Abstract
Animal bile is popularly used as a traditional medicine in China, and bile acids are their major bioactive constituents. In the present study, effects of bile extract from crocodile gallbladder on QBC939 cell growth, cell cycle, and apoptosis were investigated by MTT assay, inverted microscopy, fluorescence microscopy, transmission electron microscopy, scanning electron microscopy, PI single- and FITC/PI double-staining flow cytometry, and western blotting. Our data have revealed that bile extract inhibited cells growth significantly, and the cell cycle was arrested in G1 phase. Bile extract induced QBC939 cell apoptosis, which was associated with collapse of the mitochondrial membrane potential and increase of ROS. In bile extract-treated cells, it was observed that the expression of bcl-2 decreased and cytochrome c released to cytosol, but the expression of bax remained unchanged. The data indicated that mitochondrial pathway might play an important role in bile extract-induced apoptosis in QBC939 cells. These results provide significant insight into the anticarcinogenic action of bile extract on cholangiocarcinoma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal bile, such as bear bile and snake bile, contain high amounts of bile acids and have been used as traditional medicines for a long history in China. Both bear and snake bile solutions were shown to have anti-inflammatory, anticonvulsion, and analgesic effects. It has been reported that several unconjugated bile acids may play some roles in the development of intestinal tumors [1]. Meanwhile, these acids showed anticancer activities [2]. It is worth mentioning that the ursodeoxycholic acid was used for the prevention of gastrointestinal disorders in patients having various cancers (stomach, colon, lung, breast, and liver) [3]. It has been evaluated in clinical phase III trial and has statistically significant 39% reduction in recurrence of adenomas with high-grade dysplasia [4].
Crocodylus siamensis is one of the species of freshwater crocodile that was originally distributed throughout South East Asia. Now, the crocodile can be farmed with providing a suitable habitat. Commercial crocodile farms produce hides and meat as major products. Bile from Alligator mississippiensis was found to contain a mixture of more than 20 bile acids, bile alcohols, and neutral sterols, such as chenodeoxycholic acid, ursodeoxycholic acid, cholic acid, allocholic acid, deoxycholic acid, and so on [5]. The contents of C. siamensis bile extracts are quite similar with snake bile [6]. It is reasonable to think that bile from C. siamensis also have anticancer activities. Through our research of C. siamensis bile extracts, we hope to find out whether it has anticancer activity. And if it does, what is the mechanism?
Cholangiocarcinoma (CCA) is an aggressive and lethal cancer arising from the neoplastic transformation of the epithelial cells that line the intra- and extra-hepatic bile ducts [7]. Its incidence has been increasing worldwide over the past several decades [8], and it accounts now for 10–15% of all hepatobiliary malignancies [9]. The prognosis for CCA patients is quite poor due to the lack of an early diagnosis and the fact that the tumor is relatively resistant to chemotherapy [10]. Therefore, novel treatment strategies directed against CCA are needed.
In this study, we investigated the inhibitory effects of bile extracts of Siamese crocodile on the growth of CCA cells in vitro by determining its apoptosis-inducing capabilities and its potential mechanism.
Materials and Methods
Reagents
Gallbladders of C. siamensis was supplied by Sriracha Tiger Zoo Co., Ltd. in Thailand. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), bisbenzimide (Hoechst 33258), propidium iodide (PI), and rhodamine 123 (Rh123) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Primary antibodies of bax, bcl-2, cytochrome c, β-actin, and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (CA, USA). All other reagents were of analytical reagent quality.
Extract of Bile
The bile juice was suspended in distilled water at 4 °C for 24 h. The mixture was centrifuged at 10,000 × g for 20 min at 4 °C using a Sigma Laborzentrifugen refrigerated centrifuge (Osterode Harz, Germany). The supernatant was collected and dehydrated by using vacuum freeze-drying method to produce bile powder. And then the bile powder was stored at −20 °C for further research.
Cell Culture and Treatment
QBC939 cells were provided by Professor Shu-Guang Wang from Southwest Hospital, the Third Military Medical University, Chongqing, China. The cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml of ampicillin, and 100 μg/ml of streptomycin sulfate at 37 °C in a humidified atmosphere under 5% CO2.
Cell Viability Assay
Cell proliferation was assessed by MTT method [11].
Hoechst 33258, Transmission Electron Microscopy (TEM), and Assessment of Cell Cycle and Apoptosis by Flow Cytometry
These studies were performed as previously described [12].
Scanning Electron Microscope (SEM)
Cells were fixed in 2.5% glutaraldehyde for 2 h at 4 °C, stained in 1% osmium tetroxide for 2 h at 4 °C, dehydrated using gradient alcohol, dried at the critical point, gold evaporated, and observed using a scanning electron microscope (JSM-6390; JEOL Ltd., Japan).
Reactive Oxygen Species (ROS)
Cells were incubated with 10 μM 2, 7-dichlorofluorescein diacetate (DCFH-DA) at 37 °C for 15 min. DCF fluorescence was detected by flow cytometry. The fluorescence was measured at excitation 488 nm and emission 525 nm.
Mitochondrial Transmembrane Potential (ΔΨm)
Cells were incubated with 10 μg/ml rhodamine 123 (Rh123) at 37 °C for 30 min. The changes in ΔΨm were analyzed by flow cytometry, with the single beam at 488 nm excitation wavelength and 530 nm emission wavelength.
Western Blotting Analysis
Cells were lysed in RIPA buffer [10 mM Tris (pH 7.4), 150 mM NaCl, 0.5% NP-40, 0.1% SDS, 0.1% deoxycholate, 1 mM PMSF, 2 mM sodium fluoride, and 1 mM sodium orthovanadate] for 40 min. Cell fractionation was performed with a mitochondria/cytosol fractionation kit (BioVision, USA). Samples (15–20 μg) were subjected to 10–15% SDS–PAGE gel and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA), which were then incubated with specific primary antibodies. Blots were then incubated with horseradish peroxidase-conjugated secondary antibodies and detected using ECL system (Pierce Co., USA).
RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted from cells using the RNAiso plus Kit (Takara, Japan) following the manufacturer’s instructions and reverse-transcribed in 20 μl total volume using the SYBR PrimeScript RT-PCR kit. The process of reverse transcription was 37 °C for 15 min (reverse transcription reaction) and one cycle at 85 °C for 5 s (denaturation of reverse transcriptase). The synthesized cDNA was stored at −80 °C. The mRNA levels for bax, bcl-2 in each sample were determined by a quantitative real-time PCR. The primers used for real-time PCR were as follows: bax primers (forward 5′-TTTGCTTCAGGGTTTCATCC-3′ and reverse 5′-CAGTTGAAGTTGCCGTCAGA-3′); bcl-2 primers (forward 5′-ACTTGTGGTCCAGATAGG-3′ and reverse 5′-CGACTTCGCCGAGATGTC-3′). β-Actin (internal standard) primers (forward 5′-CATGTACGTTGCTATCCAGGC-3′ and reverse 5′-CTCCTTAATGTCACGCACGAT-3′). Real-time quantitative PCR was performed using the SYBR PrimeScript RT-PCR Kit in accordance with the manufacturer’s instructions. Then, PCR was carried out in a Rotor-Gene 6000 (Corbett Research, Australia) according to the following protocol: 30 s at 95 °C, one cycle; 10 s at 95 °C and 40 s at 57 °C, 45 cycles. Fluorescence was detected at the annealing stage of each cycle. A melting curve was generated during the reactions to check for the possibility of primer–dimer formation. The 2−△△Ct method was used to calculate the relative mRNA level of each gene.
Caspase 3 Activity Assay
Caspase 3 activity in the treated QBC939 cells was assayed according to the caspase 3 colorimetric assay kit (Kaiji Bio Co., Nanjing, China). Cells were lysed by incubation with cell lysis buffer on ice for 1 h, and then centrifuged at 10,000 × g for 1 min. Enzymatic reactions were carried out in a 96-well microplate. To each reaction sample, 50 μl cell lysate was incubated with substrate for 4 h at 37 °C before measurement of the absorbance at 405 nm. Two additional controls, one without cell lysate and the other without substrate, were included. Total protein was determined by the Coomassie brilliant blue method.
Results
Anti-proliferative Effects of Bile Extract on QBC939 Cells
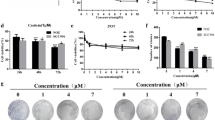
QBC939 cells were treated with 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml bile extract for 24, 48, or 72 h. Cell viability was detected by the MTT method. As shown in Fig. 1, bile extract had a significant time- and dose-dependent inhibitory effect. The inhibitory rate of 250 μg/ml on QBC939 cells at 48 h counted to 64% compared with control.
Morphological Changes of QBC939 Cells
Under the optical microscope, QBC939 cells are adherent cells with the morphology of fibroblasts, spindle and polygon in shape. After being exposed to 200 μg/ml bile extracts for 48 h, cells were generally smaller in size, and cell clone numbers decreased obviously (Fig. 2a). When cells were stained with Hoechst 33258, the structure of apoptotic bodies, a classic character of apoptotic cells, was found in bile extract-treated cells (Fig. 2b).
Morphological changes in the QBC939 cells after exposure to different concentrations (0 or 200 μg/ml) bile extract for 48 h. a Morphological changes captured using ordinary inverted microscopy without any staining (magnification ×100). b Morphological changes captured using fluorescence microscopy with Hoechst 33258 staining (magnification ×400). c Morphological changes captured using transmission electron microscope (magnification ×1,000). d Morphological changes captured using scanning electron microscope (magnification ×5,000)
After being exposed to 200 μg/ml bile extract for 48 h, under the TEM, the cells showed early changes in apoptosis with cytoplasm blebbing (Fig. 2c). When observed by SEM, the control group showed numerous microvilli over the surface of the cells. Bile extract-treated cells showed heavy shrinkage of the cell body and blebbing of the membrane surface. The surface of many cells became relatively smooth without obvious microvilli (Fig. 2d).
Bile Extract Induced Cell Cycle Arrest and Apoptosis of QBC939 Cells
Cell cycles of QBC939 cells treated with bile extract for 48 h showed an accumulation of cells in G0/G1 phase in a dose-dependent manner from 33.71% (control) to 65.71% (200 μg/ml) (Fig. 3a). Meanwhile, we measured the cells using Annexin V-FITC and PI double-staining flow cytometry. Apoptotic cells were localized in the lower right quadrant of the dot-plot graph as shown in Fig. 3b. The proportion of apoptotic cells in 0, 100, 200, and 300 μg/ml bile extracts-treated cells were 0.99%, 20.93%, 11.10%, and 8.18%, respectively.
a Cell-cycle analysis using flow cytometry with PI staining, showing DNA histograms of QBC939 cells. b Assessment of apoptosis using flow cytometry with Annexin V-FITC/PI staining, showing the dot-plot graph of QBC939 cells. c Effect of bile extract on ROS generation. QBC939 cells were treated with different concentrations of bile extract for 24 h. d Effect of bile extract on the ΔΨm of QBC939 cells. The increase in Rh123 hypofluorescence indicated the decrease in ΔΨm
Bile Extract-Induced Cell Apoptosis was Associated with the Collapse of the Mitochondrial Membrane Potential and the Increase of ROS
We investigated whether the generation of intracellular ROS is part of the mechanism by which bile extracts induced apoptosis of QBC939 cells. Treatment with 0, 100, 200, and 300 μg/ml bile extract for 24 h resulted in a dose-dependent increase in ROS level compared with control (Fig. 3c).
To further elucidate the mechanism of bile extract-induced apoptosis in QBC939 cells, we detected the alterations in ΔΨm by flow cytometry. As shown in Fig. 3d, we observed a hypofluorescence peak (reduction of Rh123 staining) after administration of bile extract for 48 h, and it became more manifest with the increasing concentration of bile extract.
Effect of Bile Extract on the Expression of Cytochrome c, bax, bcl-2, and the Activity of Caspase 3
In order to understand more about the molecular mechanism of apoptosis in QBC939 cells after bile extract treatment, we examined the effect of bile extract on bax, bcl-2 mRNA and protein expression. The results showed that bile extract down-regulated bcl-2 mRNA and protein expression, whereas the bax mRNA or protein expression remained unchanged, leading to an increase in the bax/bcl-2 ratio (Fig. 4a, b). In addition, we investigated the expression of cytochrome c. After treatment with bile extract, the amount of cytochrome c in the mitochondria of the cells was decreased, but the amount of cytochrome c in the cytosol was increased (Fig. 4a), suggesting that the cytochrome c was released from mitochondria. Meanwhile, the activity of caspase 3 increased significantly in QBC939 cells after treated with bile extract for 48 h (Fig. 4c).
a Expression of cytochrome c, bax, bcl-2 in QBC939 cells by treatment with 200 μg/ml bile extract. b Bax, bcl-2 mRNA expression in QBC939 cells by treatment with 200 μg/ml bile extract for 48 h. The net intensity values for bax and bcl-2 were normalized to the housekeeping gene β-actin, and the data are presented as the percent difference in bax/β-actin or bcl-2/β-actin gene expression from control. c Effect of bile extract on caspase 3 activity in QBC939 cells. The cells were treated with different concentrations of bile extract for 24 or 48 h and analyzed for caspase 3 activity
Discussion
Apoptosis, which plays an important role in the development and tissue homeostasis of eukaryotes, is a normal form of cell death characterized by a series of morphologic changes. Induction of apoptosis is a highly desirable goal of preventive strategies for cancer control [13].
In this study, the treatment of QBC939 cells with bile extract resulted in the inhibition of cell growth in a time- and dose-dependent manner. There were significant morphological changes in QBC939 cells after exposure to bile extract. Our further experiments showed that apoptosis induction and cell-cycle progression blockage were equally responsible for the inhibition of the tumor cell growth. The control of cell-cycle progression in cancer cells is considered an effective method to stop or slow down tumor growth. Many anticancer agents arrest the cancer cell cycle at the G1, S, and G2/M phase [14]. The current study found that the bile extract caused cell-cycle arrest in the G1 phase.
There are several signaling pathways that lead to the activation of apoptotic machinery [15]. Two major apoptotic pathways have been identified in mammalian cells. One is the intrinsic pathway and another is the extrinsic one [16, 17]. The intrinsic pathway involves the cell oxidative stress that triggers the mitochondria-dependent pathway, resulting in the induction of decreased mitochondrial membrane potential, cytochrome c release from mitochondria into cytosol. This release of cytochrome c in turn activates caspase 9. Caspase 9 can then go on to activate caspase 3 and caspase 7, which are responsible for destroying the cell [17]. Our data showed that bile extract induced a loss of mitochondria membrane potential and the release of cytochrome c to cytosol. Meanwhile, caspase 3 activity was increased significantly in QBC939 cells. The data suggested that apoptosis induced by the bile extract involved mitochondria-mediated mechanism.
In induced apoptosis, interactions between bax and bcl-2 proteins on mitochondria have been postulated to associate with apoptotic pathways [18]. The ratio of bax/bcl-2 determines survival or death following apoptotic stimulus. In the present work, bile extract decreased bcl-2 mRNA and protein expression, while the mRNA or protein expression of bax remained unchanged, leading to an increase in the bax/bcl-2 ratio. The work suggests that the apoptosis under the bile extract treatment was by regulating the ratio of bax/bcl-2.
ROS are products of normal metabolism and xenobiotic exposure, and depending on their concentration, ROS can be beneficial or harmful to cells and tissues. At physiological low levels, ROS function as “redox messengers” in intracellular signaling and regulation, whereas excess ROS induce oxidative modification of cellular macromolecules, inhibit protein function, and promote cell death. Various pathologies can result from oxidative stress-induced apoptotic signaling that is consequent to ROS increases and/or antioxidant decreases [19]. It has been shown that the accumulation of ROS could cause the loss of ΔΨm [20]. ROS generation is believed to mediate ΔΨm reduction [21], which in turn causes the release of cytochrome c and initiates the apoptotic cascade. In the present study, the results indicated that bile extract could induce the accumulation of ROS production and a decrease of ΔΨm. The data offered the proof that ROS might play an important role in bile extract-induced cancer cell apoptosis.
In conclusion, this study indicates that the crocodile bile extract have potent antitumor activity toward QBC939 cells in vitro. The results suggest that the bile extract might be a promising candidate for the treatment of QBC939 cells. Although the molecular mechanism for bile extract-induced cancer cell apoptosis is poorly understood, cytochrome c and bcl-2 might be involved. Further studies are needed to examine its possibility as a lead compound for development of novel antitumors.
Abbreviations
- CCA:
-
Cholangiocarcinoma
- ROS:
-
Reactive oxygen species
- ΔΨm:
-
Mitochondrial transmembrane potential
- PI:
-
Propidium iodide
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- HRP:
-
Horseradish peroxidase
- ECL:
-
Enhanced chemiluminescence
- TEM:
-
Transmission electron microscopy
- SEM:
-
Scanning electron microscope
- Rh123:
-
Rhodamine 123
- DCFH-DA:
-
2,7-Dichlorofluorescein diacetate
- DCF:
-
2,7-Dichlorofluorescein
References
Mahmoud, N. N., Dannenberg, A. J., Bilinski, R. T., Mestre, J. R., Chadburn, A., Churchill, M., et al. (1999). Administration of an unconjugated bile acid increases duodenal tumors in a murine model of familial adenomatous polyposis. Carcinogenesis, 20, 299–303. doi:10.1093/carcin/20.2.299.
Martinez, J. D., Stratagoules, E. D., LaRue, J. M., Powell, A. A., Gause, P. R., Craven, M. T., et al. (1998). Different bile acids exhibit distinct biological effects: The tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutrition and Cancer, 31, 111–118. doi:10.1080/01635589809514689.
Tatsumura, T., Sato, H., Yamamoto, K., & Ueyama, T. (1981). Ursodeoxycholic acid prevents gastrointestinal disorders caused by anticancer drugs. The Japanese Journal of Surgery, 11, 84–89. doi:10.1007/BF02468874.
Alberts, D. S., Martinez, M. E., Hess, L. M., Einspahr, J. G., Green, S. B., Bhattacharyya, A. K., et al. (2005). Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. Journal of the National Cancer Institute, 97, 846–853. doi:10.1093/jnci/dji144.
Tint, G. S., Dayal, B., Batta, A. K., Shefer, S., Joanen, T., Larry, M., et al. (1980). Biliary bile acids, bile alcohols, and sterols of Alligator mississippiensis. Journal of Lipid Research, 21, 110–117.
Yeh, Y. H., Wang, D. Y., Liau, M. Y., Wu, M. L., Deng, J. F., Noguchia, T., et al. (2003). Bile acid composition in snake bile juice and toxicity of snake bile acids to rats. Comparative Biochemistry and Physiology, 136, 277–284. doi:10.1016/S1532-0458(03)00230-8.
Malhi, H., & Gores, G. J. (2006). Cholangiocarcinoma: Modern advances in understanding a deadly old disease. Journal of Hepatology, 45, 856–867. doi:10.1016/j.jhep.2006.09.001.
Patel, T. (2002). Worldwide trends in mortality from biliary tract malignancies. BMC Cancer, 2, 10. doi:10.1186/1471-2407-2-10.
Lazaridis, K. N., & Gores, G. J. (2005). Cholangiocarcinoma. Gastroenterology, 128, 1655–1667. doi:10.1053/j.gastro.2005.03.040.
Gatto, M., Bragazzi, M. C., Semeraro, R., Napoli, C., Gentile, R., Torrice, A., et al. (2010). Cholangiocarcinoma: Update and future perspectives. Digestive and Liver Disease, 42, 253–260. doi:10.1016/j.dld.2009.12.008.
Hu, Y., Yang, Y., You, Q. D., Liu, W., Gu, H. Y., Zhao, L., et al. (2006). Oroxylin A induced apoptosis of human hepatocellular carcinoma cell line HepG2 was involved in its antitumor activity. Biochemical and Biophysical Research Communications, 351, 521–527. doi:10.1016/j.bbrc.2006.10.064.
Han, P., Kang, J. H., Li, H. L., Hu, S. X., Lian, H. H., Qiu, P. P., et al. (2009). Antiproliferation and apoptosis induced by tamoxifen in human bile duct carcinoma QBC939 cells via upregulated p53 expression. Biochemical and Biophysical Research Communications, 385, 251–256. doi:10.1016/j.bbrc.2009.05.059.
Farnebo, M., Bykov, V. J., & Wiman, K. G. (2010). The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochemical and Biophysical Research Communications, 396, 85–89. doi:10.1016/j.bbrc.2010.02.152.
Mork, C. N., Faller, D. V., & Spanjaard, R. A. (2005). A mechanistic approach to anticancer therapy: Targeting the cell cycle with histone deacetylase inhibitors. Current Pharmaceutical Design, 11, 1091–1104. doi:10.2174/1381612053507567.
Tompson, C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456–1462. doi:10.1126/science.7878464.
Green, D. R. (1998). Apoptotic pathways: The roads to run. Cell, 94, 695–698.
Green, D. R., & Reed, J. C. (1998). Mitochondria and apoptosis. Science, 281, 1308–1312. doi:10.1126/science.281.5381.1309.
Zong, W. X., Li, C., Hatzivassiliou, G., Lindsten, T., Yu, Q. C., Yuan, J., et al. (2003). Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. The Journal of Cell Biology, 162, 59–69. doi:10.1083/jcb.200302084.
Circu, M. L., & Aw, T. Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology & Medicine, 48, 749–762. doi:10.1016/j.freeradbiomed.2009.12.022.
Sakon, S., Xue, X., Takekawa, M., Sasazuki, T., Okazaki, T., Kojima, Y., et al. (2003). NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. The EMBO Journal, 22, 3898–3909. doi:10.1093/emboj/cdg379.
Chen, Y., & Gibson, S. B. (2008). Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy, 16, 246–248.
Acknowledgments
The present investigation was supported by Grant 81072014 of the Natural Science Foundation of China, National Foundation for fostering talents of basic science (J1030626) and supported by Sriracha Tiger Zoo Co., Ltd. Sriracha Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin-He Kang and Wen-Qing Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kang, JH., Zhang, WQ., Song, W. et al. Apoptosis Mechanism of Human Cholangiocarcinoma Cells Induced by Bile Extract from Crocodile. Appl Biochem Biotechnol 166, 942–951 (2012). https://doi.org/10.1007/s12010-011-9482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9482-x