs of fermented soybean meal by Y. lipolytica and non-fermented soybean meal. a Details of the growth of Y. lipolytica in fermentation conditions described in control (Table 5). b Surface detail of a non-fermented soybean meal. c Details of the growth of Y. lipolytica in fermentation conditions described in Assay 2 (Table 5). d Details of filamentous Y. lipolytica in a closer view
Fickers et al. [78] studying LIP7 and LIP8 from Y. lipolytica found a band with apparent molecular mass of ~ 41 kDa, while Kamoun et al. [63] found for LIP2 and LIP8 molecular masses around 37 and 38 kDa, respectively. In another study, LIP2 expressed and secreted by Pichia pastoris demonstrated apparent molecular weight of 39 kDa [64]. In the same way, Yu et al. [56] found a very similar result for an extracellular lipase from Y. lipolytica (LIP2) with a molecular mass of 38 kDa. The two stronger bands found (Fig. 7, line 5), therefore, indicate similar molecular weight than LIP2 since both presented ~ 40 and 37 kDa. Another point is that lipases/esterases produced by SSF in the present study did not present bands with molecular masses different from those usually found for Y. lipolytica when lipase production is performed in liquid medium. Considering these results, future studies should be performed aiming to identify which lipase genes are being expressed by Y. lipolytica IMUFRJ 50682 wild strain and which lipases are synthesized in SSF.
Solid Biocatalyst Scanning Electron Microscopy
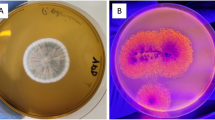
The use of the solid biocatalyst is an interesting way to reduce costs on ester synthesis production. Few steps are necessary and also this biocatalyst has a high load of lipases on its surface. The use of a solid biocatalyst obtained in SSF by Y. lipolytica to perform wax and flavor ester synthesis has not been reported before. Nevertheless, the use of fermented solids as biocatalysts has already been reported in biodiesel production, presenting a great potential for this purpose. In a work carried out by Aguieiras et al. [79], dry babassu cake with lipase activity from Rhizomucor miehei was employed aiming at ethyl ester production using free fatty acids from previously hydrolyzed macauba oil. Fermented solids obtained using a mixture of sugarcane bagasse and sunflower seed meal by Burkholderia cepacia were employed on biodiesel production (ethyl esters) from soybean soapstock acid oil. Best results were achieved in a solvent-free system, with 92% conversion in 31 h, at 50 °C [80]. In order to evaluate the Y. lipolytica growth morphology in soybean meal, scanning electron micrographs of the dry fermented soybean meal (solid biocatalyst) were obtained.
Figure 8a shows an intense growth on the surface of the agro-industrial co-product if compared to Fig. 8c which shows the non-fermented soybean meal. Figure 8d shows in a closer view the morphology presented by Y. lipolytica in SSF conditions. Differently from SmF which presents yeast cells, in SSF, hyphae morphology is predominant, since Y. lipolytica is dimorphic yeast and is able to change its morphology according to stress or environmental conditions
Solid Biocatalyst Reuse in Esterification Reactions
The reuse of biocatalyst is directly attributed to cost reduction in a synthetic process. Reuse of solid biocatalyst (Fig. 9) was tested for ethyl octanoate and cetyl stearate production. Conversion of octanoic acid showed a strong and rapid decay in the first reuse to produce ethyl octanoate, presenting a value of 16.5 ± 1.8%, indicating that many lipases could be inactivated after their first use.
Stability of the reused solid biocatalyst in different batches for ethyl octanoate (squares) and cetyl stearate (diamonds) production. Ethyl octanoate reaction was performed in 1:1 substrate molar ratio, using 10% (w/v) of solid biocatalyst at 38 °C for 6 h. Cetyl stearate reaction was performed in 1:1 substrate molar ratio, using 10% (w/v) of solid biocatalyst at 38 °C for 6 h. The solid biocatalyst was produced in the following conditions: 14 h of fermentation, 3% (w/w) soybean oil, inoculum concentration of 2.1 mgdry biomass/gsoybean meal, and 55% of moisture at 28 °C
The wax ester reaction showed a better performance reaching in the first reuse 51.5 ± 0.1% conversion of the substrates which was kept until its fourth reuse. It shows that substrates used in ethyl octanoate synthesis were more detrimental if compared to those used in wax ester synthesis, since the same temperature conditions were used. Furthermore, ethyl octanoate reaction was conducted for 4 h, whereas cetyl stearate reaction for 6 h, both in the presence of hexane. The stability of the biocatalyst over multiple reuses was inferior to the biocatalyst recycles described by other authors in ethyl octanoate [70], saturated wax ester [25], and biodiesel [79] synthesis.
Ester Synthesis in Solvent-Free Medium
In this study, the production of flavor and wax esters was evaluated in a solvent-free medium as shown in Fig. 10. After 4 h, substrate conversion of 7.1 ± 1.8% was achieved for ethyl octanoate reaction and this result suggests strong interference of the substrates on lipase activity due to the higher concentration used, since the same conditions of time and temperature than in hexane reactions were used.
Evaluation of ester production in a solvent-free system (gray bars) and in the presence of hexane (dark gray bars). In the solvent-free system, ethyl octanoate reaction was performed in 1:1 substrate molar ratio, using 15% (w/v) of solid biocatalyst at 38 °C for 4 h. Stearyl palmitate and cetyl stearate reactions were performed in 1:1 substrate molar ratio, using 10% (w/v) of solid biocatalyst at 55 °C for 6 h. In the presence of hexane, ethyl octanoate reaction was performed in the same conditions mentioned before. Stearyl palmitate and cetyl stearate reactions were performed in 1:1 substrate molar ratio, using 10% (w/v) of solid biocatalyst at 38 °C for 6 h. The solid biocatalyst was produced in the following conditions: 14 h of fermentation, 3% (w/w) soybean oil, inoculum concentration of 2.1 mgdry biomass/gsoybean meal, and 55% of moisture at 28 °C
It has been demonstrated that solvent-free reactions could lead to the decrease of ester formation as shown by Salah et al. [81] who achieved maximum conversion of 60% in a solvent-free medium against 80 and 76% in heptane and hexane, respectively, aiming at butyl acetate synthesis using R. oryzae lipase. The production of (Z)-3-hexen-1-yl butyrate by C. antarctica (Novozyme 435) was affected in the same way when no solvent was used in the reaction medium reaching a yield of 80% against 92% in 6 h using hexane [82].
The synthesis of several flavor esters such as isoamyl acetate, ethyl valerate, and butyl acetate using C. rugosa lipase and porcine pancreatic lipase entrapped in Ca-Alg in the absence of hexane was inferior to that of reactions using the organic solvent where authors attributed higher conversions in the presence of a solvent to a shift of equilibrium towards ester synthesis and consequently a total transfer of ester into the organic phase [83]. The negative effect of high acid concentration on solvent-free system is also a factor that can support enzyme deactivation and therefore a decrease on ester synthesis [8].
Wax ester synthesis was also affected in the absence of organic solvent medium, where substrate mixtures for cetyl stearate and stearyl palmitate production presented 35.7 ± 0.1 and 32.6% of conversion after 6 h of reaction, respectively. Since hexane was not employed, it was necessary to increase the reaction temperature to 55 °C due to high melting point of substrates. A long exposure to higher temperature could lead to a decrease of solid biocatalyst activity. Moreover, the medium viscosity may have interfered on its activity. On the other hand, the use of organic solvents provided better solubility of reactants and decreased the medium viscosity, increasing the accessibility of substrates to the enzymatic catalytic site which justifies its employment on saturated wax ester synthesis [84].
Conclusion
In this study, Y. lipolytica IMUFRJ 50682 showed great ability for lipase production by SSF using soybean meal. Furthermore, a solid biocatalyst with high lipase activity and able to synthesize flavor and wax ester was obtained. The solid biocatalyst performed well in several conditions tested, showing great lipase activity under vegetable oils and in the presence of hydrophobic and hydrophilic solvents such as hexane, heptane, decane, acetonitrile, and DMSO, respectively. However, alcohols with more than two atoms in the carbon chain and chloroform affected it negatively. None of the metal ions evaluated improved significantly its lipase activity. Detergents above its CMC also decreased the lipase activity. The solid biocatalyst showed higher lipase activity in alkaline conditions at 25 and 37 °C but also performed well at 50 °C in neutral pH. High storage stability was observed for the solid biocatalyst, holding approximately 80% of its initial activity after 300 days at 28 °C. Lipase produced by Y. lipolytica in SSF showed very similar mass weight than other lipases produced by the same microorganism in SmF (around 30–45 kDa). In esterification reactions, high substrate conversion was achieved for ethyl octanoate (87.3% ± 0.4) production after 4 h and for cetyl stearate (92.9 ± 0.1%) and stearyl palmitate (90.2%) production after 6 h, where all reactions were conducted in medium using hexane. To reach these results, the solid biocatalyst was produced using the following SSF conditions: 14 h of fermentation, 55% of moisture, 3% (w/w) soybean oil, and inoculum concentration of 2.1 mgdry biomass/gsoybean meal at 28 °C. Therefore, this solid biocatalyst appears to be an interesting alternative to synthesize useful high-added value esters applied in the food industry, through a system production employing a GRAS status microorganism.
References
De Barros, D. P., Azevedo, A. M., Cabral, J. M., & Fonseca, L. P. (2012). Optimization of flavor esters synthesis by Fusarium solani pisi cutinase. Journal of Food Biochemistry, 36(3), 275–284.
Güvenç, A., Kapucu, N., & Mehmetoğlu, Ü. (2002). The production of isoamyl acetate using immobilized lipases in a solvent-free system. Process Biochemistry, 38(3), 379–386.
IAL Consultants, An overview of the global flavours and fragnances market, 10th Edition (2016). http://www.ialconsultants.com/uploads/CUBE_press_relese/2016-12-21/FFPressRelease2016.pdf (accessed 10 June 2018).
BCC Research (2016). Global markets for flavors and fragrances. https://www.bccresearch.com/market-research/chemicals/flavors-fragrances-markets-report-chm034e.html (accessed 10 June 2018).
Martins, A. B., da Silva, A. M., Schein, M. F., Garcia-Galan, C., Ayub, M. A. Z., Fernandez-Lafuente, R., & Rodrigues, R. C. (2014). Comparison of the performance of commercial immobilized lipases in the synthesis of different flavor esters. Journal of Molecular Catalysis B: Enzymatic, 105, 18–25.
Brault, G., Shareck, F., Hurtubise, Y., Lépine, F., & Doucet, N. (2014). Short-chain flavor ester synthesis in organic media by an E. coli whole-cell biocatalyst expressing a newly characterized heterologous lipase. PLoS One, 9(3), e91872.
Matte, C. R., Bordinhão, C., Poppe, J. K., Rodrigues, R. C., Hertz, P. F., & Ayub, M. A. (2016). Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. Journal of Molecular Catalysis B: Enzymatic, 127, 67–75.
SÁ, A. G. A., de Meneses, A. C., de Araújo, P. H. H., & de Oliveira, D. (2017). A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends in Food Science & Technology, 69, 95–105.
Lozano, P., Bernal, J. M., & Navarro, A. (2012). A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chemistry, 14(11), 3026–3033.
Berger, R. G. (2015). Biotechnology as a source of natural volatile flavours. Current Opinion in Food Science, 1, 38–43.
Ungcharoenwiwat, P., Canyuk, B., & Aran, H. (2016). Synthesis of jatropha oil based wax esters using an immobilized lipase from Burkholderia sp. EQ3 and Lipozyme RM IM. Process Biochemistry, 51(3), 392–398.
Sellami, M., Aissa, I., Frikha, F., Gargouri, Y., & Miled, N. (2011). Immobilized Rhizopus oryzae lipase catalyzed synthesis of palm stearin and cetyl alcohol wax esters: optimization by response surface methodology. BMC Biotechnology, 11(1), 68.
Aslan, S., Hofvander, P., Dutta, P., Sun, C., & Sitbon, F. (2015). Increased production of wax esters in transgenic tobacco plants by expression of a fatty acid reductase: wax synthase gene fusion. Transgenic Research, 24(6), 945–953.
Food and Drug Administration, Electronic code of federal regulations. URL https://www.ecfr.gov/cgi-bin/ECFR?page=browse (accessed 10 june 2018).
Santala, S., Efimova, E., Koskinen, P., Karp, M. T., & Santala, V. (2014). Rewiring the wax ester production pathway of Acinetobacter baylyi ADP1. ACS Synthetic Biology, 3(3), 145–151. https://doi.org/10.1021/sb4000788.
Lopes, D. B., Duarte, M. C. T., & Macedo, G. A. (2011). Biosynthesis of oleyl oleate wax ester by non-commercial lipase. Food Science and Biotechnology, 20(5), 1203–1209.
Bart, J. C., Gucciardi, E., & Cavallaro, S. (2012). Biolubricants: science and technology (first ed.). Cambridge: Woodhead Publishing.
Trivedi, J., Aila, M., Sharma, C. D., Gupta, P., & Kaul, S. (2015). Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. Springerplus, 4(1), 165.
Poppe, J. K., Matte, C. R., Peralba, M. D. C. R., Fernandez-Lafuente, R., Rodrigues, R. C., & Ayub, M. A. Z. (2015). Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases. Applied Catalysis A: General, 490, 50–56.
Garlapati, V. K., & Banerjee, R. (2013). Solvent-free synthesis of flavour esters through immobilized lipase mediated transesterification. Enzyme Research, 2013(367410), 6.
Ghamgui, H., Karra-Chaâbouni, M., Bezzine, S., Miled, N., & Gargouri, Y. (2006). Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzyme and Microbial Technology, 38(6), 788–794.
Grosso, C., Ferreira-Dias, S., & Pires-Cabral, P. (2013). Modelling and optimization of ethyl butyrate production catalysed by Rhizopus oryzae lipase. Journal of Food Engineering, 115(4), 475–480.
Li, D., Xiaojing, W., Kaili, N., Fang, W., Junfeng, L., Pu, W., & Tianwei, T. (2011). Synthesis of wax esters by lipase-catalyzed esterification with immobilized lipase from Candida sp. 99–125. Chinese Journal of Chemical Engineering, 19(6), 978–982.
Kuo, C. H., Ju, H. Y., Chu, S. W., Chen, J. H., Chang, C. M. J., Liu, Y. C., & Shieh, C. J. (2012). Optimization of lipase-catalyzed synthesis of cetyl octanoate in supercritical carbon dioxide. Journal of the American Oil Chemists' Society, 89(1), 103–110.
Guncheva, M., Dimitrov, M., & Kambourova, M. (2013). Excellent stability and synthetic activity of lipase from B. stearothermophilus MC7 immobilized on tin dioxide in environmentally friendly medium. Biotechnology & Biotechnological Equipment, 27(6), 4317–4322.
dos Santos, J. B. C., da Silva Cruz, R. G., & Tardioli, P. W. (2017). Production of whole-cell lipase from Streptomyces clavuligerus in a bench-scale bioreactor and its first evaluation as biocatalyst for synthesis in organic medium. Applied Biochemistry and Biotechnology, 183(1), 218–240.
Zhuang, S., Fu, J., Powell, C., Huang, J., Xia, Y., & Yan, R. (2015). Production of medium-chain volatile flavour esters in Pichia pastoris whole-cell biocatalysts with extracellular expression of Saccharomyces cerevisiae acyl-CoA: ethanol O-acyltransferase Eht1 or Eeb1. Springerplus, 4(1), 467.
Jin, Z., Ntwali, J., Han, S. Y., Zheng, S. P., & Lin, Y. (2012). Production of flavor esters catalyzed by CALB-displaying Pichia pastoris whole-cells in a batch reactor. Journal of Biotechnology, 159(1–2), 108–114.
Chen, J. P., & Wang, J. B. (1997). Wax ester synthesis by lipase-catalyzed esterification with fungal cells immobilized on cellulose biomass support particles. Enzyme and Microbial Technology, 20(8), 615–622.
Soccol, C. R., da Costa, E. S. F., Letti, L. A. J., Karp, S. G., Woiciechowski, A. L., & de Souza Vandenberghe, L. P. (2017). Recent developments and innovations in solid state fermentation. Biotechnology Research and Innovation, 1(1), 52–71.
Oliveira, F., Souza, C. E., Peclat, V. R., Salgado, J. M., Ribeiro, B. D., Coelho, M. A. Z., Venâncio, A., & Belo, I. (2017). Optimization of lipase production by Aspergillus ibericus from oil cakes and its application in esterification reactions. Food and Bioproducts Processing, 102, 268–277.
Souza, C. E. C., Farias, M. A., Ribeiro, B. D., & Coelho, M. A. Z. (2017). Adding value to agro-industrial co-products from canola and soybean oil extraction through lipase production using Yarrowia lipolytica in solid-state fermentation. Waste and Biomass Valorization, 8(4), 1163–1176.
Godoy, M. G., Gutarra, M. L., Castro, A. M., Machado, O. L., & Freire, D. M. (2011). Adding value to a toxic residue from the biodiesel industry: Production of two distinct pool of lipases from Penicillium simplicissimum in castor bean waste. Journal of Industrial Microbiology & Biotechnology, 38(8), 945–953.
Liu, Y., Li, C., Meng, X., & Yan, Y. (2013). Biodiesel synthesis directly catalyzed by the fermented solid of Burkholderia cenocepacia via solid state fermentation. Fuel Processing Technology, 106, 303–309.
Boratyński, F., Szczepańska, E., Grudniewska, A., Gniłka, R., & Olejniczak, T. (2018). Improving of hydrolases biosynthesis by solid-state fermentation of Penicillium camemberti on rapeseed cake. Scientific Reports, 8(1), 10157.
Boratyński, F., Szczepańska, E., Grudniewska, A., & Olejniczak, T. (2018). Microbial kinetic resolution of aroma compounds using solid-state fermentation. Catalysts, 8(1), 28.
Zinjarde, S. S. (2014). Food-related applications of Yarrowia lipolytica. Food Chemistry, 152, 1–10.
Brígida, A. I., Amaral, P. F., Coelho, M. A., & Goncalves, L. R. (2014). Lipase from Yarrowia lipolytica: production, characterization and application as an industrial biocatalyst. Journal of Molecular Catalysis B: Enzymatic, 101, 148–158.
Hagler, A. N., & Mendonça-Hagler, L. C. (1981). Yeasts from marine and estuarine waters with different levels of pollution in the state of Rio de Janeiro, Brazil. Applied and Environmental Microbiology, 41(1), 173–178.
Freire, D. M. G., Teles, E. M. F., Bon, E. P. S., & Sant’Anna, G. L., Jr. (1997). Production by Penicillium restrictum in a bench-scale fermentere effect of carbon and nitrogen nutrition, agitation, and aeration.Applied biochemistry and biotechnology, 63-65, 409–421
Gutarra, M. L., Godoy, M. G., Maugeri, F., Rodrigues, M. I., Freire, D. M., & Castilho, L. R. (2009). Production of an acidic and thermostable lipase of the mesophilic fungus Penicillium simplicissimum by solid-state fermentation. Bioresource Technology, 100(21), 5249–5254.
Bradford, M. M. (1976). A rapid sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Sun, S. Y., Xu, Y., & Wang, D. (2009). Novel minor lipase from Rhizopus chinensis during solid-state fermentation: biochemical characterization and its esterification potential for ester synthesis. Bioresource Technology, 100(9), 2607–2612.
Dheeman, D. S., Antony-Babu, S., Frías, J. M., & Henehan, G. T. (2011). Purification and characterization of an extracellular lipase from a novel strain Penicillium sp. DS-39 (DSM 23773). Journal of Molecular Catalysis B: Enzymatic, 72(3–4), 256–262.
Giles, N. M., Watts, A. B., Giles, G. I., Fry, F. H., Littlechild, J. A., & Jacob, C. (2003). Metal and redox modulation of cysteine protein function. Chemistry & Biology, 10(8), 677–693.
Sheng, J., Wang, F., Wang, H., & Sun, M. (2012). Cloning, characterization and expression of a novel lipase gene from marine psychrotrophic Yarrowia lipolytica. Annals of Microbiology, 62(3), 1071–1077.
Gaur, R., Gupta, A., & Khare, S. K. (2008). Purification and characterization of lipase from solvent tolerant Pseudomonas aeruginosa PseA. Process Biochemistry, 43(10), 1040–1046.
Fickers, P., Ongena, M., Destain, J., Weekers, F., & Thonart, P. (2006). Production and down-stream processing of an extracellular lipase from the yeast Yarrowia lipolytica. Enzyme and Microbial Technology, 38(6), 756–759.
Sivaramakrishnan, R., & Muthukumar, K. (2012). Isolation of thermo-stable and solvent-tolerant Bacillus sp. lipase for the production of biodiesel. Applied Biochemistry and Biotechnology, 166(4), 1095–1111.
Ateşlier, Z. B. B., & Metin, K. (2006). Production and partial characterization of a novel thermostable esterase from a thermophilic Bacillus sp. Enzyme and Microbial Technology, 38(5), 628–635.
Moh'd, A. S., & Wiegel, J. (2007). Purification and characterization of two highly thermophilic alkaline lipases from Thermosyntropha lipolytica. Applied and Environmental Microbiology, 73(23), 7725–7731.
Syal, P., & Gupta, R. (2015). Cloning, expression, and biochemical characterization of an enantioselective lipase, YLIP9, from Yarrowia lipolytica MSR80. Applied Biochemistry and Biotechnology, 176(1), 110–124.
Fickers, P., Marty, A., & Nicaud, J. M. (2011). The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnology Advances, 29(6), 632–644.
Syal, P., & Gupta, R. (2017). Heterologous expression of lipases YLIP4, YLIP5, YLIP7, YLIP13, and YLIP15 from Yarrowia lipolytica MSR80 in Escherichia coli: substrate specificity, kinetic comparison, and enantioselectivity. Biotechnology and Applied Biochemistry, 64(6), 851–861.
Yu, M., Qin, S., & Tan, T. (2007). Purification and characterization of the extracellular lipase Lip2 from Yarrowia lipolytica. Process Biochemistry, 42(3), 384–391.
Glogauer, A., Martini, V. P., Faoro, H., Couto, G. H., Müller-Santos, M., Monteiro, R. A., Mitchell, D. A., de Souza, E. M., Pedrosa, F. O., & Krieger, N. (2011). Identification and characterization of a new true lipase isolated through metagenomic approach. Microbial Cell Factories, 10(1), 54.
Aloulou, A., Puccinelli, D., De Caro, A., Leblond, Y., & Carrière, F. (2007). A comparative study on two fungal lipases from Thermomyces lanuginosus and Yarrowia lipolytica shows the combined effects of detergents and pH on lipase adsorption and activity. Biochim. Biophys. Acta-Molecular and Cell Biology of Lipids., 1771(12), 1446–1456.
Golaki, B. P., Aminzadeh, S., Karkhane, A. A., Yakhchali, B., Farrokh, P., Khaleghinejad, S. H., Tehrani, A. A., & Mehrpooyan, S. (2015). Cloning, expression, purification, and characterization of lipase 3646 from thermophilic indigenous Cohnella sp. A01. Protein Expression and Purification, 109, 120–126.
Yang, Y., Wang, D., Zhang, X., Fang, J., Shen, Z., & Lin, C. (2014). Transgenic rice as bioreactor for production of the Candida antarctica lipase B. Plant Biotechnology Journal, 12(7), 963–970.
Kumari, A., & Gupta, R. (2012). Extracellular expression and characterization of thermostable lipases, LIP8, LIP14 and LIP18, from Yarrowia lipolytica. Biotechnology Letters, 34(9), 1733–1739.
Bordes, F., Tarquis, L., Nicaud, J. M., & Marty, A. (2011). Isolation of a thermostable variant of Lip2 lipase from Yarrowia lipolytica by directed evolution and deeper insight into the denaturation mechanisms involved. Journal of Biotechnology, 156(2), 117–124.
Kamoun, J., Schué, M., Messaoud, W., Baignol, J., Point, V., Mateos-Diaz, E., Mansuelle, P., Gargouri, Y., Parsiegla, G., Cavalier, J., Carrière, F., & Aloulou, A. (2015). Biochemical characterization of Yarrowia lipolytica LIP8, a secreted lipase with a cleavable C-terminal region. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1851(2), 129–140.
Yu, M., Lange, S., Richter, S., Tan, T., & Schmid, R. D. (2007). High-level expression of extracellular lipase Lip2 from Yarrowia lipolytica in Pichia pastoris and its purification and characterization. Protein Expression and Purification, 53(2), 255–263.
Darvishi, F., Destain, J., Nahvi, I., Thonart, P., & Zarkesh-Esfahani, H. (2012). Effect of additives on freeze-drying and storage of Yarrowia lipolytica lipase. Applied Biochemistry and Biotechnology, 168(5), 1101–1107.
Sun, J., Chen, Y., Sheng, J., & Sun, M. (2015). Immobilization of Yarrowia lipolytica lipase on macroporous resin using different methods: characterization of the biocatalysts in hydrolysis reaction. BioMed Research International, 2015(139179), 7.
Kumari, A., Verma, V. V., & Gupta, R. (2012). Comparative biochemical characterization and in silico analysis of novel lipases Lip11 and Lip12 with Lip2 from Yarrowia lipolytica. World Journal of Microbiology and Biotechnology, 28(11), 3103–3111.
Pereira-Meirelles, F., Rocha L., & Sant’ Anna, G. (1997). A stable lipase from Candida lipolytica: cultivation conditions and crude enzyme characteristics.Applied Biochemistry and Biotechnology63–65, 73–85.
Yadav, K. S., Adsul, M. . G., Bastawde, K. B., Jadhav, D. D., Thulasiram, H. V., & Gokhale, D. V. (2011). Differential induction, purification and characterization of cold active lipase from Yarrowia lipolytica NCIM 3639. Bioresource Technology, 102(22), 10663–10670.
Patel, V., Gajera, H., Gupta, A., Manocha, L., & Madamwar, D. (2015). Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: process parameters and reusability studies. Biochemical Engineering Journal, 95, 62–70.
Yan, H. D., Zhang, Q., & Wang, Z. (2014). Biocatalytic synthesis of short-chain flavor esters with high substrate loading by a whole-cell lipase from Aspergillus oryzae. Catalysis Communications, 45, 59–62.
Xu, Y., Wang, D., Mu, X. Q., Zhao, G. A., & Zhang, K. C. (2002). Biosynthesis of ethyl esters of short-chain fatty acids using whole-cell lipase from Rhizopus chinensis CCTCC M201021 in non-aqueous phase. Journal of Molecular Catalysis B: Enzymatic, 18(1–3), 29–37.
Lozano, P., Piamtongkam, R., Kohns, K., De Diego, T., Vaultier, M., & Iborra, J. L. (2007). Ionic liquids improve citronellyl ester synthesis catalyzed by immobilized Candida antarctica lipase B in solvent-free media. Green Chemistry, 9(7), 780–784.
Dheeman, D. S., Henehan, G. T., & Frías, J. M. (2011). Purification and properties of Amycolatopsis mediterranei DSM 43304 lipase and its potential in flavour ester synthesis. Bioresour Technol, 102(3), 3373–3379.
Guncheva, M. H., & Zhiryakova, D. (2008). High-yield synthesis of wax esters catalysed by modified Candida rugosa lipase. Biotechnol Lett, 30(3), 509–512.
Bi, Y., Yu, M., Zhou, H., Zhou, H., & Wei, P. (2016). Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J Mol Catal B Enzym, 133, 1–5.
Ahmed, E. H., Raghavendra, T., & Madamwar, D. (2010). An alkaline lipase from organic solvent tolerant Acinetobacter sp. EH28: application for ethyl caprylate synthesis. Bioresour Technol, 101(10), 3628–3634.
Fickers, P., Fudalej, F., Le Dall, M. T., Casaregola, S., Gaillardin, C., Thonart, P., & Nicaud, J. M. (2005). Identification and characterisation of LIP7 and LIP8 genes encoding two extracellular triacylglycerol lipases in the yeast Yarrowia lipolytica. Fungal Genet Biol, 42(3), 264–274.
Aguieiras, E. C. G., Cavalcanti-Oliveira, E. D., de Castro, A. M., Langone, M. A. P., & Freire, D. M. G. (2014). Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel, 135, 315–321.
Soares, D., Pinto, A. F., Gonçalves, A. G., Mitchell, D. A., & Krieger, N. (2013). Biodiesel production from soybean soapstock acid oil by hydrolysis in subcritical water followed by lipase-catalyzed esterification using a fermented solid in a packed-bed reactor. Biochem Eng J, 81, 15–23.
Salah, R. B., Ghamghui, H., Miled, N., Mejdoub, H., & Gargouri, Y. (2007). Production of butyl acetate ester by lipase from novel strain of Rhizopus oryzae. J Biosci Bioeng, 103(4), 368–372.
Bourg-Garros, S., Razafindramboa, N., & Pavia, A. A. (1997). Synthesis of (Z)-3-hexen-1-yl butyrate in hexane and solvent-free medium using Mucor miehei and Candida antarctica lipases. J Am Oil Chem Soc, 74(11), 1471–1475.
Ozyilmaz, G., & Gezer, E. (2010). Production of aroma esters by immobilized Candida rugosa and porcine pancreatic lipase into calcium alginate gel. J Mol Catal B Enzymatic, 64(3–4), 140–145.
Alves, M. D., Cren, E. C., & Mendes, A. A. (2016). Kinetic, thermodynamic, optimization and reusability studies for the enzymatic synthesis of a saturated wax ester. J Mol Catal B Enzym, 133, S377–S387.
Acknowledgments
The authors would like to thank the Brazilian research funding agencies FAPERJ, CNPq, and CAPES for the financial support.
The authors thank Prof. Marcelo Prado and Flávio Ramos (Laboratory of Scanning and Transmission Electron Microscopy from Military Engineering Institute, Rio de Janeiro, Brazil) for assisting with the scanning electron microscopy analysis presented in this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, C.E.C., Ribeiro, B.D. & Coelho, M.A.Z. Characterization and Application of Yarrowia lipolytica Lipase Obtained by Solid-State Fermentation in the Synthesis of Different Esters Used in the Food Industry. Appl Biochem Biotechnol 189, 933–959 (2019). https://doi.org/10.1007/s12010-019-03047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03047-5