Abstract
This study aimed to evaluate the use of a lyophilized fermented solid (named solid enzymatic preparation, SEP), with lipase activity, as a low-cost biocatalyst for esterification reactions of fatty acids present in acid raw materials for biodiesel synthesis. The SEP was obtained by solid-state fermentation (SSF) of soybean bran using the strain of Yarrowia lipolytica IMUFRJ 50682 and contains the lipases secreted by this yeast. The esterification reaction of ethanol and the predominant fatty acids present in different acid oil sources for biodiesel production (oleic, linoleic, stearic and palmitic acids) was investigated. Oleic acid conversion of above 85% was obtained after 24 h, using 30 wt% of SEP and ethanol/oleic acid molar ratio of 1, at 30 °C, in a reaction medium with and without solvent (n-hexane). Similar results were achieved with stearic (79%), palmitic (82%) and linoleic (90%) acids. The reusability of SEP was investigated over ten successive batches by washing it with different solvents (ethanol, water or n-hexane) between the cycles of ethyl oleate synthesis. Washing with water allowed the SEP to be reused for six cycles maintaining over 80% of the conversion reached in the first cycle. These results show the potential of this biocatalyst to reduce the content of free fatty acids in acid oils for biodiesel synthesis with a potential to be applied in a broad plethora of raw materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a biodegradable and non-toxic biofuel composed of a mixture of methyl or ethyl esters of long-chain fatty acids derived from renewable sources (e.g., vegetable oils, animal fats or microbial oils). This biofuel was introduced in the Brazilian energy matrix in 2005 and Brazil is currently one of the largest producers and consumers of biodiesel in the world (Bergmann et al. 2013; Colombo et al. 2018).
The main resource for biodiesel production in Brazil is soybean oil. However, alternative sources of raw materials for oil production have been studied to meet the growing demand for biodiesel in the country. In this context, the use of acid oils such as macauba (Acrocomia aculeata), palm (Elaeis guineensis), and jatropha (Jatropha curcas) oils as a raw material has been investigated (D’Agosto et al. 2015; Colombo et al. 2018; Ribeiro et al. 2011). The high concentration of free fatty acids (FFA) is one of the major disadvantages of these feedstocks, which prejudice the conventional homogeneous alkali-catalyzed process for biodiesel production.
The use of lipases to reduce the FFA content present in acid raw materials is a very promising approach (Jegannathan et al. 2011; Hama and Kondo 2013). Lipases can simultaneously catalyze the transesterification of triglycerides (TAG) and the esterification of the FFAs present in acid oils (Aguieiras et al. 2017a, b; Christopher et al. 2014). However, immobilized microbial commercial lipases still present high costs (Firdaus et al. 2016). Thus, the use of non-commercial lipases, such as those produced by Yarrowia lipolytica, a non-conventional yeast and one of the most widely studied oleaginous microorganisms (Ledesma and Nicaud 2016), has been investigated to minimize the costs of the biocatalyst (Fickers et al. 2011; Yu et al. 2007).
The direct use of dried fermented solids, obtained by solid-state fermentation (SSF), has also been used in biodiesel synthesis (Aguieiras et al. 2017a, b, 2014; Soares et al. 2015). The fermented solid, named solid enzymatic preparation (SEP), can be obtained from solid agroindustrial wastes, by-products or products which are abundant in Brazil, such as soybean bran, serving as a nutrient source for solid-state fermentation as well as enzyme support (Ferreira-Leitão 2017). Enzyme immobilization is a requisite for scale applications considering the advantages that an immobilized biocatalyst offers: enhances their operational stability, allows the reuse of biocatalysts and enables continuous processes (Hanefeld et al. 2009; Rodrigues et al. 2013). Therefore, the natural immobilization of lipases on the fermented solid enables its direct use in the reaction medium, and avoids the steps of extraction, purification and enzyme immobilization, lowering even more the cost of the final biocatalyst (Aguieiras et al. 2015, 2019; Fernandes et al. 2007; Liu et al. 2013; Veerabhadrappa et al. 2014). However, the enzyme release can occur, and the stability of the biocatalyst has to be investigated.
Therefore, the objective of this study was to evaluate the catalytic activity of the SEP for fatty acid esterification, considering its use in biodiesel production from acid oils. The biocatalyst SEP consisted of lyophilized fermented solid obtained by SSF of Yarrowia lipolytica IMUFRJ 50,682 in soybean bran, and containing the lipases secreted by this yeast. The esterification between oleic acid and ethanol was chosen as a model reaction since oleic acid is one of the predominant fatty acids in vegetable oils used for biodiesel synthesis and it is liquid at ambient temperature. Most common feedstocks present fatty acid profiles consisting mainly of palmitic, stearic, oleic and linoleic acids (Aarthy et al. 2014). Therefore, the esterification of palmitic, stearic and linoleic acids with ethanol was also investigated using the SEP of Yarrowia lipolytica.
Materials and methods
Solid enzymatic preparation (SEP)
The fermented solid was obtained by solid-state fermentation (SSF) of soybean bran (kindly supplied by ADM do Brasil), using a strain of Yarrowia lipolytica IMUFRJ 50,682. The SSF was done in tray-type bioreactors containing 10 g of soybean bran supplemented with 4 wt% of soy soapstock. Phosphate buffer solution at 100 mmol L−1 (pH 7) was added to obtain 58% moisture. These reactors were autoclaved at 121 °C and 1 atm for 20 min. The soybean bran was inoculated with 6.67 g L−1 (dry weight) of Yarrowia lipolytica cells and cultivated at 28 °C under humidified air injection. After 14 h, the fermented solids were lyophilized, stored at 4 °C, and named solid enzymatic preparation (SEP). These fermentation processes were previously investigated by Souza et al. (2017), in very similar conditions, when it was observed by zymography that multiple lipases/esterases were produced. Concerning this characteristic, SEP could be considered a combi-lipase biocatalyst as described by Poppe et al. (2018), since a combination of different lipases can be part of its composition, which could represent more effectiveness on heterogeneous substrates if compared to one specific lipase.
The esterification activity of the SEP was determined by measuring the consumption of acid, after 6 h, in the esterification reaction oleic acid/ethanol at the equimolar ratio, with 20 wt% of SEP (mass SEP/mass reaction medium), at 30 °C. One esterification unit (U) was defined as the enzyme amount that catalyzes the conversion of 1 µmol of fatty acid per minute under the experimental conditions. The esterification activity of SEP was 16 U g−1 (µmoles of acid·min−1 g−1).
Raw material and reagents
Oleic acid (C18:1), linoleic acid (C18:2), stearic acid (C18:0), palmitic acid (C16:0), ethanol 99.8%, ethanol 95%, ethyl acetate, n-hexane (99%), sodium hydroxide and Karl Fischer solution (without pyridine) 1 mL = 5.0 mg, with analytical grade, were obtained from Vetec Fine Chemicals Ltda. (Rio de Janeiro, Brazil). Ethyl oleate (98%) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Esterification reactions
Esterification reactions employing FFAs and ethanol were carried out in closed 15-mL batch reactors magnetically stirred and thermostated. The medium was composed of the SEP and reagents. Reaction progress was monitored by sample withdrawal, in duplicates, at fixed intervals. Samples were centrifuged for 5 min at 7000 rpm (Eppendorf centrifuge MiniSpin), and subsequently analyzed for unreacted FFAs and formed esters contents. Each parameter investigated is described in more detail as follows.
Amount and method of ethanol addition
The effects of ethanol amount were studied considering ethanol/oleic acid molar ratio (R) of 1 (stoichiometric molar ratio) and 2, using 0.02 mol of oleic acid. The ethanol addition method (single or stepwise) was also studied. The total volume of ethanol was added at the beginning of the reaction (t = 0 h) for single addition, or in a stepwise manner, in which 50% of ethanol volume was added at the beginning of the reaction (t = 0 h), and the other 50% was added after 3 h. All reactions were performed at 30 °C, for 48 h, using 30 wt% of SEP (based on the total mass of the reaction medium).
Temperature
The effect of temperature (30, 40, 50, 60, and 75 °C) was studied using 30 wt% of SEP and the stoichiometric ratio of the reagents (0.02 mol of oleic acid and 0.02 mol of ethanol), for 48 h. All ethanol volume was added at the beginning of the reaction (t = 0 h). The blank test (without biocatalyst) was carried out at 75 °C.
Concentration and method of SEP addition
The effect of biocatalyst concentration was studied using 20 and 30 wt% of SEP. The method of biocatalyst addition (single or stepwise) was also evaluated. In a single addition, 30 wt% of the biocatalyst was added at the beginning of the reaction (t = 0 h), while in stepwise method, 20 wt% was added at the beginning of the reaction (t = 0 h) and the other 10 wt% was added after 5 h. The reactions were carried out with the stoichiometric ratio (0.02 mol of oleic acid and 0.02 mol of ethanol) of the reactants for 48 h, at 30 °C.
Water concentration in ethanol
The effect of the water concentration in ethanol (0.2, 5.8 and 11.2 wt%) was studied using as reactants either anhydrous (99.8%v/v) or hydrous (95 and 90%v/v) ethanol. The reactions were carried out with the stoichiometric molar ratio of the reactants (0.02 mol of oleic acid and 0.02 mol of ethanol), for 48 h, at 30 °C. The water concentration was determined by titration using Karl Fisher Titrator Mettler Toledo T50.
Fatty acid chain
The effects of type (saturated and unsaturated) and length of the fatty acid chain were studied in esterification reactions of oleic (C18:1), linoleic (C18:2), stearic (C18:0) and palmitic (C16:0) acids with ethanol, using ethanol/fatty acid molar ratio of 1 (0.02 mol of each reagent), for 48 h. The reactions were carried out in a solvent-free system at 30 °C for oleic and linoleic acids and at 75 °C, for oleic, stearic and palmitic acids. Some experiments were carried out using a solvent (n-hexane, 80% v/v), at 30 °C, as indicated in the Results and discussion section.
Reuse of SEP
For the study of the SEP reuse, the ethyl oleate synthesis was repeated for ten cycles, each one of 26 h. The reaction was carried out with ethanol/oleic acid molar ratio of 1 and 30 wt% of SEP, at 30 °C. After each batch, the SEP was washed with 20 mL of solvent (ethanol, n-hexane, or water), vacuum filtered and placed in a desiccator (at 8 °C, 24 h) until the following reaction.
Analyses
Esterification progress was monitored by the quantification of the unconverted FFAs present in the reaction medium by volumetric neutralization, taking duplicate samples (50 µL) during all the reaction time. The titrating solution was NaOH 0.02 mol L−1, and the analysis was carried out in an automatic titrator Mettler Toledo T50. The conversion was defined as the number of moles of fatty acids reacted per mole of fatty acids fed to the system.
Oleic acid and fatty acid ethyl esters (FAEE) were analyzed by gas chromatography (GC) in a gas chromatograph Varian model CP 3800 equipped with a CP-WAX 52 CB column (30 m × 0.25 mm × 0.25 µm) and flame ionization detector (FID) in split injection system (1:50). The temperatures of the injector and detector were maintained at 250 °C. The flow rate of carrier gas (H2) was 2 mL min−1, and the pressure in the system was 12 psi. The oven temperature was initially maintained at 170 °C for 3 min, and after that increased to 245 °C at 15 °C min−1 and kept at this temperature for 8 min. A computer equipped with the Star Workstation 6.2 software was connected to the gas chromatograph using the interface module (Star 800), for automatic integration of peaks. Before analysis, samples (100 or 200 µL) were centrifuged and diluted (1:5) with ethyl acetate:n-hexane solution 1:1 (v/v). Concentrations were calculated based on peak areas using calibration curves. According to the results of the chromatographic analysis, all consumed FFA was converted to FAEE. Thus, the yield of FAEE, calculated as the relationship between the number of moles of FAEE formed and the initial number of moles of FA, was equivalent to the FFA conversion in the experiments.
Results and discussion
Influence of the amount and method of ethanol addition
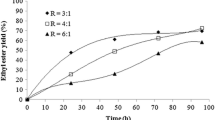
The reaction of an alcohol with a carboxylic acid is a reversible reaction. The equilibrium is shifted towards products using a large excess of one reactant (usually alcohol is used as the reaction solvent) and also removing the water formed. In this work, using Le Chatelier’s principle to shift the equilibrium to ester synthesis, the use of an excess of ethanol was investigated. However, the enzyme activity depends on the nature of the solvent (Carrea and Riva 2008). In general, enzyme activity and stability are lower in hydrophilic solvents (log P < 2), and ethanol is known to cause inhibition and denaturation of lipases. One of the strategies used to minimize enzyme inactivation and/or inhibition induced by hydrophilic solvents is the stepwise addition of the alcohol (Shimada et al. 2002; Bernardes et al. 2007; Nielsen and Rancke-Madsen 2011; Meng et al. 2011; Gog et al. 2012; Aguieiras et al. 2015). Therefore, the influence of the amount of ethanol and its addition method (single or stepwise) was evaluated using two different ethanol/oleic acid molar ratios (1 and 2). The results are shown in Fig. 1.
It can be seen in Fig. 1 that the stepwise addition of ethanol allowed a higher reaction rate up to 4 h, indicating that a lower concentration of ethanol in contact with lipase reduced enzyme inhibition and/or inactivation. However, after 48 h, the oleic acid conversion values were similar for both conditions. Meng et al. (2011) also studied the stepwise addition of ethanol, using ethanol:FFA molar ratio of 2, in one, three, five and ten steps. The authors also observed that the esterification reaction was inhibited when ethanol was added by one-step method. The esterification degree was about 50% in this condition. However, the esterification degree increased to 81.6% with the ethanol addition in ten steps.
The use of an excess of alcohol has been one method widely used to increase the reaction rate and the conversion, by shifting the equilibrium towards product formation. However, high concentrations of alcohol in reaction medium affect lipase activity (Sun et al. 2012, 2013; Yu et al. 2007). As shown in Fig. 1, the addition of an excess of ethanol, even in stepwise addition, did not result in an increase in the acid conversion, which reached 64% after 48 h. This value was lower than that achieved for the reaction carried out with the ethanol/oleic acid at the stoichiometric ratio.
Influence of the temperature
The influence of temperature was studied in the esterification reaction of oleic acid and ethanol using an ethanol/oleic acid molar ratio of 1 (single addition), and the results are shown in Fig. 2. A blank test (without biocatalyst) was also carried out at 75 °C and the oleic acid concentration remained almost constant (acid conversion near to 0%) during 48 h.
For reaction times (tr) lower than 6 h, it can be observed in Fig. 2a that the higher oleic acid conversions were obtained at 40 °C. However, for higher reaction times (tr > 6 h), the oleic acid conversion decreases as temperature increases, and higher conversions were reached at 30 °C. Similar trends were observed for the initial reaction rates. According to Fig. 2b, the increase in temperature promoted an increase in the initial reaction rate up to 40 °C, and above this temperature, it was observed a considerable drop in these values. This effect was more pronounced at 75 °C, and the oleic acid conversion decreased approximately 39% when compared to the reaction carried out at 30 °C (Fig. 2b).
For the studied reaction system, the two main effects caused by temperature increase in enzyme-catalyzed reactions were: (1) increase in the reactivity of the enzyme–substrate complex, directly related to the reaction rate and (2) enzyme denaturation due to loss of their native three-dimensional structure. The first effect is not affected by reaction time, but the second effect is strongly influenced by it (Huang et al. 2012). Yu et al. (2007) observed that the optimum temperature of the Y. lipolytica extracellular and purified lipase (YlLip2) was 40 °C. Above 45 °C, the activity was reduced, and at 60 °C, no activity was observed.
Influence of the concentration and addition method of SEP
The effects of biocatalyst concentration and its addition method (single or stepwise) were evaluated in the esterification reaction of oleic acid with ethanol, using R = 1, at 30 °C. SEP amount higher than 30 wt% promoted restrictions on mass transfer due to the difficulty of homogenization of the reaction medium. Thus, the SEP concentrations tested were 20 and 30 wt% and the oleic acid conversion obtained after 24 h was 65% and 85%, respectively, as shown in Fig. 3.
According to the results illustrated in Fig. 3, in the conditions employed, it can be seen that the equilibrium condition was achieved with 85% acid conversion using 30 wt% of SEP, with all the biocatalyst added at the beginning of the reaction. A reduction in the reaction rate was observed after 6 h using 20 wt% of SEP (Fig. 3), probably due to enzyme denaturation. So, fresh biocatalyst was added at 5 h. In this experiment (stepwise addition of biocatalyst), 20 wt% of SEP was added at the beginning of the reaction (t = 0 h), and more 10 wt% was added after 5 h. As expected, the oleic acid conversion was higher than with 20 wt% of SEP after 24 h. However, it was lower than those observed with single 30 wt% SEP addition. Two effects can explain these results: (i) the lower amount of biocatalyst clearly affects the reaction kinetics, and (ii) the water produced in the esterification reaction can be adsorbed in the SEP. Therefore, a lower amount of SEP leads to water accumulation in the bulk reaction medium. The presence of water may affect reaction rate by its participation as a reactant (reverse reaction), as well as via effects on biocatalyst hydration and hence activity (Halling 1994).
Esterification of an alcohol and an organic acid involves a reversible equilibrium; therefore, these reactions usually do not go to completion. They are limited by thermodynamic equilibrium. There are some strategies to enhance the conversion of esterification reaction by removing one of the products formed. In general, to maximize the reaction conversion, the water is removed by the use of desiccants (molecular sieves, silica gel, alumina), vacuum or pervaporation (Chandane et al. 2016). The sorption of water onto fermented solids has been already reported (Soares et al. 2015; Halling 1994) and could shift the equilibrium towards ester synthesis if the solid acts as a desiccant removing water from the reaction medium. On the other hand, in the esterification reaction using immobilized lipases, water produced may be accumulated in the enzyme environment, producing its inhibition or even its inactivation (Alves et al. 2014). According to the results shown in Fig. 3, an increase of equilibrium conversion with the increase of SEP amount was observed, therefore, indicating that the adsorbed water did not decrease enzymatic activity.
Influence of the water concentration in ethanol
The amount of water present in the ethanol decreases the yield of the esterification of fatty acids since water is a by-product, and a reagent of the reverse hydrolysis reaction, and affects enzyme activity. However, the use of anhydrous ethanol impacts on production cost.
Water plays two significant roles in the enzymatic esterification reaction: (1) it is essential for lipase conformation and, therefore, its catalytic activity and stability (Carrea and Riva 2008; Gog et al. 2012; Stergiou et al. 2013; Christopher et al. 2014), and (2) is the product of esterification reaction and directly affects the equilibrium of the reaction (Halling 1994; Meng et al. 2011).
Therefore, the effect of the water concentration in the alcohol was studied in the esterification reaction of oleic acid with ethanol having different amounts of water (0.2, 5.8 and 11.2 wt%). The results obtained in the reactions carried out with the stoichiometric ratio of reagents (R = 1), at 30 °C, are shown in Fig. 4. The conversion values after 48 h were similar for all alcohol sources tested. These results are interesting, considering biodiesel production cost. The use of ethanol 95% is advantageous because the price of this azeotropic mixture is lower than the price of anhydrous ethanol.
Influence of free fatty acid chain
The specificity of Yarrowia lipolytica lipase toward FFAs was examined in the esterification reaction with ethanol. FFAs with different chain sizes (C16 and C18) and unsaturations (C18:0, C18:1, C18:2) were tested using 30 wt% of SEP. These FFAs are those predominant in a wide range of feedstock: edible oils, non-edible oils, animal fat and oleaginous yeasts (Aarthy et al. 2014). Palmitic (C16) and stearic (C18) acids are solids at 30 °C, and their melting ranges are 59–62 °C and 68–71 °C, respectively. Therefore, the esterification reactions with these acids were studied in a solvent medium (n-hexane, 80% v/v), at 30 °C, or in a solvent-free system, at 75 °C.
Usually, organic solvents are used for enzymatic synthesis of biodiesel, because they improve the solubility of hydrophilic alcohols and hydrophobic triglycerides, and also enable the decrease of enzymatic denaturation when a high concentration of alcohol is employed. The organic solvents most suitable for use in the enzymatic synthesis of biodiesel are hydrophobic solvents such as isooctane, n-heptane, petroleum ether, n-hexane and cyclohexane (Gog et al. 2012). In this work, n-hexane was the solvent chosen, but it was observed that it did not influence on the oleic acid conversion (Fig. 5).
Figure 5 also shows the results of the experiments carried out at 30 °C with the different FFAs, and in Fig. 6 it can be seen the results of the experiments performed at 75 °C for oleic, stearic and palmitic acids.
For lower reaction times (tr < 8 h), the Y. lipolytica lipase showed higher specificity for the unsaturated fatty acid chain, as can be seen in Figs. 5 and 6. After 48 h, the acid conversion values were similar for the tested acids. The same effect was also observed for reactions carried out at 75 °C (Fig. 6). Regarding the size of the acid chain, the highest conversion was observed with the higher chain length acid (C18), at 75 °C. Brígida et al. (2014) examined the selectivity of the extracellular lipase of Y. lipolytica (Lip2p) towards substrates of different sizes and observed that this lipase prefers substrates containing long-chain fatty acids (C12, C14, and C16). Yu et al. (2007) also observed an increase in the activity of purified extracellular lipase of Y. lipolytica (YlLip2) for methyl esters of C12–C16, confirming the preference of this enzyme towards FFAs of a higher carbon chain.
Considering these results, the use of the dried fermented solid (SEP) of Y. lipolytica IMUFRJ 50,682 proved to be effective for the esterification reaction of the predominant FFAs in oil sources.
Reuse of the SEP
One of the significant disadvantages of the enzymatic route for biodiesel synthesis is the cost of the biocatalyst. To minimize the process cost, the biocatalyst must be reused. Therefore, the SEP reusability was investigated in this work, over ten successive batches. After each cycle, the SEP was washed with solvent (ethanol, n-hexane, water). Ethanol was chosen as a washing solvent considering the possibility of its reuse as a substrate for ethyl oleate synthesis. n-Hexane is known as a solvent suitable for lipase stability, whereas water is the less expensive and most environmentally friendly solvent. The reactions were carried out at the best conditions found: ethanol/oleic acid molar ratio of 1, at 30 °C, with all ethanol volume and the biocatalyst (30 wt% of SEP) added at the beginning of the reaction. Reactions were carried out for 26 h to ensure that equilibrium was achieved.
The initial rates, as well as the relative conversion after 26 h of reaction (Fig. 7), indicate that the use of n-hexane and ethanol resulted in a pronounced decrease of acid conversion after the first cycle. On the other hand, water was the best solvent used for washing the biocatalyst along the ten cycles since the initial rate decreases smoothly and gradually, whereas a small drop on the oleic acid relative conversion was observed up to the 3rd cycle and at the 6th cycle relative conversions higher than 80% were still observed. The oleic acid relative conversion at the end of the reaction was 31, 13 and 8% after ten batches using water, n-hexane, and ethanol, respectively. These results showed that the lipase secreted by Yarrowia lipolytica remains adhered in the lyophilized fermented matrix during the reaction and after washing it with solvent. However, the reduction in the activity due to a partial enzyme release is likely to occur even in the systems with a small content of water.
The choice of washing solvent is not an easy task because the enzyme activity in organic solvents varies significantly according to the solvent used. This choice must take into account important factors such as the removal of the contaminants adsorbed on the support and the inactivation of the enzyme. The solvent can change the native conformation of the protein through the disruption of hydrophobic interactions and hydrogen bonds, as well as affect the enzyme–water interactions by removing the essential layer of water, which can dramatically reduce the activity and stability of the enzyme (Carrea and Riva 2008; Aguieiras et al. 2016).
Usually, the use of a hydrophobic solvent (log P > 4) is recommended for enzyme systems to avoid enzyme denaturation. Rodrigues et al. (2008) studied the reuse of the commercial lipases Lipozyme RM-IM, Lipozyme TL-IM and Novozym 435 using washing solvents (n-hexane, ethanol, propanol, and water) during the transesterification reaction of soybean oil. In all cases, washing with n-hexane caused higher retention of enzymatic activity of the lipase. According to the authors, the main reaction components are nonpolar and the use of nonpolar solvents (e.g., n-hexane) aid in the removal of these compounds that could be adsorbed onto the biocatalyst surface. Aguieiras et al. (2017b) also observed that the efficiency of the washing solvent (hexane, ethanol) in esterification reaction had been related to the stability of the biocatalyst (dry lipases immobilized in fermented solid obtained by SSF of Rhizomucor miehei) and the composition of the reaction medium.
The major components that may have remained adsorbed onto the biocatalyst surface after esterification of oleic acid and ethanol are polar (ethyl oleate, ethanol, and water). Therefore, the use of water and ethanol as solvents would be more suitable for the removal of these components than n-hexane.
Lin et al. (2014) investigated the reuse of immobilized lipase from Y. lipolytica in the transesterification of olive oil and methanol after washing with water. They studied the effect of six Fe-MCM-41 carriers with different pore sizes. They found that the lipase immobilized on the carrier with a pore size of 4.27 nm could be reused in biodiesel production for a total of nine cycles and that the conversion yield was still higher than 90% after six cycles. In contrast, the other lipase preparations showed poor reusability. Thus, the support used for enzyme immobilization also influences the reusability of the biocatalyst. As reported by Rodrigues et al. (2013), immobilization can alter the physicochemical properties of the enzyme surroundings, producing a more hydrophobic or hydrophilic environment, resulting in a partition of some compounds away or towards the enzyme. Soares et al. (2015) observed that part of the reaction medium was sorbed on the fermented solid (ferment solid containing lipases produced by Burkholderia cepacia LTBE 11), forming a sorbed phase during the ethyl esterification of fatty acids in a packed-bed reactor. The sorbed phase was composed predominantly of water and ethanol, and the molar ratio of ethanol to fatty acid in this sorbed phase was higher than those in the bulk reaction medium.
Conclusions
The results showed the technical feasibility of using a cost-effective and eco-friendly catalyst, such as SEP, obtained by solid-state fermentation of soybean bran with Yarrowia lipolytica IMUFRJ 50,682, for enzymatic synthesis of fatty acid ethyl esters. The use of the SEP allowed obtaining oleic acid conversions higher than 80% after 24 h, under mild conditions (30 °C). Moreover, the performance of this biocatalyst was not affected by the presence of water in concentrations of up to 11 wt% in the reaction medium, and it can be reused after washing with water up to six consecutive batches, retaining more than 80% of its initial activity.
References
Aarthy M, Saravanan P, Gowthaman MK, Rose C, Kamini NR (2014) Enzymatic transesterification for production of biodiesel using yeast lipases: an overview. Chem Eng Res Des 92:1591–1601
Aguieiras ECG, Cavalcanti-Oliveira ED, Castro AM, Langone MAP, Freire DMG (2014) G. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hidroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 135:315–321
Aguieiras ECG, Cavalcanti-Oliveira ED, Freire DMG (2015) Current status and new developments of biodiesel production using fungal lipases. Fuel 159:52–67
Aguieiras ECG, Ribeiro DS, Couteiro PP, Bastos CMB, Queiroz DS, Parreira JM, Langone MAP (2016) Investigation of the reuse of immobilized lipases in biodiesel synthesis: influence of different solvents in lipase activity. App Biochem Biotechnol 179:485–496
Aguieiras ECG, Cavalcanti-Oliveira ED, Castro AM, Langone MAP, Freire DMG (2017a) Simultaneous enzymatic transesterification and esterification of an acid oil using fermented solid as biocatalyst. J Am Oil Chem Soc 94:551–558
Aguieiras ECG, Barros DSN, Sousa H, Fernandez-Lafuente R, Freire DMG (2017b) Influence of the raw material on the final properties of biodiesel producedusing lipase from Rhizomucor miehei grown on babassu cake as biocatalyst of esterification reactions. Renew Energy 113:112–118
Aguieiras ECG, Barros DSN, Fernandez-Lafuente R, Freire DMG (2019) Production of lipases in cottonseed meal and application of the fermented solid as biocatalyst in esterification and transesterification reactions. Renew Energy 130:574–581
Alves JS, Garcia-Galan C, Schein MF, Silva AM, Barbosa O, Ayub MAZ, Fernandez-Lafuente R, Rodrigues RC (2014) Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 19:9562–9576
Bergmann JC, Tupinamba DD, Costa OYA, Almeida JRM, Barreto CC, Quirino BF (2013) Biodiesel production in Brazil and alternative biomass feedstocks. Renew Sust Energy Rev 21:411–420
Bernardes OL, Bevilaqua JV, Leal MCMR, Freire DMG, Langone MAP (2007) Biodiesel fuel production by the transesterification reaction of soybean oil using immobilized lipase. Appl Biochem Biotechnol 136–140:105–114
Brígida AIS, Amaral PFF, Coelho MAZ, Gonçalves LRB (2014) Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J Mol Cat B 101:148–158
Carrea G, Riva S (2008) Organic Synthesis with Enzymes in Non-aqueous Media. Wiley, Weinheim
Chandane VS, Rathod AP, Wasewar KL (2016) Enhancement of esterification conversion using pervaporation membrane reactor. Resour Effic Technol 2:S47–S52
Christopher LP, Kumar H, Zambare VP (2014) Enzymatic biodiesel: challenges and opportunities. Appl Energy 119:497–520
Colombo CA, Berton LHC, Diaz BG, Ferrari RA (2018) Macauba: a promising tropical palm for the production of vegetable oil. Oilseeds Fats Crop Lipid 25:1. https://doi.org/10.1051/ocl/2017038
D’Agosto MA, Silva MAV, Oliveira CM, Franca LS, Marques LGC, Murta ALS, Freitas MAV (2015) Evaluating the potential of the use of biodiesel for power generation in Brazil. Renew Sust Energy Rev 43:807–817
Fernandes MLM, Saad EB, Meira JA, Ramos LP, Mitchell DA, Krieger N (2007) Esterification and transesterification reactions catalysed by addition of fermented solids to organic reaction media. J Mol Cat B: 44:8–13
Ferreira-Leitão VS, Cammarota MC, Aguieiras ECG, Sá LRV, Fernandez-Lafuente R, Freire DMG (2017) The protagonism of biocatalysis in green chemistry and its environmental benefits. Catalysts 7:9:1–34
Fickers P, Marty A, Nicaud JM (2011) The lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotech Adv 29:632–644
Firdaus MY, Guo Z, Fedosov SN (2016) Development of kinetic model for biodiesel production using liquid lipase as a biocatalyst, esterification step. Biochem Eng J 105:52–61
Gog A, Roman M, Tosa M, Paizs C, Irimie FD (2012) Biodiesel production using enzymatic transesterification—current state and perspectives. Renew Energy 39:10–16
Halling PJ (1994) Thermodynamic predictions for biocatalysis in nonconventional media: Theory, tests, and recommendations for experimental design and analysis. Enzyme Microb Technol 16:178–206
Hama S, Kondo A (2013) Enzymatic biodiesel production: an overview of potential feedstocks and process development. Bioresour Technol 135:386–395
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilization. Chem Soc Rev 38:453–468
Huang D, Han S, Han Z, Lin Y (2012) Biodiesel production catalyzed by Rhizomucor miehei lipase-displaying Pichia pastoris whole cells in an isooctane system. Biochem Eng J 63:10–14
Jegannathan KR, Eng-Seng C, Ravindra P (2011) Economic assessment of biodiesel production: comparison of alkali and biocatalyst processes. Renew Sust Energy Rev 15:745–751
Ledesma-Amaro R, Nicaud JM (2016) Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Progr Lipid Res 61:40–50
Lin J, Zhao B, Cao Y, Xu H, Ma S, Guo M, Qiao D, Cao Y (2014) Rationally designed Fe-MCM-41 by protein size to enhance lipase immobilization, catalytic efficiency and performance. Appl Catal A 478:175–185
Liu Y, Li C, Meng X, Yan Y (2013) Biodiesel synthesis directly catalyzed by the fermented solid of Burkholderia cenocepacia via solid state fermentation. Fuel Proc Technol 106:303–309
Meng Y, Wang G, Yang N, Zhou Z, Li Y, Liang X, Chen J, Li Y, Li J (2011) Two-step synthesis of fatty acid ethyl ester from soybean oil catalyzed by Yarrowia lipolytica lipase. Biotech Biofuel 4:6
Nielsen PM, Rancke-Madsen A (2011) Enzymatic large-scale production of biodiesel. Lipid Technol 23:230–233
Poppe JK, Matte CR, Fernandez-Lafuente R, Rodrigues RC, Ayub MAZ (2018) Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl Biochem Biotechnol 1–14
Ribeiro BD, Castro AM, Coelho MAZ, Freire DMG (2011) Production and use of lipase in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res 2011:1–16. https://doi.org/10.4061/2011/615803
Rodrigues RC, Volpato G, Wada K, Ayub MAZ (2008) Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J Am Oil Chem Soc 85:925–930
Rodrigues RC, Ortiz C, Berenguer-Murcia Á, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Ver 42:6290–6307
Shimada Y, Watanabe Y, Sugihara A, Toninaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J Mol Catal B: Enzym 17:133–142
Soares D, Serres JDS, Corazza ML, Mitchell DA, Gonçalves AG, Krieger N (2015) Analysis of multiphasic behavior during the ethyl esterification of fatty acids catalyzed by a fermented solid with lipolytic activity in a packed-bed bioreactor in a closed-loop batch system. Fuel 159:364–372
Souza CEC, Farias MA, Ribeiro BD, Coelho MAZ (2017) Adding value to agro-industrial co-products from canola and soybean oil extraction through lipase production using Yarrowia lipolytica in solid-state fermentation. Waste Biomass Valor 8:1163–1176
Stergiou PY, Foukis A, Filippou M, Koukouritaki M, Parapouli M, Theodorou LG, Hatziloukas E, Afendra A, Pandey A, Papamichael EM (2013) Advances in lipase-catalyzed esterification reactions. Biotech Adv 31:1846–1859
Sun J, Yu B, Curran P, Liu SQ (2012) Lipase-catalysed transesterification of coconut oil with fusel alcohols in a solvent-free system. Food Chem 134:89–94
Sun J, Yu B, Curran P, Liu SQ (2013) Lipase-catalysed ester synthesis in solvent-free oil system: is it esterification or transesterification? Food Chem 141:2828–2832
Veerabhadrappa MB, Shivakumar SB, Devappa S (2014) Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J Biosci Bioeng 117:208–214
Yu M, Qin S, Tan T (2007) Purification and characterization of the extracellular lipase Lip2 from Yarrowia lipolytica. Proc Biochem 42:384–391
Acknowledgements
The authors would like to thank PETROBRAS and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) for the financial support received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
da Silva, J.R., de Souza, C.E.C., Valoni, E. et al. Biocatalytic esterification of fatty acids using a low-cost fermented solid from solid-state fermentation with Yarrowia lipolytica. 3 Biotech 9, 38 (2019). https://doi.org/10.1007/s13205-018-1550-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1550-2