Abstract
Despite great advances in our understanding of the molecular causes of liver cancer, significant gaps still remain in our knowledge of the disease pathogenesis and development of effective strategies for early diagnosis and treatment. The present study was conducted to evaluate the chemopreventive activity of ellagic acid (EA) against experimental liver cancer in rats. This is the first report that implies a possible role of EA in controlling liver cancer through activation of mitochondrial outer membrane permeability via activating proteins such as Bax, bcl-2, cyt-C, and caspase-9, which play important roles in apoptosis. Downregulation of NF-κB, cyclin D1, cyclin E1, matrix metalloproteinases (MMP)-2, MMP-9, and proliferating cell nuclear antigen (PCNA) were noted in EA-treated experimental rats and controlled inflammation mediated liver cancer when compared to the diethylnitrosamine (DEN)-induced group. Transmission electron microscopy (TEM) analysis of the livers of experimental rats demonstrated that EA treatment renovated its internal architecture. Overall, these results demonstrate the value of molecular approaches in identifying the potential role of EA as an effective chemopreventive agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a major healthcare problem worldwide. Specifically, HCC is the fifth most malignant neoplasm in the world and an important cause of death in Asian and western countries, accounting for as many as one million deaths worldwide annually. In some parts of the world, HCC is the most common form of internal malignancy [1]. In India, liver cancer is the third most common form of cancer in men [2]. Well-known risk factors of hepatocellular carcinoma include hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, and oral contraceptives. Smoking, androgenic steroids, and diabetes mellitus are also suspected risk factors [3].
Diethylnitrosamine (DEN) is found in a variety of food products, which is the most important environmental carcinogen among nitrosamines and primarily induces tumors of the liver [4, 5]. The presence of nitrosamines and their precursors in the human body have led to the suggestion that they are involved in HCC [6]. Accordingly, DEN is now widely used as a standard experimental model for HCC [7, 8]. DEN-induced lesions and tumors in rodents show similar biochemical, histological and molecular changes to HCC [6, 9].
Ellagic acid (EA) is a polyphenol compound with strong antioxidant and antitumor properties that can be applied to improve the body’s defense against cancer. Ellagic acid is found as ellagitannins in the fruits and nuts of several plants [10]. Oral administration of EA protects the system from alcohol toxicity by decreasing liver marker enzymes, lipid peroxidative markers, and NO and increasing the antioxidant cascade [11].
Current scientific interest regarding the management of cancer is directed toward utilization of naturally occurring compounds for chemotherapeutic. A successful anticancer drug should kill or incapacitate cancer cells without causing excessive damage to normal cells, thereby inducing apoptosis in cancer cells via modulation of cell signaling [12].
There has recently been growing interest in the role and mechanism of phytochemicals. Among phytochemicals, EA has been receiving a great deal of attention because of its wide array of biological characteristics, which include radical scavenging, chemopreventive, antiviral, and antibacterial properties [13]. Inflammation is a critical component of tumor progression that is now recognized as one of the hallmarks of cancer. Mast cells are one of the major effector cells in the immune response system. Activated mast cells release proinflammatory factors [14], which disrupt cell cycle regulatory proteins.
The restriction point “R” at the transition between the G1- and S-phases of the cell cycle have been recognized as the end point of regulatory pathways that are critical for growth control and thus for the prevention of excessive or unrestricted growth. Deregulation of these pathways or the deletion or overexpression of particular factors has been linked to malignant transformation of cells and the development of cancer. Angiogenesis is associated with the rapid proliferation of cancer cells, and matrix metalloproteinases (MMPs) play a substantial role in angiogenesis. Matrix metalloproteinases comprise a family of zinc-containing endopeptidases that degrade various components of the extracellular matrix (ECM). A number of MMPs have been strongly implicated in multiple stages of cancer progression, including the acquisition of invasive and metastatic properties [15, 16]. However, this is the first study to investigate the role of EA in regulating mitochondrial outer membrane permeability in carcinogen mediated (DEN) experimental liver cancer.
Materials and Method
Animals
Male Wistar albino rats weighing 150–180 g obtained from the Small Animal Breeding Centre, Agricultural University, Mannuthy, Kerala, India was used. Rats were acclimatized under standard laboratory conditions at 25 ± 2 °C with a normal photoperiod. The rats were provided with standard rat chow and water ad libitum until 18–24 h before the experiment, at which time food was withdrawn. The care and use of laboratory rats was conducted according to the guidelines of the Council Directive CPCSEA, India (No: 659/02/a) pertaining to Good Laboratory Practices (GLPs) during animal experimentation. All animal experiments were performed in the laboratory according to the ethical guidelines suggested by the Institutional Animal Ethics Committee (IAEC).
Experimental Design
Experimental rats were divided into four groups of six rats each that were subjected to the following treatments for a total experimental period of 16 weeks: group1, normal control rats fed standard rat chow and drinking water. Group 2, rats orally administered EA alone (30 mg/kg body weight) in the form of aqueous suspension once a day for 16 weeks. The EA dose was set based on our previous report [17]. Group 3, rats provided with DEN (0.01 %) alone in drinking water for 16 weeks to induce hepatocarcinogenesis. Group 4, rats were administered DEN (0.01 %) in drinking water for the first 10 weeks, then EA as in group 2 for the remaining 6 weeks. At the end of the experimental period all rats were anesthetized with chloroform after overnight fasting and euthanized. After euthanization, blood samples were collected and liver tissue was removed, washed in ice-cold saline, part of the liver tissue was taken for histopathological and transmission electron microscopic analysis. Remaining liver samples were stored at −80 °C until further analysis.
Transmission Electron Microscopy
For transmission electron microscopy (TEM) analysis, the liver samples were fixed in Karnovsky’s fixative immediately after euthanization of rats for 6–8 h at 4 °C. These samples were then postfixed in 1 % osmium tetroxide in 0.1 M phosphate buffer for 2 h at 4 °C, dehydrated in ascending grades of acetone, infiltrated and embedded in araldite CY212, and polymerized at 60 °C for 72 h. Thin (60–70 nm) sections were then cut with an ultramicrotome, mounted on copper grids, stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope, according to methods described previously reported [18].
Mast Cell Staining
Histochemical analysis of mast cells was carried out as described by Ranieri et al. [19]. Briefly, 5-μm thick sections were dewaxed in xylene and then washed by reducing concentrations of ethanol in distilled water. The sections were subsequently stained with toluidine blue for 1 min, after which they were washed with distilled water and exposed to light green SF stain for 20 s. The samples were then washed again with distilled water, after which they were dried out in increasing concentrations of alcohol followed by xylene and mounted using DPX. Finally, quantitative analysis was conducted under a light microscope at 100 × magnification.
Immunohistochemistry of PCNA
Immunohistochemical staining was achieved using the method described by Ramakrishnan et al. [20]. The tissue sections were deparaffinized by xylene at 60 °C for 15 min each and then hydrated through decreasing concentrations of alcohol. For antigen retrieval, the slides were placed in buffer solution (citrate buffer pH 6) and then incubated for 5 min (3 cycles) in a microwave oven. Next, the slides were allowed to cool at room temperature, after which they were rinsed with 1× Tris-buffered saline (TBS) followed by treatment of the sections with 0.3 % H2O2 in methanol for 10 min to block the endogenous peroxidase activity. The sections were subsequently placed in 3 % bovine serum albumin (BSA) at room temperature for 1 h to block the nonspecific binding, after which they were incubated at 4 °C with a 1:5,000 dilution of proliferating cell nuclear antigen (PCNA) rabbit polyclonal antibody (Santa Cruz Biotechnology, USA) overnight. After washing with TBS, the slides were incubated with a 1:5,000 dilution of anti-rabbit HRP-labeled secondary antibody (Genei, Bangalore, India) for 1 h at room temperature. Next, the slides were treated with 3,3′-diaminobenzidine tetrahydrochloride (SRL, Mumbai, India) and then counterstained with Meyer’s hematoxylin to visualize the peroxidase activity. Negative controls were incubated with TBS instead of primary antibodies. Quantitative analysis was conducted in a blinded manner under a light microscope at 100 × magnification.
Western Blot Analysis
Western blot was conducted as previously described [21]. For groups 1 and 2, a portion of the liver tissue (100 mg) was cut into very small pieces, homogenized using ice-cold homogenizing buffer (0.1 M Tris, pH 7.4), then centrifuged at 10,000 rpm for 10 min at 4 °C to obtain the supernatant, which was the total cell lysate. For groups 3 and 4, equal amounts of tumorous and non-tumorous liver tissues were weighed and the total cell lysate was prepared as mentioned above. The protein content of the supernatant was determined by Lowry’s method using BSA as the standard. The supernatant was then subjected to immunoblotting and the expression patterns of Bax, Bcl-2, cyt C, caspase-9, Cyclin-D1, Cyclin-E1, MMP-2, MMP-9, and NF-κB (Santa Cruz Biotechnology, USA) were detected as follows. Briefly, supernatant protein (30 μg) was loaded onto 12 % polyacrylamide gels and separated by SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes and blocked with 5 % BSA in TBS-T-buffered saline at room temperature for 1 h. The membranes were then probed with their corresponding primary antibodies diluted in TBS-T (1:5,000 dilution of each) and incubated overnight at 4 °C with gentle shaking. The membranes were subsequently washed with TBS-T gently and incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (1:5,000 dilutions) for 1 h. Finally, protein, antibody complexes were detected by the addition of diaminobenzidine (DAB) as a substrate.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) and groups were compared with the least significant difference (LSD) test using the SPSS/10 software. P < 0.05 was considered significant. All the results were expressed as the mean ± S.D.
Results
Transmission electron microscopic (Fig. 1) analysis of rats showed normal nuclei and cytoplasm (Fig. 1A). Furthermore, rats treated with EA alone showed a similar architecture to control rats (Fig. 1B), while the presence of multiple nuclei with irregular cytoplasm was noted in DEN induced rats (Fig. 1C, D). Cell shrinkage, apoptotic bodies, chromatin condensation, and mitochondrial swelling were observed (Fig. 1E, F) after EA treatment. These findings clearly substantiated the initiation of apoptosis in EA-treated rats.
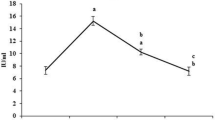
Histochemical staining of liver mast cells of control and experimental rats with toluidine blue revealed no major changes in response to treatment with EA alone (Fig. 2B) relative to control rats (Fig. 2A). DEN-treated rats (Fig. 2C) showed high accumulation of mast cells with increased cell density (P < 0.05) relative to the normal control group. EA-treated rats (Fig. 2D) showed significantly decreased mast cell density relative to DEN induced rats, confirming the possible role of EA against inflammation during carcinogenesis.
Immunohistochemical analysis of changes in the expression of PCNA in DEN-induced (Fig. 3C) rats showed a significant increase in the expression of PCNA-positive nuclei relative to the control group (Fig. 3A). No significant difference was observed among rats treated with EA alone (Fig. 3B) and the control group; however, EA-treated (Fig. 3D) rats were found to have significantly fewer PCNA-positive nuclei than DEN-induced rats.
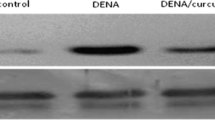
Immunoblot analysis of NF-κB, Cyclin-D1, Cyclin-E1, MMP-2, and MMP-9 (Fig. 4) in the liver of control and experimental rats showed upregulation in DEN-induced rats when compared to the control group. No significant changes in expression were noted between control rats and those treated with EA alone; however, these proteins were downregulated in EA-treated rats. These findings confirm that EA attenuated cell proliferation in DEN-induced rats.
Protein signaling in inflammation-mediated cell proliferation. Lane 1 : control (group 1); Lane 2 : drug-control (group 2); Lane 3 : DEN-induced (group 3); Lane 4 : DEN + EA (group 4); Immunoblot and densitometric analysis of A Cyclin-D1; B: Cyclin-E1; C MMP-2; D MMP-9; E NF-κB-p65; F β-actin. AU represents arbitrary units. Values are expressed as mean ± S.D. (n = 6) p < 0.05. Comparisons are made as group 1 (a) (control) vs group 3 (DEN-induced); group 3 (b) (DEN-induced) vs group 4 (DEN + EA)
In this study, the expression levels of proapoptotic protein Bax, antiapoptotic protein Bcl-2, apoptogenic factor cyt-C, and caspase-9 (Fig. 5) of the experimental groups favored normalization of the mitochondrial outer membrane. No significant changes in expression were observed between control rats and those treated with EA alone. A simultaneous decrease in the expression of Bax and increase in the expression of Bcl-2 was observed in DEN-treated rats; however, these levels were normalized in EA-treated rats. The Bcl-2/Bax ratio (Fig. 5C) was increased in the DEN-induced group, which established the activation of antiapoptotic factors, resulting in the prevention of apoptosis. The expression levels of cyt-C and caspase-9 were downregulated in DEN-treated rats and then upregulated after EA administration. These results demonstrated that EA regulates mitochondrial permeabilization and favors the stimulation of apoptosis.
Protein signaling in mitochondrial permeability. Lane 1: control (group 1); Lane 2: drug-control (group 2); Lane 3: DEN-induced (group 3); Lane 4: DEN + EA (group 4). Immunoblot and densitometric analysis of Bax (A); Bcl-2 (B); densitometric representation of Bcl2/Bax ratio (C); Cyt-C (D), caspase-9 (E), and β-actin(F). AU represents arbitrary units. Values are expressed as mean ± S.D. (n = 6). Comparisons are made as group 1 (a) (control) vs group 3 (DEN-induced); group 3 (b) (DEN-induced) vs group 4 (DEN + EA)
Discussion
The chemopreventive nature of EA in DEN-induced hepatoma bearing rats were investigated in this study and ultrastructural studies were conducted to confirm the morphological changes at the cellular level. Rats treated with DEN showed irregular shaped-nuclei with irregular cytoplasm and metastatic potential, which might have been due to excessive free radical generation during DEN administration. These findings are similar to those reported in a previous study [22]. The control and EA alone groups showed a similar cell architecture, while DEN-treated rats showed altered morphological structures such as dysplastic nuclei and membrane changes with irregular cytoplasm. Stimulation of apoptosis was also indicated by the presence of liver cells with shrunken nuclei, condensed chromatin, membrane blebbing, and apoptotic bodies in EA-treated rats. Overall, these findings confirm stimulation of apoptosis by EA.
Mast cells (MCs) perform a significant role in the inflammation-mediated cell proliferation. Conversely, mast cells are the unique target for the treatment of cancer [23]. MCs circulates in the blood as progenitors and mature when they enter into the tissue [24]. However, matured cells secrete several proangiogenic factors, leading to initiation of the tumor angiogenesis switch [25]. In the present study, MCs was shown to be involved in initiation of angiogenesis through secretion of inflammatory factors, resulting in activation of the NF-κB. Conversely, EA administration decreased the mast cell density, demonstrating that EA plays a key role in mast cell inhibition.
Cell proliferation is thought to play an important role in several steps of the carcinogenic process; therefore, exploration of drugs that can affect malignant proliferation of liver cells is of primary importance in the prevention of liver cancer. PCNA, a polypeptide chain that directly involved in DNA replication and an important marker for evaluation of the proliferation of several cancers, including HCC [26, 27]. In this study, detection of PCNA helped to confirm the transformation of normal cells into malignant cells [28]. Similarly, the high expression of PCNA positive hepatocytes in DEN-treated rats indicated the increased rate of intensity of cell proliferation and transformation of normal hepatocytes into malignant [29]. Overall, the results of the present study showed that the administration of EA led to a remarkable decrease in PCNA expression in HCC bearing rats, suggesting that EA restrains the malignant proliferation of hepatocytes in experimental liver cancer.
NF-κB is a proinflammatory transcription factor that connects inflammation and tumorigenesis and plays an important role in innate immunity, liver inflammation, fibrosis, and antiapoptosis [30]. Hence, NF-κB activation induced cellular transformation, mediated cellular proliferation, and concurrently prevented the abolition of preneoplastic and fully malignant cells by upregulating the antiapoptosis proteins. Furthermore, NF-κB regulated the expression of cyclins (D1and E1) and MMPs (2 and 9), which are involved in cell proliferation and metastasis. These findings suggest a potential molecular bridge between inflammation and cancer [31]. Moreover, the translational confirmation of NF-κB-p65 overexpression in DEN-induced rats indicated that inflammation plays a role in HCC. Additionally, the results presented herein confirm the role of EA against inflammation mediated cell proliferation by downregulation of NF-κB in EA-administered rats.
Growth factors synthesized by mast cells activate NF-κB, which then binds to the promoter Cyclin-D1. Similarly, such deregulation in cyclin pathways is linked to the development of cancer [32]. Therefore, highly expressed cyclins (D1 and E1) in DEN-induced rats controlled the reentry of cells into the normal cell cycle and enhanced the oncogenic transformation concurrently [33]. Cyclin D1 and Cyclin E1 were upregulated in the DEN-treated group, suggesting deregulation in the cell cycle and progression of cell proliferation. Conversely, EA administration significantly lowered the expression of these cyclins, thereby hampering oncogenic transformation, confirming the antiproliferative activity of EA.
Matrix metalloproteinases (MMPs) play an important role in the early and later stages of cancer development [34, 35]. DEN-induced experimental animals showed elevated expressions of MMP-2 and MMP-9, suggesting degradation of the main basement membrane component. Several studies have reported that angiogenesis require membrane damage, which leads to malignant transformation [36, 37]. Conversely, EA administration downregulated the expression of MMPs, demonstrating that EA can restrain angiogenesis and exert tumor inhibitory effects. Members of the Bcl-2 family protein play an important role in the maintenance of cell death [38] and dysregulation in the cell cycle leads to, alteration of Bcl-2 family protein expression and defective apoptosis [39]. Modification of the Bcl-2/Bax ratio alters the release of cytochrome C, thereby influencing the mitochondrial membrane permeability in DEN-treated rats [40]. Following EA treatment, the expression of Bcl-2 proteins was altered to near normal levels, demonstrating the involvement of EA in stopping the growth of cancer cells.
Activation of caspase-3 plays a central role in the initiation of apoptosis. Caspase activation occurs through the release of cytochrome C from the mitochondria and hence, mitochondrial outer membrane permeability and cyt C release is directly related to apoptosis [17, 41]. The activated caspase-9 then cleaves and activates effector caspases, such as caspase-3 and 7, which execute the apoptotic program [42, 43]. The expression of cyt C and caspase-9 were downregulated in the DEN-induced group, while EA treatment regulated the mitochondrial membrane permeability by enhancing the release of cyt C and thus controlled cell proliferation as an effective chemotherapeutic agent.
Conclusion
The results of this study demonstrate that EA hinders hepatic cancer through inhibition of mast cell expression, which controls the release of proinflammatory factors. Hence, activation of NF-κB, cyclins, and MMPs was halted, which led to the conservation of the basement membrane and inhibited angiogenesis. Conversely, EA regulates normalization of the mitochondrial outer membrane permeability by increasing the release of cytochrome, which subsequently activates apoptotic proteins, resulting in the programmed death of malignant cells. These effects indicate that EA has anticancer activity. Furthermore, EA exhibited chemopreventive activity through the regulation of genetic factors involved in cell propagation, inflammation, angiogenesis, and apoptosis. Overall, the results of this investigation demonstrate the potential role of EA as an effective chemopreventive agent that should be considered in future drug development.
References
Zhao, J. L., Zhao, J., & Jiao, H. J. (2013). Applied Biochemistry and Biotechnology, 172, 784–791.
Khan, M. S., Halagowder, D., & Niranjali, S. D. (2011). Food and Chemical Toxicology, 49, 173–178.
Ford, N. A., Lashinger, L. M., Allott, E. H., & Hursting, S. D. (2013). Frontiers Oncology, 3, 209.
Jakszyn, P. G., Allen, N. E., Barroso, L. L., et al. (2012). Cancer Epidemiology Biomarkers, 21, 547–551.
Mittal, G., Brar, A. P., & Soni, G. (2006). Pharmacological Reports, 58, 413–419.
Mittal, G., Brar, A. P., & Soni, G. (2006). Pharmacological Reports, 58, 413–419.
Sivaramakrishnan, V., & Devaraj, S. N. (2009). Chemico-Biological Interactions, 180, 353–359.
Al-Kaseem, M., Al-Assaf, Z., & Karabet, F. (2013). Pharmacology and Pharmacy, 4, 611–618.
Lee, L., Wang, K., Li, G., Xie, Z., Wang, Y., Xu, J., et al. (2011). BMC Genomics, 12(S3), 1–13.
Kim, J. Y., Ok, E., Kim, Y. J., Choi, K. S., & Kwon, O. (2013). Nutrition Research and Practice, 7(3), 153–159.
Umesalma, S., & Sudhandiran, G. (2010). Basic and Clinical Pharmacology and Toxicology, 107, 650–655.
Jiang, W., Zhu, Z., McGinley, J. N., Bayoumy, K. E., Manni, A., & Thompson, H. J. (2013). Cancer Research, 72(15), 3795–806.
Vanella, L., Giacomo, C. D., Acquaviva, R., Barbagallo, I., Volti, G. L., Cardile, V., et al. (2013). Cancers, 5, 726–738.
Pittoni, P., & Colombo, M. P. (2012). Cancer Research, 72, 831–835.
Choi, Y. J., Li, X., Hydbring, P., Sanda, T., Stefano, J., Christie, A. L., et al. (2012). Cancer Cell, 22(4), 438–451.
Herszényi, L., Hritz, I., Lakatos, G., Varga, M. Z., & Tulassay, Z. (2012). International Journal of Molecular Sciences, 13, 13240–13263.
Srigopalram, S., Ilavenil, S., & Indira, A. J. (2012). Biomedicine and Preventive Nutrition, 2, 1–8.
Nelson, L. J., Treskes, P., Howie, A. F., Walker, S. W., Hayes, P. C., & Plevris, J. N. (2013). Scientific Reports, 3, 2735.
Ranieri, G., Labriola, A., Achille, G., Florio, G., Zito, A. F., & Grammatica, L. (2002). International Journal of Oncology, 21(6), 1317–1323.
Ramakrishnan, G., Elinos-Baaez, C. M., Jagan, S., Augustine, T. A., Kamaraj, S., Anandakumar, P., et al. (2008). Molecular and Cellular Biochemistry, 313(1–2), 53–61.
Ramakrishnan, G., Jagan, S., Kamaraj, S., Anandakumar, P., & Devaki, T. (2009). Investigational New Drugs, 27, 233–240.
Hassoun, E. A., & Cearfoss, J. (2011). Toxicological and Environmental Chemistry, 93(2), 332–344.
Gounaris, E., Erdman, S. E., Restaino, C., Gurish, M. F., Friend, D. S., & Gounari, F. (2007). Proceedings of the National Academy of Sciences of the United States of America, 104(50), 19977–19982.
Grizzi, F., Di Caro, G., Laghi, L., Hermonat, P., Mazzola, P., Nquyen, D. D., et al. (2013). Immunity & Ageing, 10(1), 1–10.
Hodges, K., Kennedy, L., Meng, F., Alpini, G., & Francis, H. (2012). Translation of Gastrointestinal Cancer, 1(2), 138–150.
Kang, J. S., Kang, H. G., Park, Y. I., Lee, H., Park, K., Lee, Y. S., et al. (2013). Experimental and Therapeutic Medicine, 5, 138–142.
Sun, H., Yu, L., Wei, H., & Liu, G. (2012). Journal of Biomedicine & Biotechnology, 2012(584728), 1–9.
Qasim, B. J., Ali, H. H., & Hussein, A. G. (2012). Saudi Journal of Gastroenterology, 18, 268–76.
Zhao, H., Ho, P. C., Lo, Y. H., Espejo, A., Bedford, M. T., Hung, M. C., et al. (2012). PloS One, 7(e29416), 1–7.
Muriel, P. (2009). NF-kB in liver diseases: a target for drug therapy. Journal of Applied Toxicology, 29, 91–100.
Holdenrieder, S., & Stieber, P. (2004). Clinical Biochemistry, 37, 605–617.
Rusca, N., Dehò, L., Montagner, S., Zielinski, C. E., Sica, A., Sallusto, F., et al. (2012). Molecular and Cellular Biology, 32, 4432–4444.
Boström, P., Söderström, M., Palokangas, T., Vahlberg, T., Collan, Y., Carpen, O., et al. (2009). BMC Research Notes, 2(140), 1–8.
Thieringer, F. R., Maass, T., Anthon, B., Meyer, E., Schirmacher, P., Longerich, T., et al. (2012). Molecular Carcinogenesis, 51, 439–448.
Chantrian, C. F., Henriet, P., Jodele, S., Emonard, H., Feron, O., & Courtoy, P. J. (2006). European Journal of Cancer, 42(3), 310–318.
Huang, X. H., Chen, J. S., Wang, Q., Chen, X. L., Wen, L., Chen, L. Z., et al. (2011). The Journal of Pathology, 225(3), 463–472.
Partyka, R., Gonciarz, M., Jałowiecki, P., Kokocińska, D., & Byrczek, T. (2012). Medical Science Monitor, 18(4), 130–134.
Lo, S. J., Fan, L. C., Tsai, Y. F., Lin, K. Y., Huang, H. L., Wang, T. H., et al. (2013). Hepatology, 57(5), 1893–1905.
Yu, J. Q., Bao, W., & Lei, J. C. (2013). Phytotherapy Research, 27, 251–257.
Zhang, C. Z., Pan, Y., Cao, Y., Lai, P. B. S., Liu, L., Chen, G. G., & Yun (2012). Journal of PLoS ONE, 7(6), e39870, 1–10.
Mahdavi, M., Davoodi, J., Zali, M. R., & Foroumadi, A. (2011). Biomedicine & Pharmacotherapy, 65, 175–182.
Wang, N., Feng, Y., Zhu, M., Tsang, C. M., Man, K., Tong, Y., et al. (2010). Berberine Induces Autophagic Cell Death and Mitochondrial Apoptosis in Liver Cancer Cells: The Cellular Mechanism. Journal of Cellular Biochemistry, 111, 1426–1436.
Kang, J. H., Zhang, W. Q., Song, W., Shen, D. Y., Li, S. S., Tian, L., et al. (2012). Applied Biochemistry and Biotechnology, 166(4), 942–51.
Acknowledgments
This research was supported by Golden Seed Project (Center for Horticultural Seed Development, No. 213003-04-1-CG100), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA), and Korea Forest Service (KFS).
Conflict of Interest
The authors confirm that there is no conflict of interest associated with this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srigopalram, S., Jayraaj, I.A., Kaleeswaran, B. et al. Ellagic Acid Normalizes Mitochondrial Outer Membrane Permeabilization and Attenuates Inflammation-Mediated Cell Proliferation in Experimental Liver Cancer. Appl Biochem Biotechnol 173, 2254–2266 (2014). https://doi.org/10.1007/s12010-014-1031-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1031-y