Abstract
Proteins are one of the dynamic macromolecules that play a significant role in many physiologically important processes to sustain life on the earth. Proteins need to be properly folded into their active conformation to perform their function. Alteration in the protein folding process may lead to the formation of misfolded conformers. Accumulation of these misfolded conformers can result in the formation of protein aggregates which are attributed to many human pathological conditions including neurodegeneration, cataract, neuromuscular disorders, and diabetes. Living cells naturally have heterogeneous crowding environments with different concentrations of various biomolecules. Macromolecular crowding condition has been found to alter the protein conformation. Here in this review, we tried to show the relation between macromolecular crowding, protein aggregation, and its consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macromolecular Crowding
Natural physiology of a living cell is densely crowded with different molecules including proteins, DNA, RNA, lipids and solute particles [1].This biological molecules occupies a significant volume (in the range of 5% to 40%) of the cell [2]. The concentration of protein and RNA inside an E.coli cell can range up to 300-400 g/l [3].
Crowding deals with the available volume to a biomolecule inside the cell. Internal environment of a eukaryotic cell is far more complex in contrast to prokaryotic cell [4]. It contains variety of membrane bound organelles and cytoskeletal fibre network. The functional properties of biomacromolecules evolved in cellular milieus crowded with both soluble and insoluble macromolecules. The crowding concentration of these macromolecules can reach up to hundreds g/l. For example, hemoglobin concentration in red blood cells is about 350 g/l [5], protein content in human lens is approximately 340 g/l [6]. Different cells and compartments can differ in the level of crowdedness. Overall, a significant volume of the cell is occupied by these macromolecules, making it nearly inaccessible to the other molecules present inside the cell. Such crowded environment is termed as volume occupied rather than concentrated, as because no single species of molecules is present at a high concentration.
The term macromolecular crowding was first coined by Minton and Wilf in 1981 which brought up the importance of studying the effect of crowding on nature and interaction between biomacromolecules [7]. The term crowding is strictly related to the volume exclusion principle physically arising purely as a result of stearic repulsion. In addition to the intracellular environment, crowding is witnessed in the extracellular matrix of the tissues. For instance, the protein concentration in the blood plasma is 80 g/l [5], significantly enough to exert crowding effect. In contrast to the size of the macromolecules, the minimum distance between any two biomacromolecules under crowded environment can be much lower than themselves. Consequently, macromolecular crowding will affect any type of reactions that depends on the available accessible volume.
In contrary to macromolecular crowding, the existing knowledge about the various biological processes has been learned by research done mostly under dilute buffer conditions. The concentration of the crowding agent in the buffer system does not exceed even 10 g/l. In buffer environment, the biomolecule has enough accessible space unlikely in the crowded environment as shown in Fig. 1. This difference can significantly influence the conformations of the biomolecules. Overall, macromolecular crowding can considerably affects the biological processes like enzyme activity, protein folding, ligand–protein interactions, protein–protein and protein-nucleic acids interactions etc. [8].
In Vitro Macromolecular Crowding
In vitro crowding environment can be created using different molecular crowders of both natural and artificial origin, contrary to the in vivo where the cell is already crowded due to presence different biomolecules. The natural origin crowding agents include different types of proteins like lysozyme, serum albumins, ovalbumin, nucleic acids, lipids, etc. Whereas, the artificial crowding agents composed of synthetic inert polymers. For example: dextran, polyethylene glycol, ficoll, glycerol, etc.
Significance of Macromolecular Crowding
In contrast to the diluted buffer system, the behavior of a biomolecule of interest in the crowded environment is expected to be different. Macromolecular crowding was found to exert both positive and negative effects on biomolecules [9]. The studies conducted so far found that macromolecular crowding enhance protein association, association of monomeric proteins, increase the process rate of protein folding as well as refolding [10, 11]. It is also found to positively impact on kinetics of gene regulation constraining number of binding sites for DNA protein binding per cell [12]. It also increases self-association of fibrinogen protein [13]. It also stabilizes α-chymotrypsin against solvent induced aggregation [14]. Fascinatingly, besides stability macromolecular crowding also affects the functional properties of the protein. For instance, enzyme activity of PKG increases with increase in crowding concentration [15]. Researchers overall concluded that macromolecular crowding (i) stabilizes protein against chemical or heat induced denaturation. It is also postulated that crowding condition stabilizes globular protein via volume exclusion mechanism as native conformation occupy less volume than misfolded/unfolded form [16,17,18,19,20]; (ii) alters the rate of reactions [21, 22]; (iii) increases the catalytic activity of the enzymes [23,24,25,26]; (iv) protein aggregation inhibition of β- rich proteins [27, 28].
Conflicting to the above mentioned positive effects of macromolecular crowding, many studies has been conducted illustrating negative effects of macromolecular crowding. For instance, recent investigations reported that macromolecular crowding and confinement promotes hemoglobin aggregation and fibril formation [29, 30]. Furthermore, it also disrupt the refolding of reduced lysozyme and forms aggregates [31, 32]. Decreased activity of recombinant human brain like creatine kinase has also been reported under crowded and confinement conditions [33]. Similarly, α- lactalbumin was also destabilized thermally in the presence of crowding agent polyethylene glycol 2000 [9]. Additionally, crowding agent ficoll 70 was found to influence the process of myoglobin unfolding [34]. Likewise, dextran 70 has also been demonstrated to negatively affect the stability of the properly folded prion protein (rPrPC) [35]. Recent studies conducted by many researchers interestingly underlines the role of natural crowding agents in destabilizing protein and causing aggregation and fibril formation. This may be the consequence of the weak non-specific protein–protein interactions [2, 36, 37]. Amusingly, all these data advocate that the effect of macromolecular crowding on protein does not confined alone to stabilizing/positive or destabilizing/negative properties. For this reason, it is essential to understand both the positive and negative effect of macromolecular crowding in order to have complete scenario of what kind of effect does macromolecular crowding has on bio macromolecular properties.
The Macromolecular Crowding Agent
The crowding environment is created in the laboratory with the help of crowding agents. The crowding agents are polymer in nature made up of repeated monomeric unit typically joined by covalent linkage. Strictly, the macromolecular crowding deals with volume exclusion and confinement, so, it is utmost important to select a suitable crowding agent ensuring that it will produce the desired effect. In order to make sure that the consequences witnessed will be solely due to crowding and confinement, the crowding agent have to meet definite requirements: 1) there should be insignificant interactions between proteins and the crowding agents except steric repulsions, 2) the polymeric crowding agent should not be prone to self-aggregation, 3) it should be available in different molecular sizes, and 4) the solubility of the crowding agent in water or physiologic solution should be high.
It is now well known that ficoll 70, dextran 70, polyethylene glycol (PEG) and inert proteins are some of the most commonly used crowding agent.
Macromolecular Crowding and Protein Aggregation
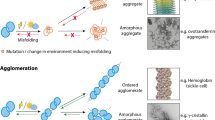
The defined ability of a protein to fold into its physiological active conformation is the most vital process in biology. The native form of protein can be turned in to unfolded or partially folded conformation due to both external and or internal factors like temperature and pH [38]. Naturally, primary sequence of a protein has the tendency to form aggregates [39]. Protein aggregate formation is responsible for many proteopathies including cardiovascular, metabolic and neurodegenerative disorders [40,41,42,43,44,45,46]. The process of protein aggregation frequently encountered both in vivo and in vitro. Significant efforts is being made to understand the basic cause and factors affecting protein aggregation. However, dilute buffer medium is being used to study the process of protein aggregation in vitro. In contrary, the natural environment wherein protein executes its function, is densely crowded making it of utmost important to consider the effect of macromolecular crowding on protein structure, stability and function. The possible effects induced by macromolecular crowding has been illustrated in Fig. 2.
The effect of macromolecular crowding on the process of protein aggregation have been extensively studied in the last few decades (Table 1).
The results revealed that macromolecular crowding enhances the process of aggregate formation of many proteins [15]. However, it is dependent on the type and physio-chemicals properties of the protein being studied. For instance, the crowding condition have been shown by our lab, to promote hemoglobin aggregation in time and concentration dependent manner [29]. In addition, aggregation of reduced lysozyme was because of the accumulation of aggregation prone intermediates [31]. In another exciting finding, both the protein and polymer based crowding agent favor aggregation during GroEL refolding [47]. Others also showed that mixed crowded conditions also promotes the aggregation of rabbit muscle creatine kinase, and reduced lysozyme [10, 48]. Though, the process of protein aggregation in the presence of mixed crowding agents is found to be less serious compared to the aggregation process in the presence of single protein crowding agent like BSA. This may be due to the more effective volume exclusion done by BSA addition to the weak protein–protein interactions. Collectively, macromolecular crowding induced protein aggregation can be attributed to the following reasons: a) increased solute concentration due to the reduction in water activity consequently leading to decreased protein solubility and increased aggregate formation, b) preferential accumulation of aggregation prone intermediates, c) slow rate of protein refolding as a result of increased viscosity with increased crowding agent concentration.
Macromolecular crowding has been reported to accelerate the process of protein fibrillation [30]. Addition of polymer based crowding agents has been shown to facilitate the fibrillation process of insulin, α-synuclein, human superoxide dismutase1 and β-lactalbumin [28, 49, 50]. Dextran 70 at a concentration of 200 g/l accelerate hemoglobin fibrillation in a time dependent manner [30]. The fibrils formed, induce redox perturbation and exerts cytotoxic effects. Dextran has also been reported to accelerate the process of reduced apo α- lactalbumin and human apolipoprotein fibrillation [51, 52]. Likewise, ficoll is another crowding agent that has also been reported to speed up protein fibrillation. At a concentration of 200 g/l, ficoll was found to hasten human prion protein, α-synuclein, and human tau protein fibrillation [50, 53]. Another crowding agent PEG has also been shown to accelerate the fibrillation process of hemoglobin [30], β- synuclein and bovine core histone proteins [49, 54]. Addition to the polymer crowders, protein based crowding agent has also been reported to influence the fibrillation of many proteins. For instance, protein crowing agents accelerates the process of hemoglobin [29], S-carboxymethyl-α-lactalbumin [49], and α-synuclein [55, 56]. Altogether, from the above facts it has been witnessed that macromolecular crowding affect the native conformation of protein and accelerates the process of protein aggregation and fibril formation.
Solvent Modulation in Presence of Crowding Agents
A number of experimental and theoretical studies have confirmed the active role of solvent in protein stability and dynamics. The macromolecular crowding has been shown to possess potential of modulating the solvent properties. Almost all the enzymes are protein in nature. They are required to be properly folded in to their active conformation in order to perform physiological function. Crowding condition increases the solvent viscosity which is one of the key factor influencing protein structure consequently influencing enzyme activities. Many researchers have reported that increased crowding concentration reduces the enzymatic activity. This can be attributed to increased viscosity. Furthermore, an increase in the Km values of few enzymes has been observed due to increased polymeric crowding concentration. Additionally, increased viscosity has been shown to decrease the mobility of the dimers consequently affecting the re-association of the denatured glyceraldehyde-3-phosphate dehydrogenase tetramer. This delayed re-association of GAPDH in to its active tetrameric form reduces its reactivation rate [12, 24, 33, 57,58,59,60].
Hydration is another key factor that can influence protein function. Hydration forces are in charge of protein structure packing and stability. Water, in particular, widely acknowledged to serve an important role in influencing the structure, stability, dynamics, and function of biological macromolecules [61]. Water of hydration and bulk water are the two types of water found in cells. The water of hydration is water that has been firmly adsorbed on macromolecules. The amount of hydration water is determined by the total concentration of macromolecules in the sample, thus, as the solute concentration increases, the hydration water volume around each macromolecule decreases [62, 63]. Water molecules can direct folding and aid packing of super-secondary structural features by mediating long-range interactions between polar charged amino acids, highlighting their importance in the folding and stability of large and multi-domain proteins. Previous research suggests that at low levels of crowding, the structure of water within the hydration shell is only marginally impacted, while at high levels of crowding, the structure of water is dramatically altered beyond the first hydration shell. Additionally, an examination of self-diffusion rates and dielectric constants demonstrated a linear decrease in hydration dynamics as crowder concentration increased [64, 65]. Moreover, the enzymatic activity of α-chymotrypsin was decreased in the presence of polymeric crowding agent PEG. This reduced activity can be attributed to the decrease in kcat, which can be consequence of the loss of critical water residues from the enzyme hydration shell. Moreover, the macromolecular crowding was found to facilitate the differential binding mechanism in anionic and cationic ligands binding to telomere and inhibiting telomerase activity. The anionic ligands were found to be more effective in inhibiting the telomerase activity in contrast to the cationic ligands. This differential binding can be attributed to the degree of hydration during the G-quadraplex / ligand complex formation [14, 66]. Despite these elegant studies, more studies are required to establish the relationship between water of hydration of particular enzymes and thus their activity with increasing crowder concentration.
Protein Aggregation, Propagation and Consequences
Proteins, one of the most important biological macromolecules. It plays a wide range of physiological functions essential for all biological processes. Protein functions as a transporter, storage of molecules such as oxygen, movement generation, nerve impulse transmission, immune responses, neural control and coordination during growth and differentiation [67]. For a protein, it requires to be folded in a proper conformation to execute any function. The process of protein folding typically very well organized, forming a specific functionally active conformation as illustrated in Fig. 3.
Though, the course of folding is not fail proof and undeniably a percentage of all the proteins synthesized in the cell did not fold correctly. Inappropriate folding of protein may results in formation of abnormal structured conformations whose accumulation can lead to many pathophysiological consequences [68]. Misfolded conformers are known to form due to factors like temperature fluctuations, oxidative stress, genetic mutations, and alterations in the cellular environments due to ageing [69,70,71].
Cells normally challenged with the continuous flow of misfolded forms of proteins arising from different factors like mutation or physiological stressors. Protein quality control system is a mechanism developed by the cells to deal with misfolded conformers of the protein (Fig. 4). This system consists of both proteases and chaperones serves as regulating agents [72,73,74]. In a crowded cellular environment, misfolded proteins can form amorphous aggregates and or ordered elongated amyloid fibrils. These highly ordered aggregates are not easily degraded by protein quality control system and starts to accumulate in specific organ or tissue. Accumulation of these aggregates results in pathological conditions known as amyloidosis. More than 20 such proteins associated with severe diseases have been identified (Table 2) which includes islet amyloid peptide with type 2 diabetes, prion protein with spongiform encephalitis and Aβ with Alzheimer’s disease [75,76,77,78,79].
Displaying the protein quality control system (PQCS). It involves the degradation of the misfolded conformers of protein by different proteolytic cellular pathways. Misfolded conformers are at first recognized by molecular chaperones carrying the substrates to the ubiquitin–proteasome system (UPS), chaperone mediated autophagy (CMA) or macro-autophagy subjected to the nature of size, solubility and misfolding
Additionally, the amyloid aggregates are thermodynamically highly stable which contributes to their property of converting native form of protein into amyloid forms [80]. This acts like a key factor in propagation of pathogenic protein species in prion like manner. Studies conducted in past have shown that these pathogenic aggregates can spread from one neuron to other neuron and neighboring glial cells. Researcher have also found that one form of disease causing protein can trigger the misfolding of other aggregate prone proteins. Recent studies also suggested that the spread of these misfolded protein from one cell to other involves activity dependent secretion by exosomes and or chaperone mediated pathways [81,82,83,84].
Failure of a cell to prevent the process of protein misfolding and or degradation of the misfolded protein subsequently forming toxic protein aggregates forms the basis of the pathology [85,86,87]. The cause of protein aggregation is dependent on many factors which includes both environmental and genetic origin (Fig. 5) [69, 88].
The protein abnormality caused by genetic mutations may be of autosomal recessive and or autosomal dominant [89]. Redox perturbation can be the cause of nongenetic protein misfolding and conformational disorders. Reactive oxygen species can potentially damage the biomacromolecules of the cell including proteins. The partially unfolded or misfolded protein conformers further modified by these reactive oxygen species consequently leading to protein aggregation [30, 69, 90].
Therapeutic Approaches Towards Amyloidogenic Disorders
For the treatment of amyloidosis, many therapeutic approaches have been advised which includes increasing the rate of degradation of misfolded and aggregated protein conformers, increasing stability of aggregation prone proteins, inhibiting the production of amyloidogenic forms of protein and it’s self-assembly. From the previous studies, it has been reported that plant derived phenolic compounds have the potential to inhibit the amyloid aggregate formation. It has also been shown that these compounds possesses cytoprotective activity against aggregates induced cytotoxic effects [30, 73, 91, 92]. These assumptions should be highly relevant for the future de novo design of small molecule inhibitors for the treatment of amyloidogenic diseases.
Industrial Application of Studying Macromolecular Crowding
With the advancement of the technological knowledge, the use of protein based therapeutics for the treatment of various human pathological disorders was came into existence. Interferons, monoclonal antibodies, cytokines, anticoagulants, bone morphogenetic proteins engineered proteins, scaffolds, enzymes, growth factors and hormones are some of the examples of macromolecular therapeutic proteins. These therapeutic proteins have high activity and specificity but they have some limitations too. Addition to the short half-life and low solubility, one of the prominent limitation is self-aggregation and poor stability. Since protein therapeutics are self-crowded during the industrial synthesis process, there can be a significant effect of macromolecular crowding can exists that may be the reason for its poor stability and self-aggregation. So, from industrial point of view, it becomes more important to study the behavior of protein in crowded condition so that the unfavorable consequences could be avoided.
Current Challenges and Future Perspective
From the past decades, scientists were making every effort to understand the process of protein folding, unfolding and aggregation. Protein aggregation has been associated with many pathological conditions making life of elderly miserable. It is very difficult to study the process of protein misfolding and aggregation inside the cell, so researchers are trying to imitate the in vivo like conditions in vitro. One of the most noticeable factor of in vivo environment is molecular crowding. The effect of crowding on structure, stability and interactions of proteins with other biomolecules is of utmost important to study. Crowding condition in vitro has been successfully achieved with the help of both natural and artificial molecular crowders. The study involves use of both homogenous (single type of crowder) and heterogeneous (mixed crowders). However, the exact scenario of in vivo crowded milieu is far away from that being created in vitro. The in vitro crowding conditions has many limitations which needs to be answered with prime concern. In future, much of the work has to be done to create an in vitro system which should be if not exactly, nearly identical with that of in vivo cellular conditions. This can be achieved with growing advanced technology like in vitro organ developments. Once we able to create similar in vivo like environment in vitro, then it will be more easier to study the process of protein folding, unfolding and aggregation. The amelioration of amyloid aggregate formation by various molecules can also be tested under these in vitro created crowded conditions. The positive results obtained via this study could be successfully implemented for the treatment of protein conformational disorders.
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
References
Van Den Berg J, Boersma AJ, Poolman B (2017) Microorganisms maintain crowding homeostasis. Nat Rev Microbiol 15:309–318
Miklos AC, Sarkar M, Wang Y, Pielak GJ (2011) Protein crowding tunes protein stability. J Am Chem Soc 133:7116–7120
Zimmerman SB, Trach SO (1991) Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol 222:599–620
Szathmáry E, Smith JM (1995) The major evolutionary transitions. Nature 374:227–232
Ellis RJ (2001) Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol 11:114–119
Harding J (1991) Biochemistry epidemiology and pharmacology. Cataract 195–217
Minton AP, Wilf J (1981) Effect of macromlecular crowding upon the structure and function of an enzyme: glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 20:4821–4826
Christiansen A, Wang Q, Samiotakis A et al (2010) Factors defining effects of macromolecular crowding on protein stability: an in vitro/in silico case study using cytochrome c. Biochemistry 49:6519–6530
Mittal S, Singh LR (2014) Macromolecular crowding induces holo α-lactalbumin aggregation by converting to its apo form. PLoS ONE 9:e114029
Du F, Zhou Z, Mo ZY et al (2006) Mixed macromolecular crowding accelerates the refolding of rabbit muscle creatine kinase: implications for protein folding in physiological environments. J Mol Biol 364:469–482
Kozer N, Kuttner YY, Haran G, Schreiber G (2007) Protein-protein association in polymer solutions: from dilute to semidilute to concentrated. Biophys J 92:2139–2149
Wenner JR, Bloomfield VA (1999) Crowding effects on EcoRV kinetics and binding. Biophys J 77:3234–3241
Rivas G, Fernandez JA, Minton AP (1999) Direct observation of the self-association of dilute proteins in the presence of inert macromolecules at high concentration via tracer sedimentation equilibrium: theory, experiment, and biological significance. Biochemistry 38:9379–9388
Verma PK, Rakshit S, Mitra RK, Pal SK (2011) Role of hydration on the functionality of a proteolytic enzyme α-chymotrypsin under crowded environment. Biochimie 93:1424–1433
Mittal S, Chowhan RK, Singh LR (2015) Macromolecular crowding: Macromolecules friend or foe. Biochim Biophys Acta (BBA)-Gen Subj 1850:1822–1831
Mittal S, Singh LR (2013) Denatured state structural property determines protein stabilization by macromolecular crowding: a thermodynamic and structural approach. PLoS ONE 8:e78936
Stagg L, Zhang S-Q, Cheung MS, Wittung-Stafshede P (2007) Molecular crowding enhances native structure and stability of α/β protein flavodoxin. Proc Natl Acad Sci 104:18976–18981
Roque A, Ponte I, Suau P (2007) Macromolecular crowding induces a molten globule state in the C-terminal domain of histone H1. Biophys J 93:2170–2177
Perham M, Stagg L, Wittung-Stafshede P (2007) Macromolecular crowding increases structural content of folded proteins. FEBS Lett 581:5065–5069
Cheung MS, Klimov D, Thirumalai D (2005) Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci U S A 102:4753–4758
Muramatsu N, Minton AP (1988) Tracer diffusion of globular proteins in concentrated protein solutions. Proc Natl Acad Sci 85:2984–2988
Han J, Herzfeld J (1993) Macromolecular diffusion in crowded solutions. Biophys J 65:1155–1161
Dhar A, Samiotakis A, Ebbinghaus S et al (2010) Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding. Proc Natl Acad Sci 107:17586–17591
Derham BK, Harding JJ (2006) The effect of the presence of globular proteins and elongated polymers on enzyme activity. Biochim Biophys Acta (BBA)-Proteins Proteomics 1764:1000–1006
Jiang M, Guo Z (2007) Effects of macromolecular crowding on the intrinsic catalytic efficiency and structure of enterobactin-specific isochorismate synthase. J Am Chem Soc 129:730–731
Norris MGS, Malys N (2011) What is the true enzyme kinetics in the biological system? An investigation of macromolecular crowding effect upon enzyme kinetics of glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun 405:388–392
Mittal S, Singh LR (2014) Macromolecular crowding decelerates aggregation of a β-rich protein, bovine carbonic anhydrase: a case study. J Biochem 156:273–282
Ma B, Xie J, Wei L, Li W (2013) Macromolecular crowding modulates the kinetics and morphology of amyloid self-assembly by β-lactoglobulin. Int J Biol Macromol 53:82–87
Siddiqui GA, Naeem A (2018) Aggregation of globular protein as a consequences of macromolecular crowding: A time and concentration dependent study. Int J Biol Macromol 108:360–366
Siddiqui GA, Naeem A (2020) The contrasting effect of macromolecular crowding and confinement on fibril formation of globular protein: Underlying cause of proteopathies. J Mol Liq 114602
van den Berg B, Ellis RJ, Dobson CM (1999) Effects of macromolecular crowding on protein folding and aggregation. EMBO J 18:6927–6933
van den Berg B, Wain R, Dobson CM, Ellis RJ (2000) Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell. EMBO J 19:3870–3875
Fan Y-Q, Liu H-J, Li C et al (2012) Effects of macromolecular crowding on refolding of recombinant human brain-type creatine kinase. Int J Biol Macromol 51:113–118
Malik A, Kundu J, Mukherjee SK, Chowdhury PK (2012) Myoglobin unfolding in crowding and confinement. J Phys Chem B 116:12895–12904
Huang L, Jin R, Li J et al (2010) Macromolecular crowding converts the human recombinant PrPC to the soluble neurotoxic β-oligomers. FASEB J 24:3536–3543
Harada R, Tochio N, Kigawa T et al (2013) Reduced native state stability in crowded cellular environment due to protein–protein interactions. J Am Chem Soc 135:3696–3701
Sarkar M, Smith AE, Pielak GJ (2013) Impact of reconstituted cytosol on protein stability. Proc Natl Acad Sci 110:19342–19347
Chi EY, Krishnan S, Randolph TW, Carpenter JF (2003) Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res 20:1325–1336
Castillo V, Graña-Montes R, Sabate R, Ventura S (2011) Prediction of the aggregation propensity of proteins from the primary sequence: aggregation properties of proteomes. Biotechnol J 6:674–685
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10:S10–S17
Kholova I, Niessen HWM (2005) Amyloid in the cardiovascular system: a review. J Clin Pathol 58:125–133
Williams RA, Mamotte CDS, Burnett JR (2008) Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev 29:31
Xu J, Reumers J, Couceiro JR et al (2011) Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol 7:285–295
Chowhan RK, Warepam M, Dar TA, Singh LR (2013) Recent trends in treating neuronal proteinopathies. J Proteins Proteomics 4
Chowhan RK, Mittal S, Dar TA et al (2014) Ignored avenues in alpha-synuclein associated proteopathy. CNS Neurol Disord Targets (Formerly Curr Drug Targets-CNS Neurol Disord) 13:1246–1257
Ahmed A, Shamsi A, Bano B (2017) Characterizing harmful advanced glycation end-products (AGEs) and ribosylated aggregates of yellow mustard seed phytocystatin: Effects of different monosaccharides. Spectrochim Acta Part A Mol Biomol Spectrosc 171:183–192
Galán A, Sot B, Llorca O et al (2001) Excluded volume effects on the refolding and assembly of an oligomeric protein: GroEL, a case study. J Biol Chem 276:957–964
Zhou BR, Liang Y, Du F et al (2004) Mixed macromolecular crowding accelerates the oxidative refolding of reduced, denatured lysozyme: Implications for protein folding in intracellular environments. J Biol Chem 279:55109–55116
Munishkina LA, Ahmad A, Fink AL, Uversky VN (2008) Guiding protein aggregation with macromolecular crowding. Biochemistry 47:8993–9006
Ma Q, Fan J-B, Zhou Z et al (2012) The contrasting effect of macromolecular crowding on amyloid fibril formation. PLoS ONE 7:e36288
Hatters DM, Minton AP, Howlett GJ (2002) Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J Biol Chem 277:7824–7830
Ghahghaei A, Divsalar A, Faridi N (2010) The effects of molecular crowding on the amyloid fibril formation of α-lactalbumin and the chaperone action of α-casein. Protein J 29:257–264
Zhou Z, Fan J-B, Zhu H-L et al (2009) Crowded cell-like environment accelerates the nucleation step of amyloidogenic protein misfolding. J Biol Chem 284:30148–30158
Yamin G, Munishkina LA, Karymov MA et al (2005) Forcing nonamyloidogenic β-synuclein to fibrillate. Biochemistry 44:9096–9107
Munishkina LA, Cooper EM, Uversky VN, Fink AL (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit 17:456–464
Uversky VN, Cooper EM, Bower KS et al (2002) Accelerated α-synuclein fibrillation in crowded milieu. Febs Lett 515:99–103
Homchaudhuri L, Sarma N, Swaminathan R (2006) Effect of crowding by dextrans and Ficolls on the rate of alkaline phosphatase–catalyzed hydrolysis: A size-dependent investigation. Biopolym Orig Res Biomol 83:477–486
Gellerich FN, Laterveer FD, Korzeniewski B et al (1998) Dextran strongly increases the Michaelis constants of oxidative phosphorylation and of mitochondrial creatine kinase in heart mitochondria. Eur J Biochem 254:172–180
Pastor I, Vilaseca E, Madurga S et al (2011) Effect of crowding by dextrans on the hydrolysis of N-succinyl-l-phenyl-ala-p-nitroanilide catalyzed by α-chymotrypsin. J Phys Chem B 115:1115–1121
Ren G, Lin Z, Tsou C, Wang C (2003) Effects of macromolecular crowding on the unfolding and the refolding of D-glyceraldehyde-3-phosophospate dehydrogenase. J Protein Chem 22:431–439
Levy Y, Onuchic JN (2004) Water and proteins: A love–hate relationship. Proc Natl Acad Sci 101:3325–3326
Rupley JA, Careri G (1991) Protein hydration and function. Adv Protein Chem 41:37–172
Frölich A, Gabel F, Jasnin M et al (2009) From shell to cell: neutron scattering studies of biological water dynamics and coupling to activity. Faraday Discuss 141:117–130
Mansell JL, Clegg JS (1983) Cellular and molecular consequences of reduced cell water content. Cryobiology 20:591–612
Harada R, Sugita Y, Feig M (2012) Protein crowding affects hydration structure and dynamics. J Am Chem Soc 134:4842–4849
Yaku H, Murashima T, Tateishi-Karimata H et al (2013) Study on effects of molecular crowding on G-quadruplex-ligand binding and ligand-mediated telomerase inhibition. Methods 64:19–27
Shah H, Rawat K, Ashar H et al (2019) Dual role for fungal-specific outer kinetochore proteins during cell cycle and development in Magnaporthe oryzae. J Cell Sci 132:jcs224147
Radwan M, Wood RJ, Sui X, Hatters DM (2017) When proteostasis goes bad: protein aggregation in the cell. IUBMB Life 69:49–54
Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81:678–699
Gregersen N, Bolund L, Bross P (2005) Protein misfolding, aggregation, and degradation in disease. Mol Biotechnol 31:141–150
Ilyinsky NS, Nesterov SV, Shestoperova EI et al (2021) On the role of normal aging processes in the onset and pathogenesis of diseases associated with the abnormal accumulation of protein aggregates. Biochem 86:275–289
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Sweeney P, Park H, Baumann M et al (2017) Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener 6:1–13
Gregersen N, Bross P (2010) Protein misfolding and cellular stress: an overview. Protein Misfolding Cell Stress Dis Aging 3–23
Furkan M, Siddiqi MK, Zakariya SM et al (2019) An In Vitro elucidation of the antiaggregatory potential of Diosminover thermally induced unfolding of hen egg white lysozyme; A preventive quest for lysozyme amyloidosis. Int J Biol Macromol 129:1015–1023
Arfin S, Siddiqui GA, Naeem A, Moin S (2018) Inhibition of advanced glycation end products by isoferulic acid and its free radical scavenging capacity: An in vitro and molecular docking study. Int J Biol Macromol 118:1479–1487
Fazili NA, Siddiqui GA, Bhat SA et al (2015) Rifampicin induced aggregation of ovalbumin: Malicious behaviour of antibiotics. Protein Pept Lett 22
Knaupp AS, Bottomley SP (2009) Serpin polymerization and its role in disease—The molecular basis of α1-antitrypsin deficiency. IUBMB Life 61:1–5
Amani S, Naeem A (2013) Understanding protein folding from globular to amyloid state: aggregation: darker side of protein. Process Biochem 48:1651–1664
Meersman F, Dobson CM (2006) Probing the pressure–temperature stability of amyloid fibrils provides new insights into their molecular properties. Biochim Biophys Acta (BBA)-Proteins Proteomics 1764:452–460
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol cell Biol 11:301–307
Moreno-Gonzalez I, Soto C (2011) Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. In Seminars in cell & developmental biology. Elsevier, pp 482–487
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51
Davis AA, Leyns CEG, Holtzman DM (2018) Intercellular spread of protein aggregates in neurodegenerative disease. Annu Rev Cell Dev Biol 34:545–568
Blancas-Mejía LM, Ramirez-Alvarado M (2013) Systemic amyloidoses. Annu Rev Biochem 82:745–774
Lee S-J, Desplats P, Sigurdson C et al (2010) Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol 6:702–706
Bucciantini M, Giannoni E, Chiti F et al (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511
Jiang T, Yu J-T, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer’s disease: from genetic to non-genetic factors. Curr Alzheimer Res 10:852–867
Monsellier E, Ramazzotti M, Taddei N, Chiti F (2008) Aggregation propensity of the human proteome. PLoS Comput Biol 4:e1000199
Hensley K, Carney JM, Mattson MP et al (1994) A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci 91:3270–3274
Siddiqui GA, Siddiqi MK, Khan RH, Naeem A (2018) Probing the binding of phenolic aldehyde vanillin with bovine serum albumin: Evidence from spectroscopic and docking approach. Spectrochim Acta - Part A Mol Biomol Spectrosc 203:40–47
Dawn A, Deep S (2020) Thinking beyond tradition: Polyphenols as effective refolding modulators. Int J Biol Macromol 148:969–978
Acknowledgements
The authors would like to acknowledge the facilities obtained at Aligarh Muslim University, Aligarh.
Author information
Authors and Affiliations
Contributions
Review article written by Gufran Ahmed Siddiqui and checked by Dr Aabgeena Naeem.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqui, G.A., Naeem, A. Connecting the Dots: Macromolecular Crowding and Protein Aggregation. J Fluoresc 33, 1–11 (2023). https://doi.org/10.1007/s10895-022-03082-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03082-2