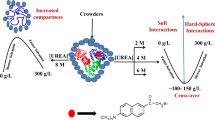
Abstract
The folding and unfolding of proteins inside a cell take place in the presence of macromolecules of various shapes and sizes. Such crowded conditions can significantly affect folding, stability, and biophysical properties of proteins. Thus, to logically mimic the intracellular environment, the thermodynamic stability of two different proteins (lysozyme and α-lactalbumin) was investigated in the presence of mixtures of three crowding agents (ficoll 70, dextran 70, and dextran 40) at different pH values. These crowders possess different shapes and sizes. It was observed that the stabilizing effect of mixtures of crowders is more than the sum effects of the individual crowder, i.e., the stabilizing effect is non-additive in nature. Moreover, dextran 40 (in the mixture) has been found to exhibit the greatest stabilization when compared with other crowders in the mixture. In other words, the small size of the crowder has been observed to be a dominant factor in stabilization of the proteins.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cell comprises cytoplasm with complex architecture including all the proteins, other biomolecules, metabolites, and all the necessary raw materials and machines entailed for the protein synthesis and folding processes. The situation present inside the cell and the condition which accounts for the dilute media, i.e., the idealized conditions, are absolutely different from each other [1,2,3]. The macromolecules which include different proteins, carbohydrates, nucleic acids, and ribosomes are present in large amount and have evolved to function in a crowded media [4]. Moreover, they are accountable for the extremely crowded intracellular milieu. The total quantity of macromolecules in the cytoplasm is estimated to be around 50 to 400 mg ml−1 [1, 3, 5, 6], corresponding to 5–40% of the total volume and defining such situation as macromolecular crowding [7]. Macromolecular crowding is caused by macromolecules or crowders that are inert in regard to any biological process taking place, resulting in an excluded volume interaction, where the available volume is decreased due to the existence of large molecules. Hence, this volume becomes unavailable to other molecules in the system [8, 9]. The level of crowding inside a cell is governed by the presence of a number of macromolecules of different sizes, shapes, and compositions occupying approximately 10–40% of the total cellular volume [10]. Although it is putative that a living cell constituted macromolecular crowders of various sizes and shapes [11], it has been seen that most of the researchers have utilized individual crowders and not their mixtures in their studies [9, 12,13,14,15,16,17,18].
One of the significant aspects is the observation that the mixed macromolecular crowding influences the properties of a protein in a different way than the individual crowders. It has been proposed that protein folding could be more favorable in mixtures of crowding agents [19,20,21]. Some of the previous studies have also demonstrated that the mixture of crowding agents inhibited the amyloid formation [22] and further stabilized the native and the mutated form [22, 23] suggesting that it is not only the total concentrations of crowders but their constitutions that might play significant roles in the crowding effects on protein folding and stability [23]. Thus, it is expected that in case of any crowding agent, the optimization of the stabilizing effect can be done by varying the sizes as well as the mixing ratio of the crowders having different sizes [24].
In this study, the effects of mixed macromolecular crowding and the extent of stabilization it provides to the proteins in comparison with the sum of individual crowding agents from a physiological point of view have been investigated. Varying concentrations of ficoll 70, dextran 70, and dextran 40 in different mixing ratios have been employed in order to see their effect on the thermodynamic stability of two well-characterized model proteins, hen egg white lysozyme and apo α-lactalbumin (α-LA), against thermal denaturation. The transition between the native (N) and the denatured (D) states of both the proteins (lysozyme and α-LA) has been reported to be a reversible and two-state process in the absence of crowding agents [25]. Several studies have been performed by our research group [26,27,28,29,30,31] as well as other researchers [32,33,34,35] on these proteins. ∆GD° (Gibbs free energy change at 25 °C) of lysozyme and α-LA in the absence and presence of mixtures of crowders at different pH values has been measured and ∆∆GD° (change in ∆GD°) was calculated so as to compare the extent of stabilization caused by the mixture and individual crowder. The crowding agents, dextran (polymer of glucose) and ficoll (a copolymer of sucrose and epichlorohydrin), exhibit distinguished characteristics in terms of flexibility, linearity, and compactness. Dextran has a rod-like shape with more flexibility and is a linear polysaccharide with some short branches; however, ficoll is more like a sphere, i.e., compact, less flexible, and highly branched [36,37,38].
In this study, we report that the mixed macromolecular crowding leads to more stabilization of the proteins as compared with the sum of the constituent crowding agent owing to more volume exclusion by the mixtures of crowders than individually, hence, defining it to be a non-additive effect.
Materials and Methods
Materials and Reagents
A commercial lyophilized form of hen egg white lysozyme, holo-α-lactalbumin from bovine milk, ficoll 70 (F70; average molecular mass 70,000 Da), dextran 40 and dextran 70 (D40, D70; average molecular mass 40,000 and 70,000 Da, respectively), and sodium cacodylate trihydrate were bought from Sigma-Aldrich Co. Sodium acetate, potassium chloride, ethylenediaminetetraacetic acid (EDTA), ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), and sodium bicarbonate were purchased from Merck (India) Ltd. Ultrapure guanidinium chloride (GdmCl) was obtained from MP Biomedicals. All chemicals were of analytical grade and used without further purification.
Preparation of Proteins and Reagents
The lyophilized powdered form of lysozyme and holo-α-lactalbumin was dissolved in their required amount in 0.1 M KCl solution at pH 7.0. We have used the apo form of α-lactalbumin (α-LA) in our study, which was prepared by adding 5 mM EGTA to the solution of holo-α-lactalbumin (Ca2+ bound). Both the protein solutions were then dialyzed against the several changes of 0.1 M KCl solution at pH 7.0 and 4 °C and then filtered using a 0.22-μm Millipore filter. The concentrations of lysozyme and α-LA solutions were determined experimentally using the molar absorption coefficient at 280-nm (ε280, M−1 cm−1) values of 39,000 and 29,210 for lysozyme [39] and α-LA [40], respectively. Protein solutions were then stored at 4 °C. The stock solutions of GdmCl and macromolecular crowding agents (F70, D70, and D40) were prepared by dissolving their requisite amount in the desired buffer solutions and were degassed. All the solutions were then filtered through Whatman filter paper No. 1 and the concentrations of GdmCl [41] and crowding agents [42, 43] were estimated by refractive index measurements.
All solutions employed for optical measurements were prepared in the desired degassed buffers. For various pH/pH ranges, the buffers used were 0.05 M acetate buffer (pH 4.0) and 0.05 M sodium cacodylate buffer (pH range 5.0–7.0), both containing 0.1 M KCl. The pH value of each solution was also measured after the denaturation experiments to make sure whether there was any change in pH values during the experiments.
Thermal Denaturation Measurements
Thermal denaturation experiments of lysozyme and α-LA were performed in a Jasco V-660 UV/Visible spectrophotometer outfitted with a Peltier-type temperature controller (ETCS-761). The change in the absorbance of lysozyme and α-LA with increasing temperature was followed at 300 and 295 nm, respectively. The concentration of the proteins used was in the range 0.5–0.4 mg ml−1. Each sample was heated from 20 to 85 °C with a heating rate of 1 °C min−1 to provide adequate time for equilibration of the sample. All the measurements were carried out in triplicate and approximately 650 data points of each transition curve were collected. Experiments were performed in the absence and presence of mixtures of varying concentrations of F70, D70, and D40 (in different combinations) at pH values 7.0 and 4.0. After denaturation, each protein sample was immediately cooled down so as to measure the reversibility of the reaction. All solution blanks were subtracted before analysis of the data. The raw absorbance data was converted into change in molar absorption coefficient (∆ελ, M−1 cm−1) at a given wavelength, λ. Each heat-induced transition curve was analyzed for Tm (midpoint of denaturation) and ΔHm (enthalpy change at Tm) using a non-linear least squares analysis according to the relation:
where y(T) is the optical property at temperature T (K), yN(T) and yD(T) are the optical properties of the native and denatured molecules of the protein at temperature T (K), and R is the gas constant. In the analysis of denaturation curve, it was assumed that a parabolic function describes the dependence of the optical properties of the native and denatured protein molecules (i.e., yN(T) = aN + bNT + cNT2 and yD(T) = aD + bDT + cDT2, where aN, bN, cN, aD, bD, and cD are temperature-independent coefficients) [44, 45]. The value of the constant-pressure heat capacity change (ΔCp) was calculated from slope of the linear plots of ΔHm versus Tm, using the relation [46]:
Using values of Tm, ΔHm, and ΔCp, the value of ΔGD at any temperature T, ΔGD(T), was determined with the help of the Gibbs-Helmholtz equation:
The reversibility of thermal denaturation was determined by cooling the heated solution of denatured protein to 25 °C and comparing its optical signals to that of the protein prior to heating.
Results
Thermal Denaturation Study
To investigate the effects of mixture of different crowding agents on the thermodynamic stability of proteins, thermal denaturation measurements of lysozyme and α-LA were carried out in the presence of mixtures of ficoll 70, dextran 70, and dextran 40 at pH values 7.0 and 4.0. It should be noted that the experiments of lysozyme were performed in the presence of 2.0 M GdmCl at the pH values of 7.0 and 4.0 (the procedure of GdmCl correction is explained in detail in our previous work [30]).
Thermal denaturation measurements of both the proteins were carried out in the presence of combinations of dextran 70 and ficoll 70, dextran 40 and ficoll 70, and dextran 70 and dextran 40 in different mixing ratios at pH values 7.0 and 4.0. In case of both the proteins, the concentration of one crowding agent was kept constant while the concentration of the other was varied and vice versa. For example, the concentration of F70 was kept constant while the concentration of D70 was varied as follows: 50 mg ml−1 D70 + 100 mg ml−1 F70, 100 mg ml−1 D70 + 100 mg ml−1 F70, 150 mg ml−1 D70 + 100 mg ml−1 F70, 200 mg ml−1 D70 + 100 mg ml−1 F70, and 250 mg ml−1 D70 + 100 mg ml−1 F70. And on the other hand, the concentration of D70 was kept constant while the concentration of F70 was varied as follows: 100 mg ml−1 D70 + 50 mg ml−1 F70, 100 mg ml−1 D70 + 100 mg ml−1 F70, 100 mg ml−1 D70 + 150 mg ml−1 F70, 100 mg ml−1 D70 + 200 mg ml−1 F70, and 100 mg ml−1 D70 + 250 mg ml−1 F70. The highest working concentration of the mixture of crowders was limited to 300 mg ml−1 due to the presence of 2.0 M GdmCl in the samples of lysozyme. Thermal denaturation experiments were carried out by following the changes in Δε300 of lysozyme and Δε295 of α-LA as a function of temperature at pH values 7.0 and 4.0 (see Figs. 1, 2, and 3). Since the increase in stabilization was more at pH 4.0 in the case of lysozyme, the experiments were performed at both pH values 7.0 and 4.0. However, the experiments of α-LA were carried out at pH 7.0 only due to maximum stabilization at this pH value. It has been observed that the temperature dependencies of yN and yD measured by both Δε300 and Δε295 do not show any dependency on the entire concentration range of mixture of crowders at both pH values (Figs. 1, 2, and 3). Thermal denaturation profiles of lysozyme and α-LA were found to be reversible in the presence of the entire range of concentration of each mixture of crowding agents at pH values 7.0 and 4.0 (data not shown).
Thermal denaturation profiles of lysozyme in the absence and presence of different concentrations of crowders in different mixing ratios at pH 7.0. Panels a and b show effect of varying concentrations of dextran 70 and constant concentration of ficoll 70 and vice versa, respectively. Panels c and d show effect of varying concentrations of dextran 40 and constant concentration of ficoll 70 and vice versa, respectively. Panels e and f show effect of varying concentrations of dextran 70 and constant concentration of dextran 40 and vice versa, respectively
Thermal denaturation profiles of lysozyme in the absence and presence of different concentrations of crowders in different mixing ratios at pH 4.0. Panels a and b show effect of varying concentrations of dextran 70 and constant concentration of ficoll 70 and vice versa, respectively. Panels c and d show effect of varying concentrations of dextran 40 and constant concentration of ficoll 70 and vice versa, respectively. Panels e and f show effect of varying concentrations of dextran 70 and constant concentration of dextran 40 and vice versa, respectively
Thermal denaturation profiles of α-LA in the absence and presence of different concentrations of crowders in different mixing ratios at pH 7.0. Panels a and b show effect of varying concentrations of dextran 70 and constant concentration of ficoll 70 and vice versa, respectively. Panels c and d show effect of varying concentrations of dextran 40 and constant concentration of ficoll 70 and vice versa, respectively. Panels e and f show effect of varying concentrations of dextran 70 and constant concentration of dextran 40 and vice versa, respectively
Thermal denaturation curves (Δε300 (or Δε295) versus T) of lysozyme and α-LA in the presence of each and every concentration of mixture of crowders were analyzed according to Eq. (1) to obtain the values Tm and ΔHm. The values of Tm and ΔHm measured for lysozyme were then corrected for the effect of 2.0 M GdmCl according to the procedure described earlier [30]. The observed (in the presence of 2.0 M GdmCl) and the corrected (in the absence of 2.0 M GdmCl) values of Tm and ΔHm of lysozyme and the values of Tm and ΔHm of α-LA in the absence and presence of varying concentrations of mixture of crowders for all the combinations at pH 7.0 and 4.0 are provided in supplementary material (Tables S1 and S2). It can be seen in Tables S1 and S2 that there is an increase in the values of Tm of both the proteins with the increasing concentration of dextran 70 and dextran 40 when compared with ficoll 70 in all the combinations at both the pH values; however, a slight change in the values of ΔHm has been observed. We have already reported the values of ∆Cp as 1.60 ± 0.09 and 1.56 ± 0.09 kcal mol−1 K−1 for lysozyme and α-LA, respectively [30], along with the explanation of the measurement of ∆Cp. Since ∆Cp was found to be independent of the concentrations of the crowding agents, we have used the same values of ∆Cp for both proteins [30] to estimate the value of ΔGD° for each mixing ratio in this study. The values of ΔGD° in the absence and presence of mixture of crowders were then estimated using Eq. (3) using values of Tm, ΔHm, and ΔCp in a given solvent condition. Values of ΔGD° are given in Tables S1 and S2. It can be seen in these tables that the maximum increase in ΔGD° was found in the mixture of dextran 70 and dextran 40 at pH 4.0 in case of lysozyme and pH 7.0 in case of α-LA. To observe whether the effects of the two crowding agents (for example, dextran 70 and ficoll 70) were additive, ∆∆GD° values of lysozyme and α-LA were determined for both crowding agents alone and in combination. Tables 1 and 2 show values of the sum of individual crowding agents (predicted) and those observed in their mixtures. A comparison of predicted and observed values shows that the stabilizing effects exerted by dextran 70 and ficoll 70, at a total concentration of 200, 250, 300, and 350 mg ml−1, are greater than the sum of the constituent crowding agents for all the combinations in the case of both the proteins and at both the pH values. Thus, this finding shows a non-additive effect of crowding agents on the stability of proteins.
Discussion
The intracellular milieu of a cell is tremendously crowded and is comprised of various soluble and insoluble macromolecules including ribosomes, proteins, DNA, RNA, and carbohydrates [1, 8, 9]. The estimated concentration of these macromolecules in the cell lies in the range of 80–400 mg ml−1 [3, 8]. There are a number of studies that have been performed using an individual crowding system, though a few applied mixtures of crowders [19,20,21,22, 24]. Thus, to understand the protein folding problem under the complicated macromolecular architecture in cells, the mixture of synthetic crowding agents has been employed in our study. Here, we have asked a question whether the effect of crowding agents alone on protein stability is different from that of these crowders in combination. It is expected that the mixed crowding agents would mimic the intracellular environment more precisely than what individual crowding agent does. Thus, the effects of mixed macromolecular crowding on the thermodynamic stability of lysozyme and α-LA from a physiological point of view were tested.
We carried out thermal denaturation of both the proteins (lysozyme and α-LA) in different mixing ratios of combinations of crowding agents (dextran 70 and ficoll 70, dextran 40 and ficoll 70, and dextran 70 and dextran 40) at pH values 7.0 and 4.0. The concentration of one crowding agent was kept constant while the other was varied and vice versa at all the combinations (described in the “Results” section). It is known that the thermal denaturation transition between the native and denatured states of these proteins in the absence [46,47,48] and presence [30] of crowding agents is a two-state process. It was observed from the thermal denaturation profiles of both the proteins that Tm increases with increase in concentration of each mixture of crowding agents (see Figs. 1 and 2 for lysozyme and Fig. 3 for α-LA). There is a slight change in the values of ΔHm (within 10% experimental errors) of both the proteins in the presence of mixtures of crowding agents at both the pH values. To estimate the value of ΔGD° of each protein in the presence of each mixture of crowding agents, we used value of ∆Cp of the protein in the absence of crowding agents (i.e., 1.60 ± 0.09 and 1.56 ± 0.09 kcal mol−1 K−1 for lysozyme and α-LA [30], respectively), for ∆Cp was found to be independent of the concentrations of the crowding agents [30]. It has been observed that stabilization of proteins (both in terms of Tm and ΔGD°) in the mixture of crowding agents increases with an increase in the concentration of the crowder at a fixed concentration of the other crowder (see Tables S1 for lysozyme and S2 for α-LA). Thus, protein stabilization has been found to be entirely entropic in nature in the presence of mixtures of crowding agents, for enthalpy change remains almost constant with increasing concentrations of mixtures of crowders.
The characteristics of crowding agents such as their shape, size, and composition play a pivotal role in the stabilization of proteins. The flexible and rod-shaped dextran in comparison with rigid and compact ficoll of sphere-like shape resulted in more volume exclusion and hence higher stabilization [30, 49, 50]. Additionally, on mass/volume scale, dextran 40 has more number of molecules that will cause maximum packing in the mixture of crowding agents and leads to the highest volume exclusion. Although the presence of other crowders like ficoll 70 and dextran 70 in a mixture contributes to the stabilization of protein, the impact of dextran 40 due to its small size in comparison with dextran 70 and due to its rod shape in comparison with spherical ficoll 70 is greater on the stabilization of both the proteins. Moreover, Alfano and co-workers [51] have investigated the influence of three crowders of distinct sizes, i.e., polyethylene glycol (PEG) 20, dextran 40, ficoll 70 and ficoll 400 at concentrations ranging from 0 to 20% (w/v), on the stability of yeast frataxin (Yfh1) in the temperature range 2–70 °C. The presence of crowders showed a significant rise in the stability at low temperature but a minor increase in the stability at high temperature which could be due to the large volume of low-temperature unfolded species than that at high temperature. They demonstrated that volume exclusion affects protein stability, and the effect is more noticeable when the size of the crowder is closer to that of the test protein [51]. It has also been found that dextran due to its crowding property decreases the positive effect of arginin on the chaperone ability of α-crystallin [52]; however, bovine serum albumin (BSA) leads to refolding and regain of enzyme activity of triosephosphate isomerase [53]. Additionally, the effect of single and mixed crowding agents (PEG 2000 and dextran 70) was investigated by Fan and co-workers [54] on recombinant human brain–type creatine kinase (rHBCK) inactivation induced by GdmCl by analyzing residual activity, reaction kinetics, intrinsic fluorescence, and phase diagram. There was a rise in the residual activity and a decay in the inactivation rate by both the crowders; however, PEG 2000 has been found to stabilize the conformation of rHBCK better than dextran 70 [54]. They suggested that mixed crowders did not perform better than single crowders, but there was an additive effect with the mixtures of crowding agents [54]. Furthermore, Kumar and associates [55] performed thermodynamic analysis of thermal denaturation curves of base-denatured ferricytochrome c (from horse heart) and hen egg white lysozyme at pH 12.9 (± 0.1) in the presence of varying concentrations of dextran 70, dextran 40, and ficoll 70. They revealed that the presence of crowders increases the thermal stability of base-denatured proteins as well as prevents the cold denaturation of ferricytochrome c. Their results further indicated that the size, shape, and nature of crowding agent also affect the crowding-mediated rise in the stabilization of the secondary structure [55].
Values of ∆∆GD° of both the proteins (lysozyme and α-LA) are obtained for all the combinations of crowding agents in different mixing ratios (designated as “observed”). Values of ∆∆GD° of both these proteins are also obtained from the sum of ∆GD° of each crowding agent in the mixture (designated as “predicted”). For an example (see Tables 1 and 2), ∆∆GD° (observed) of lysozyme in the mixture 100 mg ml−1 of dextran 70 and 200 mg ml−1 of dextran 40 at pH 4.0 has been found to be 1.64 kcal mol−1. In the presence of 100 mg ml−1 of dextran 70 alone, ∆∆GD° was found to be 0.14 kcal mol−1; and in presence of the counterpart 200 mg ml−1 of dextran 40 alone, ∆∆GD° was 0.99 kcal mol−1. On summing up these two individual ∆∆GD° values, one gets a value of 1.13 kcal mol−1 for ∆∆GD° (predicted) which is less than the ∆∆GD° (observed) value of 1.64 kcal mol−1. Observed and predicted values of ∆∆GD° are shown in Table 1 for lysozyme and Table 2 for α-LA. It can be seen in these tables that the mixtures of crowding agents exert a greater stabilizing effect than the sum of the individual crowders. Hence, the stabilization effect of mixture of crowders on proteins is non-additive in nature.
Our results are in consistency with several other studies, such as those of Zhou et al. who examined the effects of mixtures of crowders (dextran 70, ficoll 70, and BSA) and their individual forms in two different pieces of work: (i) on the amyloid formation of hen egg white lysozyme and (ii) the oxidative refolding of the reduced and denatured form of lysozyme by examining through activity assay [21, 22]. It was shown that the mixture of BSA and ficoll 70 contributes to both stabilization and inhibitory effect cooperatively [22], and also this mixture seems more favorable to the folding of lysozyme [21]. In addition, the mixture of crowders comprising PEG 2 and calf thymus DNA has been found more favorable for the refolding of GdmCl-induced unfolded form of rabbit muscle creatine kinase and caused a lesser amount of aggregation than the individual crowding agents, i.e., ficoll 70, PEG 2, dextran 70, and calf thymus DNA [19]. Similar results were obtained during the refolding and aggregation of GdmCl-induced unfolded form of recombinant human brain–type creatine kinase in the presence of PEG 2, dextran 70, and calf thymus DNA along with their distinct mixtures [20]. Moreover, Batra et al. [23] studied the folding stability of the FK506-binding protein (FKBP) in the presence of mixture of dextran 6 and ficoll 70, where the shape and size of the crowding agents differ with each other [23]. They perceived that stabilization of a protein is more in the presence of mixed crowding than the sum of two single crowders and defined it to be a non-additive effect as well. Furthermore, from their studies, they led to an assumption that not only the total concentration but also the composition of crowding agents has a substantial impact on the macromolecular crowding effect on protein stability [23]. Hence, our study validates their assumption. Thus, the non-additive effect of mixture of crowding agents has great implications for the phenomenon of macromolecular crowding inside cells.
Moreover, numerous studies by Pielak and associates [56,57,58,59,60,61,62,63,64] have focussed on various effects and consequences of macromolecular crowding resulting from two phenomena, i.e., soft (or chemical) interactions and hard-core repulsions. These soft and hard interactions are the characteristics of the enthalpic and the entropic contributions to protein stability, and their relationship administrates the excluded volume [60]. Different studies have been performed under several conditions in order to show the effect of crowding on the stability of CI2 [56,57,58,59, 62], ubiquitin [64], and Protein L [63]. They suggested that proteins possess a favorable, though weak interaction with other proteins which might overcome the stabilizing effect owing to hard-core repulsions associated to physiologically relevant macromolecular crowding [61].
Many macromolecules at different concentrations are existing inside a cell. Therefore, it is indicated that the composition of macromolecules inside a cell and their total concentration should be considered while mimicking the intracellular condition when conducting in vitro experiments. Such types of in vitro experiments try to supplement the in vivo measurements of folding and stability [65]. Furthermore, it has been suggested that the effects of crowded intracellular environment exerted on the stability of folding/unfolding process and other biophysical properties of proteins can considerably get altered with time. Hence, such kinds of variations may enact an essential role in diseases such as Alzheimer’s and/or Parkinson’s disease, associated with protein misfolding and aggregation [23].
Conclusion
This study demonstrates that mixed macromolecular crowding inside a cell plays a significant role in influencing the thermodynamic stability of proteins. The composition and varying concentrations of crowding agents in different mixing ratios have insightful insinuations on the biophysical properties of proteins. The extent of stabilization is more in the presence of mixtures of crowding agents than the sum of their constituent crowding agents owing to more volume exclusion for both the proteins at all experimental conditions. Among different combinations of mixtures such as (i) dextran 70 + ficoll 70, (ii) dextran 40 + ficoll 70, and (iii) dextran 70 + dextran 40, the mixture of dextran 70 and dextran 40 stabilizes the proteins more than their sum individually due to the factors depending on their shape and size. This shows that the stabilizing effect of mixtures of crowding agents is non-additive in nature. Thus, it can be concluded that the mixtures of crowding agents with a different architecture such as their shape and size, mimic the intracellular environment more closely than the single crowder and even complements the in vivo measurements of protein folding and stability.
Abbreviations
- GdmCl:
-
Guanidinium chloride
- UV:
-
Ultra-violet
- T m :
-
Midpoint of thermal denaturation
- ΔH m :
-
Enthalpy change at Tm
- ΔC p :
-
Constant-pressure heat capacity change
- ∆G D°:
-
Gibbs free energy change at 25 °C
- F70:
-
Ficoll 70
- D70:
-
Dextran 70
- D40:
-
Dextran 40
References
Fulton, A. (1982). How crowded is the cytoplasm ? Cell, 30(2), 345–347.
Minton, A. P. (1983). The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Molecular and Cellular Biochemistry, 55(2), 119–140.
Zimmerman, S. B., & Trach, S. O. (1991). Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. Journal of Molecular Biology, 222(3), 599–620.
Medalia, O., Weber, I., Frangakis, A. S., Nicastro, D., Gerisch, G., & Baumeister, W. (2002). Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science, 298(5596), 1209–1213.
Ellis, R. J., & Minton, A. P. (2003). Cell biology: join the crowd. Nature, 425(6953), 27–28.
Rivas, G., Ferrone, F., & Herzfeld, J. (2004). Life in a crowded world. EMBO Reports, 5(1), 23–27.
Minton, A. P. (1981). Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers, 20(10), 2093–2120.
Ellis, R. J. (2001). Macromolecular crowding: an important but neglected aspect of the intracellular environment. Current Opinion in Structural Biology, 11(1), 114–119.
Zimmerman, S. B., & Minton, A. P. (1993). Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annual Review of Biophysics and Biomolecular Structure, 22(1), 27–65.
Ellis, R. J. (2001). Macromolecular crowding: obvious but underappreciated. Trends in Biochemical Sciences, 26(10), 597–604.
Goodsell, D. S. (1991). Inside a living cell. Trends in Biochemical Sciences, 16(6), 203–206.
Chebotareva, N. A., Kurganov, B. I., & Livanova, N. B. (2004). Biochemical effects of molecular crowding. Biochemistry (Moscow), 69(11), 1239–1251.
Christiansen, A., Wang, Q., Cheung, M. S., & Wittung-Stafshede, P. (2013). Effects of macromolecular crowding agents on protein folding in vitro and in silico. Biophysical Reviews, 5(2), 137–145.
Kuznetsova, I., Turoverov, K., & Uversky, V. (2014). What macromolecular crowding can do to a protein. International Journal of Molecular Sciences, 15(12), 23090–23140.
Kuznetsova, I., Zaslavsky, B., Breydo, L., Turoverov, K., & Uversky, V. (2015). Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. Molecules, 20(1), 1377–1409.
Minton, A. P. (2001). The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. The Journal of Biological Chemistry, 276(14), 10577–10580.
Samiotakis, A., Wittung-Stafshede, P., & Cheung, M. S. (2009). Folding, stability and shape of proteins in crowded environments: experimental and computational approaches. International Journal of Molecular Sciences, 10(2), 572–588.
Zhou, H.-X., Rivas, G., & Minton, A. P. (2008). Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annual Review of Biophysics, 37(1), 375–397.
Du, F., Zhou, Z., Mo, Z. Y., Shi, J. Z., Chen, J., & Liang, Y. (2006). Mixed macromolecular crowding accelerates the refolding of rabbit muscle creatine kinase: implications for protein folding in physiological environments. Journal of Molecular Biology, 364(3), 469–482.
Fan, J., ZHOU, Z., Chen, J., YANG, Z.-Z. and Liang, Y. (2012) The contrasting effect of macromolecular crowding on protein misfolding. FEBS JOURNAL, pp. 408–408. WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030–5774, NJ USA.
Zhou, B.-R., Liang, Y., Du, F., Zhou, Z., & Chen, J. (2004). Mixed macromolecular crowding accelerates the oxidative refolding of reduced, denatured lysozyme: implications for protein folding in intracellular environments. Journal of Biological Chemistry, 279(53), 55109–55116.
Zhou, B. R., Zhou, Z., Hu, Q. L., Chen, J., & Liang, Y. (2008). Mixed macromolecular crowding inhibits amyloid formation of hen egg white lysozyme. Biochimica et Biophysica Acta, 1784(3), 472–480.
Batra, J., Xu, K., & Zhou, H. X. (2009). Nonadditive effects of mixed crowding on protein stability. Proteins, 77(1), 133–138.
Zhou, H. X. (2008). Effect of mixed macromolecular crowding agents on protein folding. Proteins, 72(4), 1109–1113.
Privalov, P. L., & Khechinashvili, N. N. (1974). A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. Journal of Molecular Biology, 86(3), 665–684.
Beg, I., Minton, A. P., Hassan, M. I., Islam, A., & Ahmad, F. (2015). Thermal stabilization of proteins by mono- and oligosaccharides: measurement and analysis in the context of an excluded volume model. Biochemistry, 54(23), 3594–3603.
Beg, I., Minton, A. P., Islam, A., Hassan, M. I., & Ahmad, F. (2017). The pH dependence of saccharides’ influence on thermal denaturation of two model proteins supports an excluded volume model for stabilization generalized to allow for intramolecular electrostatic interactions. The Journal of Biological Chemistry, 292(2), 505–511.
Beg, I., Minton, A. P., Islam, A., Hassan, M. I., & Ahmad, F. (2018). Comparison of the thermal stabilization of proteins by oligosaccharides and monosaccharide mixtures: measurement and analysis in the context of excluded volume theory. Biophysical Chemistry, 237, 31–37.
Khan, S., Bano, Z., Singh, L. R., Hassan, M. I., Islam, A., & Ahmad, F. (2013). Testing the ability of non-methylamine osmolytes present in kidney cells to counteract the deleterious effects of urea on structure, stability and function of proteins. PLoS One, 8(9), e72533.
Shahid, S., Ahmad, F., Hassan, M. I., & Islam, A. (2015). Relationship between protein stability and functional activity in the presence of macromolecular crowding agents alone and in mixture: an insight into stability-activity trade-off. Archives of Biochemistry and Biophysics, 584, 42–50.
Singh, R., Haque, I., & Ahmad, F. (2005). Counteracting osmolyte trimethylamine N-oxide destabilizes proteins at pH below its pKa: measurements of thermodynamic parameters of proteins in the presence and absence of trimethylamine N-oxide. Journal of Biological Chemistry, 280(12), 11035–11042.
Arakawa, T., & Timasheff, S. N. (1982). Stabilization of protein structure by sugars. Biochemistry, 21(25), 6536–6544.
Mittal, S., & Singh, L. R. (2013). Denatured state structural property determines protein stabilization by macromolecular crowding: a thermodynamic and structural approach. PLoS One, 8(11), e78936.
Sasahara, K., McPhie, P., & Minton, A. P. (2003). Effect of dextran on protein stability and conformation attributed to macromolecular crowding. Journal of Molecular Biology, 326(4), 1227–1237.
Sharma, G. S., Mittal, S., & Singh, L. R. (2015). Effect of dextran 70 on the thermodynamic and structural properties of proteins. International Journal of Biological Macromolecules, 79, 86–94.
Dumitriu, S. (2004). Polysaccharides: Structural diversity and functional versatility, second edition. CRC Press.
Luby-Phelps, K., Castle, P. E., Taylor, D. L., & Lanni, F. (1987). Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proceedings of the National Academy of Sciences of the United States of America, 84(14), 4910–4913.
Venturoli, D. and Rippe, B. (2005) Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. ed.
Hamaguchi, K., & Kurono, A. (1963). Structure of muramidase (lysozyme) I. The effect of guanidine hydrochloride on muramidase. The Journal of Biochemistry, 54, 111–122.
Sugai, S., Yashiro, H., & Nitta, K. (1973). Equilibrium and kinetics of the unfolding of α-lactalbumin by guanidine hydrochloride. Biochimica et Biophysica Acta (BBA) - Protein Structure, 328(1), 35–41.
Nozaki, Y. (1972). The preparation of guanidine hydrochloride. Methods in Enzymology, 26, 43–50.
A guide to multi-detector gel permeation chromatography 2012. Available from: www.agilent.com/chem.
Fissell, W. H., Hofmann, C. L., Smith, R., & Chen, M. H. (2010). Size and conformation of Ficoll as determined by size-exclusion chromatography followed by multiangle light scattering. American Journal of Physiology - Renal Physiology, 298(1), F205–F208.
Sinha, A., Yadav, S., Ahmad, R., & Ahmad, F. (2000). A possible origin of differences between calorimetric and equilibrium estimates of stability parameters of proteins. Biochemical Journal, 345(3), 711–717.
Yadav, S., & Ahmad, F. (2000). A new method for the determination of stability parameters of proteins from their heat-induced denaturation curves. Analytical Biochemistry, 283(2), 207–213.
Becktel, W. J., & Schellman, J. A. (1987). Protein stability curves. Biopolymers, 26(11), 1859–1877.
Hiraoka, Y., & Sugai, S. (1984). Thermodynamics of thermal unfolding of bovine apo-α-lactalbumin. International Journal of Peptide and Protein Research, 23(5), 535–542.
Privalov, P. L. (1979). Stability of proteins: small globular proteins. Advances in Protein Chemistry, 33, 167–241.
Homchaudhuri, L., Sarma, N., & Swaminathan, R. (2006). Effect of crowding by dextrans and Ficolls on the rate of alkaline phosphatase-catalyzed hydrolysis: a size-dependent investigation. Biopolymers, 83(5), 477–486.
Shahid, S., Hassan, M. I., Islam, A., & Ahmad, F. (2017). Size-dependent studies of macromolecular crowding on the thermodynamic stability, structure and functional activity of proteins: in vitro and in silico approaches. Biochimica et Biophysica Acta (BBA) - General Subjects, 1861(2), 178–197.
Alfano, C., Sanfelice, D., Martin, S. R., Pastore, A., & Temussi, P. A. (2017). An optimized strategy to measure protein stability highlights differences between cold and hot unfolded states. Nature Communications, 8, 15428.
Ghahghaei, A., & Mohammadian, S. (2014). The effect of Arg on the structure perturbation and chaperone activity of α-crystallin in the presence of the crowding agent, dextran. Applied Biochemistry and Biotechnology, 174(2), 739–750.
Kumar, K., Bhargava, P., & Roy, U. (2011). In vitro refolding of Triosephosphate isomerase from L. donovani. Applied Biochemistry and Biotechnology, 164(7), 1207–1214.
Fan, Y.-Q., Liu, H.-J., Li, C., Luan, Y.-S., Yang, J.-M., & Wang, Y.-L. (2013). Inactivation of recombinant human brain-type creatine kinase during denaturation by guanidine hydrochloride in a macromolecular crowding system. Applied Biochemistry and Biotechnology, 169(1), 268–280.
Kumar, R., Sharma, D., Garg, M., Kumar, V., & Agarwal, M. C. (2018). Macromolecular crowding-induced molten globule states of the alkali pH-denatured proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1866(11), 1102–1114.
Benton, L. A., Smith, A. E., Young, G. B., & Pielak, G. J. (2012). Unexpected effects of macromolecular crowding on protein stability. Biochemistry, 51(49), 9773–9775.
Charlton, L. M., Barnes, C. O., Li, C., Orans, J., Young, G. B., & Pielak, G. J. (2008). Residue-level interrogation of macromolecular crowding effects on protein stability. Journal of the American Chemical Society, 130(21), 6826–6830.
Miklos, A. C., Li, C., Sharaf, N. G., & Pielak, G. J. (2010). Volume exclusion and soft interaction effects on protein stability under crowded conditions. Biochemistry, 49(33), 6984–6991.
Miklos, A. C., Sarkar, M., Wang, Y., & Pielak, G. J. (2011). Protein crowding tunes protein stability. Journal of the American Chemical Society, 133(18), 7116–7120.
Sarkar, M., Li, C., & Pielak, G. J. (2013). Soft interactions and crowding. Biophysical Reviews, 5(2), 187–194.
Sarkar, M., Lu, J., & Pielak, G. J. (2014). Protein crowder charge and protein stability. Biochemistry, 53(10), 1601–1606.
Sarkar, M., Smith, A. E., & Pielak, G. J. (2013). Impact of reconstituted cytosol on protein stability. Proceedings of the National Academy of Sciences, 110(48), 19342–19347.
Schlesinger, A. P., Wang, Y., Tadeo, X., Millet, O., & Pielak, G. J. (2011). Macromolecular crowding fails to fold a globular protein in cells. Journal of the American Chemical Society, 133(21), 8082–8085.
Wang, Y., Sarkar, M., Smith, A. E., Krois, A. S., & Pielak, G. J. (2012). Macromolecular crowding and protein stability. Journal of the American Chemical Society, 134(40), 16614–16618.
Ignatova, Z., & Gierasch, L. M. (2004). Monitoring protein stability and aggregation in vivo by real-time fluorescent labeling. Proceedings of the National Academy of Sciences of the United States of America, 101(2), 523–528.
Funding
This work was supported by grant from the Science & Engineering Research Board (SERB), India (SR/FT/LS-48/2010), FIST Program (SR/FST/LSI-541/2012), and Council of Scientific and Industrial Research (CSIR), India (37(1604)/13/EMR-II). SS is thankful to Maulana Azad National Fellowship, University Grants Commission (Government of India), for providing fellowship. FA is grateful to Indian National Science Academy for the award of Senior Scientist Position.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Shahid, S., Ahmad, F., Hassan, M.I. et al. Mixture of Macromolecular Crowding Agents Has a Non-additive Effect on the Stability of Proteins. Appl Biochem Biotechnol 188, 927–941 (2019). https://doi.org/10.1007/s12010-019-02972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02972-9