Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. In laboratory animal models, diethylnitrosamine (DENA) is a well-known agent that has a potent hepatocarcinogenic effect that is used to induce HCC. As curcumin has a potent anti-inflammatory effect with strong therapeutic potential against a variety of cancers, our present study aims to investigate its curative effects and the possible mechanisms of action against DENA-induced HCC in male rats. Investigation of biochemical and molecular parameters of HCC animal model liver showed an overexpression of TGF-β and Akt proteins accompanied with a significant reduction of the proapoptotic marker caspase-3. DENA-induced hepatic cellular injury resulted also in a significant increase in liver function marker enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lipid peroxides in this group. Curcumin treatment partially reversed DENA-induced damage as it reduced the overexpression of the angiogenic and anti-apoptotic factors TGF-β and Akt and improved caspase-3 expression. Also, it could partially normalize the serum values of liver marker enzymes and lipid peroxidation and improve liver architecture. Curcumin shows a unique chemotherapeutic effect in reversing DENA-induced HCC in rat model. This effect is possibly mediated through its proapoptotic, antioxidant, anti-angiogenic, as well as antimitotic effects. It interferes and modulates cell signaling pathways and hence turns death signals and apoptosis on within tumor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide and accounts for about 80–90 % of all liver cancers. It is the fourth most common cause of cancer mortality [41]. Major risk factors for HCC include hepatitis viral infection, food additives, alcohol administration, aflatoxins, environmental and industrial toxic chemicals, and air and water pollutants [26].
Animal models of chemically induced liver cancer (hepatocarcinogenesis) have been widely used for investigating therapeutic strategies against HCC in vivo. To induce HCC in experimental animal models, a well-known potent hepatocarcinogenic agent (diethylnitrosamine, DENA) is frequently used. DENA can induce damage in DNA repair machinery, hepatocellular necrosis, and hence HCC without cirrhosis [3].
The cellular mechanisms contributing to HCC involve a multistep process that includes inactivation of tumor suppressor genes and dysregulation of several oncogenic pathways including Wnt/β-catenin, VEGF, FGF, MAPK, and PI3K/AKT/mTOR pathways [14]. AKT, also known as protein kinase B or PKB, comprises three closely and evolutionary related isoforms, AKT 1, 2, and 3 or PKBα/β/γ [22]. AKT appears to play an important role in keeping normal mammalian signaling; however, it becomes overexpressed in many inflammatory as well as cancer diseases [12]. Lately, AKT pathway has emerged as one of the key oncogenic pathways in breast cancer, providing the potential for therapeutic intervention [33].
The standard protocol for treating early stage HCC includes surgical resection, liver transplantation, radiofrequency ablation (RFA), and transcatheter arterial chemoembolization (TACE). However these interventions provide a cure for a limited population of patients, but unfortunately, these curative treatments are often not so successful because of the frequent recurrence of hepatic carcinoma [39]. This can be explained by the ability of the remaining liver to maintain the potential for carcinogenesis [31]. Despite being of limited value, using chemotherapeutic agents is still the strategy of choice in case of patients with unresectable HCC. However, conventional chemotherapy with cytotoxic agents is not effective enough to modulate the process of tumor progression, resulting in a relatively high mortality rate in patients diagnosed with HCC at an advanced stage [5, 56]. These facts emphasize the importance and necessity to discover new effective and well-tolerated therapeutic agents for HCC.
Curcumin, also known as 1,7-bis (4-hydroxy-3-methoxyphenol)-1,6-heptadiene-3,5-dione is the principal curcuminoid in the Asian spice turmeric (Curcuma longa). It has been shown to exert a potent anti-angiogenic, anti-inflammatory, antioxidant, and antitumor effects [34, 52, 60]. Curcumin was proven to exert a potent antitumor effects in hepatic carcinoma [13] as it inhibits the proliferation and survival of almost all types of tumor cells.
Curcumin has been shown to have a chemopreventive as well as a chemotherapeutic effect in several types of cancer and also to suppress angiogenesis and metastasis in a variety of animal tumor models. Several previous studies had linked the inhibitory effects of curcumin on cancer cells to its ability to induce apoptosis or inhibit growth/proliferation pathways [1, 2, 11, 29, 43]. This apoptosis-inducing effect of curcumin is activated through the inhibition of multiple pathways including AKT pathway. The role of AKT in angiogenesis was demonstrated by Jiang and co-workers [27], who demonstrated the angiogenesis-inducing effect of AKT in chick embryos. In 2003, Morin and his work team identified the intracellular superoxide generating capacity of curcumin to be responsible for its cytotoxic effect on tumor cells [36].
Though several theories could interpret the mechanism(s) of the cytotoxic/apoptotic effects of curcumin, the role of AKT in controlling both the angiogenic and apoptotic pathways of curcumin chemoprevention in HCC is still poorly understood and needs further investigation.
Progression of human HCC was shown to be associated with angiogenesis which plays a significant role in aggressiveness of HCC [40, 47]. Angiogenesis is induced when hepatic stellate cells and tumor inflammatory cells start to release factors like vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF- β) among others, which promote sprouting of new vessels from nearby normal existing ones [21, 28]. Using an anti-angiogenic agent for cancer treatment theoretically provides several advantages over cytotoxic chemotherapeutic approaches. These advantages include the ease of delivery of the anti-angiogenic agent to the targeted region as the agent is circulating with the blood and taken up by the vascular endothelial cells. Additionally, anti-angiogenic agents that affect cancer cells are much less toxic than the regular chemotherapeutic agents. This can be attributed to their ability to target the immature tumor vasculature which differ both structurally and functionally from the normal tissue quiescent vasculature [40]. These agents show such an anti-angiogenic effect on tumors by disrupting various signaling pathways involved in the process of tumor angiogenesis. Recently, curcumin was shown to exert its tumor suppressing effect via an anti-angiogenic effect [20, 58].
Accordingly, the objective of this research was to assess the chemotherapeutic effect of curcumin in DENA-induced HCC model and present a better understanding of the molecular mechanisms and effector molecules behind this effect. This will include its modulating effect on some angiogenic, apoptotic, anti-apoptotic, and/or fibrogenic cytokines like TGF-β, caspase-3, and AKT. In addition, the study will shed light on curcumin ability to modulate the oxidative stress state induced by the formation of reactive oxygen species (ROS) in the rat hepatocytes.
Materials and methods
Chemicals
RNA extraction kit from BioFlux, Bioer Technology Co., Ltd.; Reverse transcription using RevertAid™ First strand cDNA synthesis kit from Fermentas, Thermo Scientific, Germany; PCR using Tag master/high yield from Jena Bioscience, Germany; DNA ladder using low range DNA ladder 50-1000bp linear scale from Jena Bioscience, Germany; Protein marker using Page Ruler™ prestained protein ladder from Fermentas, Thermo Scientific, Germany; and PVDF membrane from Amersham Hybond™-P, GE Healthcare were used. All the other chemicals and solvents used in the study were of analytical grade and were obtained either from Sigma Company or commercial suppliers, unless otherwise mentioned.
Animals and maintenance
Experiments were conducted in accordance with the international ethical guidelines for animal care of the United States Naval Medical Research Centre, Unit No. 3, Abbaseya, Cairo, Egypt, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care international (AAALAC international). The adopted guidelines are in accordance with “Principles of Laboratory Animals Care” (NIH Publication No. 85–23, revised 1985). The study protocol was approved by members of “The Research Ethics Committee” and by the Pharmacology and Toxicology Department, Faculty of Pharmacy, Minia University, Egypt.
Thirty male albino male rats with average body weight (250 ± 25 g) were obtained from Animal House of Assiut University. All animals received professional humane care in compliance with the guidelines of the ethical committee.
Treatment protocols
The animals were randomly divided into three groups, each containing ten rats as the following:
-
I-
DENA group (n = 10): Each animal of the carcinogenic group received a single dose of diethylnitrosamine (DENA) 200 mg/kg body weight [15, 51] intraperitoneal and left for 3 months followed by daily administration of 0.5 ml saline for 2 weeks.
-
II-
Curcumin-treated group (n = 10): Each animal of the curcumin group received a single dose of 200 mg/kg body weight DENA intraperitoneal. after 3 months, rats were treated orally for 15 successive days with curcumin 100 mg/kg body weight in volume of 0.5 ml saline [51].
-
III-
Control group (n = 10): Each animal of the control group received a single dose of 0.5 ml saline, after 3 months, animals received 0.5 ml saline orally for 2 weeks. at the end of the experiment rats were sacrificed
Sample collection
Blood samples were collected for biochemical analysis and liver tissues were excised rapidly and used for histological investigation and RNA preparation. For protein analysis, tissue samples were homogenized in 20 mM Tris, 100 mM NaCl, 1 mM EDTA, and 0.5 % Triton X-100 buffer. Protein content of liver homogenate was determined using Biuret reagent and bovine serum albumin as standard. The protease inhibitors mix was added, divided in aliquot, and stored at −70 °C until assay.
Molecular analysis
-
1-
Detection of TGF-β and Akt by Western blotting
Fifty micrograms from each protein homogenate were denatured by boiling for 5 min in 2 % SDS and 5 % 2-mercaptoethanol and loaded in each lane [32]. Sodium dodecyl sulfate–poly acrylamide gel electrophoresis (SDS–PAGE) was done at 100 V for 2 h using 12 % gels. The electro-transfer was done using T-77 ECL semidry transfer unit (Amersham Biosciences) for 2 h. The membrane was blocked in TBS buffer that contains 0.05% Tween and 5 % non-fat milk for 1 h. The primary antibodies used were rabbit polyclonal anti-rat TGF-β (Abcam) or rabbit polyclonal anti-rat Akt, code number (#9272). Polyclonal goat anti-rabbit or anti-mouse immunoglobulin conjugated to alkaline phosphatase (Sigma-Aldrich, Schelldorf, Germany) diluted 1:5000 in the 10× diluted blocking buffer served as secondary antibody. Detection of protein bands was done by adding alkaline phosphatase buffer (100 mM tris; pH 9.5; 100 mM NaCl; 5 mM MgCl2) containing substrate, 6.6 μl NBT/ml, and 3.3 μl BCIP/ml (from stock of 50 mg/mL nitroblue tetrazolium (NTB) and 50 mg/ml 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in 70 % formamide). Color reactions were stopped by rinsing with stop buffer (10 mM Tris–Cl, pH 6.0, 5 mM EDTA).
-
2-
RNA extraction and reverse transcriptase chain reaction (RT-PCR) of caspase-3
RNA was prepared using total RNA Kit (BioFlux, Bioer Technology Co., Ltd) to provide a rapid method for the isolation of total RNA according to manual instructions. RNA was quantified by measuring absorbance at 260 nm. RT-PCR assay was performed using Tag master/high yield (Jena Bioscience) according to manual instructions. Synthetic oligonucleotides used for amplifications, NCBI reference sequence, were used to design primers for rat caspase-3 and β-actin, respectively. The primer sets are the following: caspase-3 sense: 5′-GAC CAT GGA GAA CAA CAA AAC-3′, caspas-3 antisense: 5′-GGC AGG CCT GAA TGA TGA AG-3′, β-actin sense: 5′-CAT GGA TGA CGA TAT CGC TG-3′, and β-actin antisense: 5′-CAT AGA TGG GCA CAG TGT GG-3’, where β-actin served as a control. A total 20 μl of reaction mixture contained 2 μl RT product, 2.5 U Taq DNA polymerase, 20 μmol/L dNTP, 0.1 μmol/L primer, and 1× Taq DNA polymerase buffer (Fermentas/Thermo Scientific/Germany). The amplification was carried out using Biometra cycler (Germany), programmed to predenature at 94 °C for 4 min, denature at 94 °C for 30 s, anneal at 55 °C for 30 s, and extend at 72 °C for 1 min for 30 cycles. The last cycle was followed by incubation at 72 °C for 4 min and cooling to 4 °C. The PCR products were analyzed on 1.5 % (v/v) agarose gels.
Biochemical analysis
Assessment of serum liver function tests, lipid peroxides, and glutathione S-transferase
Blood samples were collected by cardiac puncturing method, centrifuged, and sera were isolated for estimation of serum alanine aminotransferases (ALT) and aspartate aminotransferase (AST) [44]. Liver homogenate were used for lipid peroxides [38] and glutathione S-transferase estimation [54]. All assessments were done using commercially available kits according to the manufacturer’s instructions (Biodiagnostic, Egypt).
Histopathology
Formalin-fixed liver specimens were transferred to 70 % ethanol and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (HE). At least three slides were prepared from each specimen and examined under Optica B-82 microscope for detection of pathological changes.
Statistical analysis
Statistical analysis was achieved using Graph Pad InStat. Software Inc, Program, version 4.0, Philadelphia. Data were presented as mean ± standard deviation (SD), and the levels of significance were accepted with P < 0.05 Multiple comparisons were done using one way ANOVA test.
Results
-
1-
Investigation of TGF-β expression level in liver tissue
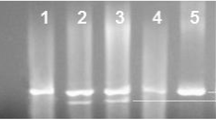
Liver tissue samples of different test groups homogenized as mentioned in “Materials and methods” were used for this experiment. After loading equal amounts of total protein on SDS–gel, the expression of TGF-β was estimated by Western blot analysis using specific antibodies. The results exhibited an overexpression of TGF-β protein in liver cells of DENA-treated group (Fig. 1 lane 2) when compared to healthy control (Fig. 1 lane 1). Interestingly, curcumin treatment resulted in a strong reduction in the expression level of the protein when compared to the DENA group that did not receive any curcumin (Fig. 1 lane 3).
Fig. 1 Analysis of TGF-β protein expression level determined by Western blot of rat hepatocellular carcinoma homogenate from control, DENA- and DENA/curcumin-treated groups. Upper panel: DENA-treated group shows an increase in TGF-β expression (lane 2) compared to healthy control group (lane 1). Curcumin treatment resulted in downregulation of TGF-β expression (lane 3). Lower panel: β-actin reprobed on the same immunoblot to correct for loading errors (n = 10 for each group)
Fig. 2 Analysis of AKT protein expression level determined by Western blot analysis in liver homogenates of control, DENA-treated, and DENA/curcumin-treated groups. AKT expression was induced as a result of DENA treatment (lane 2) when compared to control group (lane 1). Curcumin treatment resulted in a partial reduction in its expression (lane 3). β-actin reprobed on the same immunoblot was used to correct for loading errors (n = 10 for each group)
-
2-
Investigation of AKT expression level in liver tissue
As mentioned before, AKT over expression was linked to an angiogenic and anti-apoptotic effect in cancer tissues. To elucidate the possible changes in AKT expression level, homogenized liver tissues from the different test groups were used. Equal amounts of total protein were loaded onto SDS–gel and analyzed by Western blot analysis where bound antibodies were visualized using alkaline phosphatase system. Comparing the observed bands shows an overexpression of AKT protein in liver homogenates of DENA-treated group in comparison to healthy control group (Fig. 2 lanes 1 and 2). However, its expression is still higher than control group; curcumin treatment of HCC animals resulted in a partial reduction of AKT expression level when compared to DENA-treated group (Fig. 2 lane 3).
Fig. 3 Analysis of expression of caspase-3 mRNA (upper figure) in the different experimental groups (control, DENA, and curcumin group) by RT-PCR. Caspase-3 expression is decreased in DENA group in comparison to control. Curcumin-treated group shows an increased level of the apoptotic marker caspase-3 over DENA-treated group. Lower panel shows the RT-PCR product of B-actin which is used as internal control for RNA integrity
-
3-
Detection of changes in caspase-3 messenger RNA (mRNA) expression by RT-PCR
In this experiment, reverse transcriptase PCR technique as described in “Materials and methods” was utilized for detecting any possible changes in caspase-3 mRNA levels in liver homogenate prepared from the different test groups. As expected from a carcinogen, DENA caused the mRNA expression level of the apoptotic marker caspase-3 to be reduced (Fig. 3, lane 2). Interestingly, curcumin administration after HCC establishment strongly induced the overexpression of caspase-3 mRNA as can be seen in lane 3 of Fig. 3 when compared to its expression in healthy control as well as DENA-treated group (Fig. 3, lanes 1 and 2).
Fig. 4 Mean levels of serum lipid peroxide in the different animal groups (control, DENA, and curcumin). DENA group shows a highly significant increase in lipid peroxide level compared to control. Curcumin treatment resulted in a significant reduction of peroxide level compared to DENA; however, it still significantly higher than control (n = 10). P values are shown as *P < 0.05; **P < 0.01; ***P < 0.001
-
4-
Serum levels of aminotransferases
Serum levels of alanine aminotransferase and aspartate aminotransferase (ALT and AST) are usually used as indicators of hepatocellular damage. In this study, the changes in their serum level in the different test groups were measured to assess the effect of DENA alone and DENA followed by curcumin on liver integrity. Due to the hepatocellular damaging effect of DENA, significant increase in rat serum ALT and AST levels was detectable in serum isolated from DENA-treated group (P < 0.001) compared to the healthy control group (Table 1). On the other hand, curcumin treatment of animals that administered DENA previously resulted in a significant reduction of the DENA-induced elevation of ALT/AST serum levels. However, curcumin treatment as described before was yet not able to normalize ALT and AST values as their levels were still significantly higher than these of the healthy control group (P < 0.05) as shown in Table 1.
Table 1 Mean serum levels of AST and ALT (U/L) and their ratio in control, DENA, and curcumin treated groups -
5-
Serum levels of lipid peroxide and glutathione S-transferase
DENA treatment resulted in a significant reduction in GST (glutathione S-transferase) activity in comparison to control-untreated group. In addition, DENA treatment significantly increased serum levels of lipid peroxide as shown in Fig. 4 (P < 0.001) in comparison to control-treated animals. Interestingly, curcumin treatment was able to reverse the cellular damage as it resulted in a significant reduction in lipid peroxides level. In the same time, it reversed partially, yet significantly, DENA’s effect on GST activity, raising its level to be closer to normal values Fig. 5.
-
6-
Histopathological study
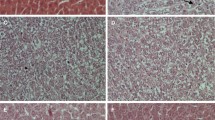
To assess the changes in parenchymal cells of the liver after DENA administration and curcumin treatment, paraffin section prepared after the course of 14 weeks of a single dose of DENA administration was stained with hematoxylin/eosin and examined, as shown in Fig. 6. Administration of DENA induced morphological deformations in the liver pronounced with degeneration of hepatocytes and liver neoplastic cellular alteration. After curcumain administration, the diseased liver had some improvements in its histological structure as the epithelium of central vein became more intact; architecture of hepatocyte arrangement became normal in some parts of the diseased area and hydropic degeneration decreased.
Fig. 6 Light photomicrograph of rat liver from the control group a showing normal histology of liver having polygonal hepatocytes radiating from the central vein (arrow head) with prominent nucleus, eosinophilic cytoplasm, and maintained sinusoidal space. b DENA group showing degenerative changes of some hepatocytes in the form of swelling of the cells, vacuolated cytoplasm, and shrunken nucleus (arrow head). c DENA group liver neoplastic cellular alteration is apparent. Hepatic cell enlargement and pleomorphism were found. The hepatic nuclei were enlarged with prominent nuclei. d Curcumin-treated group showed improvement in the hepatocytes which appeared similar to the normal, radiating from the central vein (arrow head) (H&E, ×400)
Discussion
HCC is the main form of liver cancer and is classified as the fourth leading cause in cancer-related death due to its bad prognosis [6]. HCC in rat model is best studied using DENA, which is known to be metabolized to its active ethyl radical metabolite. This reactive metabolite causes perturbations in the nuclear enzymes involved in DNA repair/replication leading to carcinogenesis, and it is normally used as a liver cancer-inducing agent in animal models [10].
In the current study, we focused on the possible chemotherapeutic effects of curcumin that could be mediated by modulating the expression of AKT, TGF-β, and caspase-3 in HCC animal model in addition to its effect on the oxidative stress condition of the liver. Curcumin is a polyphenolic compound derived from turmeric rhizome (C. longa). It has been used for thousands of years in the orient as a healing agent for a wide variety of ailments including neoplastic and neurodegenerative diseases. Research over the last few decades has shown that curcumin’s therapeutic potential against the diverse types of cancers is mediated by suppressing the activation of several transcriptional factors that are implicated in carcinogenesis [2, 46].
Xu and co-workers stated that all AKT isoforms have the ability to be overexpressed in 40 % of hepatocellular carcinoma cases. This can be explained by an overexpression or activating mutations of tyrosine kinase receptors and their ligands, all of which may lead to the activation of AKT and its overexpression [22, 57]. As a downstream effector of PI3K signaling pathway activation, AKT promotes survival of mutated and damaged cells which would normally be eliminated by apoptosis. Based on these findings, many studies postulated that AKT plays an important role in the process of carcinogenesis as it protects cancer cells form apoptosis. As apoptosis helps to establish a natural balance between cell death and cell renewal in mature animals by destroying excess, damaged, or abnormal cells, so that any disturbance in this sharp balance would direct the cells to carcinogenesis [1].
In this study, one can observe that induction of carcinogenesis by DENA administration was accompanied by a strong upregulation of AKT expression which can be explained by its anti-apoptotic role that is essential for tumor development. Interestingly, curcumin administration to HCC rats caused a reduction in AKT expression level, which agrees with its previously shown properties as an antineoplastic, apoptosis-inducing agent [48, 59]. Upon receiving a signal of apoptosis, a variety of proteases including the group of proteases called caspases (cysteinyl aspartate-specific proteases) become activated within the cells planned for this pathway [17]. Caspases are constitutively expressed as carcinoma cases. This generally require proteolytic processing for their activation and are capable of self-activation as well as activating each other in a cascade-like process [24]. In order to survive, tumors always try to escape apoptosis by several mechanisms including downregulation or complete loss of caspase-3 expression. This disruption in caspase-3 expression is usually associated with resistance to apoptosis as well as chemotherapy in different kinds of tumors [30]. Some research groups have already shown that the anti-apoptotic effect of curcumin can be linked to activation of both procaspases-3 and -8, decreased expression of anti-apoptotic members of the Bcl-2 family, and elevated expression of p53, Bax, and caspases-3 [7, 46]. Curcumin was also shown to suppress constitutively activated targets of AKT in T cells, leading to the inhibition of proliferation and induction of caspase-dependent apoptosis [23].
In the current study, hepatic cell homogenate of HCC rats showed lower expression of mRNA of caspase-3 as one of the apoptotic markers when compared to the healthy control group, but after curcumin administration, its level increased significantly supporting the apoptosis-inducing effect of curcumin. Also, rat hepatic cell homogenates of HCC model showed an increased expression level of transforming growth factor TGF-β. The increase in TGF-β expression and its release in the sera of HCC rats are not unexpected due to the known role of this factor as an initiator of a signaling cascade that is closely linked to liver fibrosis, cirrhosis, and subsequent progression to HCC. The indicated pro-fibrogenic role for TGF-β together with its newly elucidated role as an anti-apoptotic factor provide a reasonable explanation for results observed in our study [8]. The strong reduction in TGF-β expression which resulted after curcumin treatment can be correlated to the previously indicated antifibrogenic and apoptotic effects of curcumin on tumor cells [37, 45].
For most solid tumors, angiogenesis is essential for tumor growth and metastasis as blood vessels that penetrate into the cancerous growth, supply nutrients and oxygen and remove waste products [18]. TGF-β plays another unique role in the pathogenesis of HCC by promoting angiogenesis at least in part via the autocrine secretion of TGF-α, which activates PI3K/AKT pathway in addition to its role in promoting cell growth, migration, invasion, and metastasis of colon cancer [42]. This role of TGF-β might explain its upregulation in most of the HCC patients and goes in accordance with its overexpression observed in our current study on HCC animal model [25, 55]. The blockage of the angiogenic effect of TGF-β by the anti-angiogenic agent curcumin could provide a possible mechanism for the tumor suppressive effect of curcumin observed in our study in a similar way as stated previously by Mohan and co-workers [19, 35]. This anti-angiogenic effect of curcumin might be mediated by its inhibitory effect on the expression of genes involved in angiogenesis and metastasis like VEGF and MMP-9 [20, 50, 58].
Several studies demonstrated the role of oxidative stress in liver pathogenesis and proved the role of natural antioxidants in its amelioration [16]. To assess such stress, some biochemical parameters are being evaluated like aminotransferases (ALT and AST) which give an indication about liver cell damage. In addition, the estimation of lipid peroxidation and glutathione S-transferase activity in serum reflect the antioxidative capacity and hence the oxidative stress condition within the liver [9, 53].
Glutathione S-transferase is a supporting antioxidant enzyme, converting H2O2 into water and so protecting cells from reactive oxygen species. Reduction in GST activity following DENA administration could result from the increased free radical production upon DENA metabolism. The data represented here shows also that HCC was accompanied by an increased serum level of lipid peroxides, indicating oxidative stress. The DENA-induced increase in lipid peroxidation reflects cell membrane damage which results in the release of ALT and AST from the cytoplasmic compartment to blood stream together with the increased oxidative stress [49].
Interestingly, curcumin significantly reduced both aminotransferases level as well as lipid peroxidation in sera of treated groups and partially regained the depleted GST activity, indicating its ability to reduce the extent of cellular damage and oxidative stress caused by DENA. This observed effect of curcumin can be related to its reported ability to improve the enzymatic antioxidant activity by increasing cellular levels of the enzymes catalase, glutathione S-transferase, glutathione peroxidase, and superoxide dismutase and their mRNAs, in addition to its antioxidant, free radical scavenging effect that inhibits lipid peroxidation [4, 55].
Conclusion
Our investigation on both the molecular and biochemical changes associated with the administration of curcumin confirmed its anti-carcinogenic effect in DENA-induced HCC model. This effect is mediated through several mechanisms including regulation of the angiogenic, apoptotic, and anti-apoptotic factors TGF-β, caspase-3, and AKT, respectively. Also, it could function by improving the oxidative stress state and architecture of cancerous liver. These findings highlight the possible therapeutic applications of curcumin as a promising natural chemotherapeutic agent in treating HCC. However, further investigations are required to discover the optimal therapeutic strategy and possible further mechanisms.
Abbreviations
- DENA:
-
Diethylnitrosamine
- EGF:
-
Endothelial growth factor
- FGF:
-
Fibroblast growth factor
- HCC:
-
Hepatocellular carcinoma
- MDA:
-
Malondialdehyde
- MMP-9:
-
Matrix metalloproteinase-9
- RT/PCR:
-
Reverse transcriptase Polymerase Chain Reaction
- SDS–PAGE:
-
Sodium dodecyl sulfate–poly acrylamide gel electrophoresis
- TGF-β:
-
Transforming growth factor beta
- ROS:
-
Reactive oxygen species
- TBA:
-
Thiobarbituric acid
- VEGF:
-
Vascular endothelial growth factor
References
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98.
Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8.
Bhosale PML, Ingle AD, Gadre RVB, Rao KV. Protective effect of Rhodotorula glutinis NCIM3353 on the development of hepatic preneoplastic lesions. Curr Sci. 2002;83:303–8.
Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41.
Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Bush JA, Cheung Jr KJ, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–14.
Caja L, Ortiz C, Bertran E, Murillo MM, Miro-Obradors MJ, Palacios E, et al. Differential intracellular signalling induced by TGF-beta in rat adult hepatocytes and hepatoma cells: implications in liver carcinogenesis. Cell Signal. 2007;19:683–94.
Caraglia M, Giuberti G, Marra M, Addeo R, Montella L, Murolo M, et al. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011;2:e150.
Chakraborty T, Chatterjee A, Rana A, Dhachinamoorthi D, Kumar PA, Chatterjee M. Carcinogen-induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: chemopreventive role of vanadium on this process. Biochim Biophys Acta. 2007;1772:48–59.
Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–607.
Clarke RB, Anderson E, Howell A, Potten CS. Regulation of human breast epithelial stem cells. Cell Prolif. 2003;36 Suppl 1:45–58.
Dai XZ, Yin HT, Sun LF, Hu X, Zhou C, Zhou Y, et al. Potential therapeutic efficacy of curcumin in liver cancer. Asian Pac J Cancer Prev. 2013;14:3855–9.
Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, et al. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–6.
El-Shahat M, El-Abd S, Alkafafy M, El-Khatib G. Potential chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis in rats: myrrh (Commiphora molmol) vs. turmeric (Curcuma longa). Acta Histochem. 2012;114:421–8.
Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon. 2012;12:160–7.
Fabregat I, Roncero C, Fernandez M. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 2007;27:155–62.
Folkman J. Angiogenesis-dependent diseases. Semin Oncol. 2001;28:536–42.
Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–30.
Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–42.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64.
Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27.
Hussain AR, Al-Rasheed M, Manogaran PS, Al-Hussein KA, Platanias LC, Al Kuraya K, et al. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006;11:245–54.
Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406.
Ito N, Kawata S, Tamura S, Takaishi K, Shirai Y, Kiso S, et al. Elevated levels of transforming growth factor beta messenger RNA and its polypeptide in human hepatocellular carcinoma. Cancer Res. 1991;51:4080–3.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–53.
Jung JO, Gwak GY, Lim YS, Kim CY, Lee HS. Role of hepatic stellate cells in the angiogenesis of hepatoma. Korean J Gastroenterol. 2003;42:142–8.
Khar A, Ali AM, Pardhasaradhi BV, Begum Z, Anjum R. Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS Lett. 1999;445:165–8.
Kolenko V, Uzzo RG, Bukowski R, Bander NH, Novick AC, Hsi ED, et al. Dead or dying: necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Cancer Res. 1999;59:2838–42.
Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anticancer Drugs. 2007;18:861–74.
Masuelli L, Benvenuto M, Fantini M, Marzocchella L, Sacchetti P, Di Stefano E, et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J Biol Regul Homeost Agents. 2013;27:105–19.
Mohan R, Sivak J, Ashton P, Russo LA, Pham BQ, Kasahara N, et al. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J Biol Chem. 2000;275:10405–12.
Morin D, Barthelemy S, Zini R, Labidalle S, Tillement JP. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495:131–6.
Muriel P. Cytokines in liver diseases. In: Sahu S, editor. Hepatotoxicity: from genomics to in vitro and in vivo models. West Sussex: Wiley; 2007.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Okuda H. Hepatocellular carcinoma development in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:161–73.
Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–67.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Ramamoorthi G. & Sivalingam N. (2014) Molecular mechanism of TGF-beta signaling pathway in colon carcinogenesis and status of curcumin as chemopreventive strategy. Tumour Biol
Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? Aaps J. 2009;11:495–510.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63.
Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, et al. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417–27.
Scott DW, Loo G. Curcumin-induced GADD153 gene up-regulation in human colon cancer cells. Carcinogenesis. 2004;25:2155–64.
Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–80.
Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10.
Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991;151:260–5.
Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human umbilical vein endothelial cell. Cancer Lett. 1996;107:109–15.
Sreepriya M, Bali G. Effects of administration of embelin and curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol Cell Biochem. 2006;284:49–55.
Suckow BK, Suckow MA. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. Int J Biomed Sci. 2006;2:402–5.
Tanaka S, Miyanishi K, Kobune M, Kawano Y, Hoki T, Kubo T, et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J Gastroenterol. 2013;48:1249–58.
Vaubourdolle M, Chazouilleres O, Briaud I, Legendre C, Serfaty L, Poupon R, Giboudeau J. Plasma alpha-glutathione S-transferase assessed as a marker of liver damage in patients with chronic hepatitis C. Clin Chem 1995;41:1716–9.
Vinals F, Pouyssegur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol. 2001;21:7218–30.
Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2011;29:4989–5005.
Xu X, Sakon M, Nagano H, Hiraoka N, Yamamoto H, Hayashi N, et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol Rep. 2004;11:25–32.
Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109–15.
Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–20.
Zhang CY, Zhang L, Yu HX, Bao JD, Lu RR. Curcumin inhibits the metastasis of K1 papillary thyroid cancer cells via modulating E-cadherin and matrix metalloproteinase-9 expression. Biotechnol Lett. 2013;35:995–1000.
Acknowledgments
This work was supported by Deanship of Scientific Research, Taibah University, El- Madinah El-Munawarah, P.O. Box 30001-Saudi Arabia, Faculty of Pharmacy Al-azhar University Assuit Branch-Egypt, and Faculty of Pharmacy Minia University, Mina, Egypt.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abouzied, M.M.M., Eltahir, H.M., Abdel Aziz, M.A. et al. Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT, and caspase-3 expression in experimental rat model. Tumor Biol. 36, 1763–1771 (2015). https://doi.org/10.1007/s13277-014-2778-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2778-z