Abstract
Multipoint covalent bonding of glucose oxidase (EC 1.1.3.4) to hydrophilic natural polymer dextran and optimization of procedures to obtain, with enhanced temperature and pH stabilities, were studied. Purified enzyme was conjugated with various molecular weight dextrans (17.5, 75, and188 kD) in a ratio of 20:1, 10:1, 1:1, 1:5, 1:10, 1:15, and 1:20. After 1 h of incubation at pH 7, the activities of purified enzyme and conjugates were determined at different temperatures (25°C, 30°C, 35°C, 40°C, 50°C, 60°C, 70°C, and 80°C), and the results were evaluated for thermal resistance. Increases in temperature from 25°C to 50°C did not change the activities of the conjugates. The conjugate, which was prepared with 75 kDa dextran in a molar ratio of 1:5, showed the highest thermal resistance and even the activity still remains at 80°C at pH 7.0. This conjugate also displayed activity in a wide pH range (pH 4.0–7.0) at high temperatures. Conjugate, which was synthesized with 75 kDa dextran in a molar ratio of 1:5, appears to be feasible and useful for biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inactivation factors affect the operational stability of enzymes during their industrial applications [1, 2]. The lack of stability strongly limits their usages because of nonconventional media or extreme conditions [3–5]. Enzyme immobilization can improve performance of enzymes such as activity, stability, or selectivity [6–8]. Additional stabilization of immobilized enzymes may also enhance their resistance against destructive effects [9, 10].
Formations of additional inter- and intramolecular bridges during chemical modification of enzymes with polysaccharides may improve enzyme stability. Multipoint attachment of polysaccharides increases the rigidity, as well as hydration of these molecules [10–12]. Dextrans are a kind of polysaccharides containing mainly α-1,6 and variable numbers of α-1,2, α-1,3, and α-1,4 linkages between glucose units [13, 14]. Modification of glucose oxidase with 20 kDa dextran aldehyde may exert a highly stabilizing effect against both mechanic stirring in aqueous systems and n-heptane/aqueous buffer stirred systems [1, 9].
Glucose oxidase (GOD, EC 1.1.3.4) is a flavoprotein that catalyzes the oxidation of β-D-glucose to D-glucono-δ-lactone using molecular oxygen as the electron acceptor and releases hydrogen peroxide [15, 16]. The enzyme is composed of two identical subunits with two moles of flavin adenine dinucleotide (FAD) located inside deep pockets in the protein structure [17]. GOD is used in a large scale of technological applications, such as removing residual glucose and oxygen from beverages, wine and foodstuff, in bleaching cellulose fibers, and production of gluconic acid and is also used as a food preservative [15, 17, 18]. However, the most significant GOD application is in biosensors for the monitoring of glucose levels in body fluids during fermentation of beverages or in miniaturized biofuel cells [19–22, 24].
The stabilization of GOD against destructive factors improves its characteristics and broadens its applicability. Hence, in this study we aimed to enhance the stability of GOD against temperature and pH changes. So, various GOD-dextran covalent conjugates were prepared by using three different molecular weight dextrans (17.5, 75, and 188 kD). The stability of conjugates was displayed by the activities determined at different temperatures and pHs after 1 h of incubation.
Materials and Methods
Materials
Glucose oxidase from Aspergillus niger (M w = 186 kDa; FL.49180), Sephadex G-50 (FL.84946), and o-dianisidine (FL.33430) were purchased from Fluka. D-(+)-glucose (G 7528) and dextrans from Leuconostoc mesenteroides with different molecular weights (M w = 17.5, M w = 75, and M w = 188 kDa; SI.D4626, SI.D3759, and SI.D4876, respectively) were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). All other chemicals used were analytical grade.
Purification
The protein content of the purchased GOD enzyme was determined as 90% (A 280/1.34). High performance liquid chromatography chromatograms also confirmed the presence of impurities in this commercial enzyme. For conjugate synthesis; commercial enzyme was purified by using Biorad model 2110 purification system with Sephadex G-50 column (1.5 × 30); fractions were eluted with PBS buffer at pH 7.0, and appropriate fractions were pooled for the conjugate synthesis.
Preparation of Dextran Aldehyde Derivatives
Freshly prepared NaIO4 solution (8 g dissolved in 70 ml distilled water) was added slowly over dextran solution (3.33 g dissolved in 30 ml distilled water) and kept stirred in dark for 24 h at room temperature. At the end of this period, the solution was dialyzed against distilled water for 24 h, and aldehyde derivative of dextran was recovered by freeze drying [9, 23].
Preparation of GOD-dextran Aldehyde Conjugates
Amount of dextran aldehyde derivatives needed for the preparation of conjugates was calculated according to the formula given below for constant enzyme concentration (0.2 mg/ml).
where c shows concentration (mg/ml) while M is molar weight.
Dextran aldehyde and enzyme solutions were freshly prepared in PBS buffer at pH 7.0. Afterwards, the reaction was initiated by mixing enzyme (2 ml) and dextran aldehyde (2 ml) solutions and incubating in a water bath at 25°C for 16 h. As a result, a Schiff base was formed between aldehyde groups of dextran derivative and amine groups of enzyme. Then, 5.6 ml cold (+4°C) 100 mM sodium bicarbonate solution at pH 8.5 was added to stop the reaction. To reduce the amount of Schiff base formed and unreacted aldehyde groups of dextran derivative, 9.6 mg of sodium borohydride was added. This solution was stirred for 15 min and then, again, 9.6 mg of sodium borohydride was added to the reaction medium. Reduction reaction was continued at +4°C for 15 min, and pH of the final solution was adjusted to 7.0 [9].
Activity Determination
The test tubes containing 780 µl PBS buffer were incubated in a stirring water bath at working temperatures for 5 min. The reaction was started at working temperatures (25°C, 30°C, 35°C, 40°C, 50°C, 60°C, and 70°C) by adding 50 µl glucose (%25 w/v), 25 µl o-dianisidine (10 mM), 15 µl HRP (0.005 mg/ml), and 30 µl GOD solution (0.0025 mg/ml), respectively, for the initiation of this reaction. After 10 min, reaction was stopped by adding 100 µl 2 M H2SO4 solution. The total volume was completed to 1 ml [25, 26]. Afterwards, UV-visible spectra of these reaction products and their A 400 values were recorded. Activities in units were calculated according to the formula given below.
- ε :
-
Molar absorption coefficient of o-dianisidine at 400 nm (17,500)
- t :
-
Incubation time (min)
- c GOD :
-
GOD concentration (mg/ml)
- A 400 :
-
Absorbance at 400 nm
Stability Studies
To show the thermal resistance of enzyme and all conjugates, activity values were determined after incubating enzyme and conjugate solutions in a water bath for 1 h at pH 7.0. Meanwhile, to display the pH stability of enzyme and conjugate, activity of conjugate n GOX/n D 1:5 was also determined by repeating the same procedure at different pHs.
Characterization of the Conjugate with GPC (Gel-permeation Chromatography) and Fluorescence Techniques
Molecular weight distributions of dextran aldehyde, purified enzyme, and selected conjugate with the ratio n E/n D 1:5 were determined by gel-permeation chromatography (Viscotek GPCmax VE2001 GPC solvent/sample module) on a 7.9 mm × 50 cm Shim-Pack Diol 300 column with ultraviolet (UV) and refractive index (RI) detectors. The fractions were eluated at 1 ml min−1 with 0.1 M PBS buffer (pH 7) containing 0.15 M NaCl and 7.5 mM NaN3.
Fluorescence measurements of the enzyme and mentioned conjugate above were carried out using a PTI QM-4 steady state spectrofluorophotometer. The excitation and emission slit widths were set to 2 nm. Sample excitation was performed at 450 nm, while the emission spectrum was recorded in the range of 524 nm. The working temperature of the cell was maintained at 60°C by circulating water through the thermostatted cuvette holder.
Results and Discussion
The modification of glucose oxidase by covalent conjugation with 17.5, 75, and 188 kDa dextrans using different molar ratios (n E/n D 20:1, 10:1, 1:1, 1:5, 1:10, 1:15, and 1:20) was studied. The peak of aldehyde observed at about 2,934 cm−1 of FTIR spectra disappeared when covalent bonding occurred between aldehyde groups of dextran derivatives and primer amino groups of enzyme molecules (results were not submitted). The stabilities of conjugates were evaluated with the activities determined at different temperatures and pHs after 1 h of incubation.
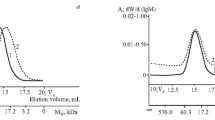
One of the most fundamental parameters for characterizing macromolecules is their molecular weight and molecular weight distribution [27]. However, gel-permeating chromatography has been a widely used technique for estimating these characteristics of proteins, polysaccharides, and protein-polymer conjugates in their native forms based on their elution positions [28, 29]. The size exclusion chromatograms of the enzyme-dextran conjugate (n E/n D 1:5), purified enzyme, and aldehyde derivative of dextran were recorded by using UV and RI detectors (Fig. 1). Elution position of the conjugate, which was in front of glucose oxidase and aldehyde derivative of dextran, relates the formation of covalently bonded macromolecules with higher molecular weight than the constituents.
The fluorescence prosthetic group FAD exhibits different spectral characteristics in different proteins reflecting the specific environmental property of isoalloxazine, which is the chromophore present in the molecule. Each subunit of dimeric GOD contains one tightly bound flavin adenine dinucleotide as cofactor and ten tryptophan residues. The energy transfer probability from tryptophan residues to the flavin cofactor could be affected from both distance and orientation due to structural perturbation of this enzyme [30–32]. In this work, FAD fluorescence intensity of purified GOD and the conjugate (75 kDa dextran and n E/n D 1:5) was measured for 1 h at 60°C; pH 4, 5, 6, 7, and 8 (Fig. 2).
The fluorescence intensities of GOD and conjugates were affected by pHs of the aqueous media. FAD fluorescence intensity of pure enzyme increased with incubation time, whereas, FAD fluorescence intensity of conjugate showed almost constant values. This is related to resistance of the conjugate against high temperature denaturation (Figs. 2 and 3).
In order to reveal thermal stabilities of conjugates, their activities were determined after 1 h of incubation at different temperatures for pH 7 (Fig. 4).
Thermal stabilities of conjugates were influenced by both molar weights of dextrans and n E/n D ratios used in the synthesis reactions. It was observed that activity of conjugates was slightly less than purified enzyme, but their activities were not significantly changed before 50°C. Pure enzyme shows maximum activity around pH 4–5, but thermal resistance is high only at pH 7. Conjugates obtained with 75 kDa dextran showed higher activity than the conjugates synthesized with 17.5 kDa and 188 kDa dextrans. This conjugate (n E/n D 1:5) showed highest thermal resistance even at high temperatures (70°C and 80°C) in a wide pH range (pH 4–8; Fig. 5).
Pure GOD and GOD-dextran conjugate (n E/n D 1:5, 75 kDa dextran) were incubated at pH 7 in PBS buffer at room temperature for a given period of time as shown in Fig. 6 to evaluate the shelf lives. There was an instantaneous and a regular decrease in the activity of pure enzyme, whereas, the activity of the conjugate was constant for nearly a week (Fig. 6).
Conclusion
Covalent conjugation of glucose oxidase with different molar weight dextrans in various molar ratios provided an enhanced stability of enzyme against temperature and pH. Especially conjugate n E/n D 1:5 obtained with 75 kDa dextran showed high thermal resistance and good stability in a wide pH range. This conjugate may be potentially useful in preparing clinical diagnostic kits and biosensors.
References
Betancor, L., Fuantes, M., Dellamora-Ortiz, G., López-Gallego, F., Hidalgo, A., Alonso-Morales, N., et al. (2005). Journal of Molecular Catalysis. B Enzymatic, 32, 97–101.
de la Casa, R. M., Guisán, J. M., Sánchez-Montero, J. M., & Sinisterra, J. V. (2002). Enzyme and Microbial Technology, 32, 30–40.
Abian, O., Wilson, L., Mateo, C., Fernández-Lorente, G., Palomo, J. M., Fernández-Lafuente, R., et al. (2004). Journal of Molecular Catalysis. B Enzymatic, 19–20, 295–303.
Longo, M. A., & Combes, D. (1999). Journal of Chemical Technology and Biotechnology, 74, 25–32.
Christakopoulos, P., Kourentzi, E., Hatzinikolou, D. G., Claeyssens, M., Kekos, D., & Macris, B. J. (1998). Carbohydrate Research, 314, 95–99.
Lopez-Serrano, P., Cao, L., van Rantwijk, F., & Sheldon, R. A. (2002). Biotechnological Letters, 24, 1379–1383.
Sheldon, R. A., Schoevaart, R., & van Langen, L. M. (2005). Biocatalysis and Biotransformation, 23(3/4), 141–147.
Mislovicova, D., Michalkova, E., & Vikartovska, A. (2007). Process Biochemistry, 42, 704–709.
Betancor, L., López-Gallego, F., Hidalgo, A., Alonso-Morales, N., Fuentes, M., Fernández-Lafuente, R., et al. (2004). Journal of Biotechnology, 110, 201–207.
Fuentes, M., Segura, R. L., Abian, O., Betancor, L., Hidalgo, A., Mateo, C., et al. (2004). Proteomics, 4, 2602–2607.
Bolivar, J. M., Wilson, L., Ferrarotti, S. A., Guisán, J. M., Fernández-Lafuente, R., & Mateo, C. (2006). Journal of Biotechnology, 125, 85–94.
Irazoqui, G., Giacomini, C., Batista-Viera, F., & Brena, B. M. (2007). Journal of Molecular Catalysis B Enzymatic, 46, 43–51.
Gil, E. C., Colarte, A. I., EI Ghzaoi, A., Durand, D., Delarbe, J. L., & Battaille, B. (2008). European Journal of Pharmaceutics and Biopharmaceutics, 68, 319–329.
Karmarkar, S., Garber, R., Kluza, J., & Koberda, M. (2006). Journal of Pharmaceutical and Biomedical Analysis, 41, 1260–1267.
Zakhartsev, M., & Momeu, C. (2007). Journal of Chromatography B, 858, 151–158.
Simpson, C., Jordan, J., Gardiner, N. S., & Whiteley, C. (2007). Protein Expression and Purification, 51, 260–266.
Hecht, H. J., Kalisz, H. M., Hendle, J. H., & Schmid, R. D. (1993). Journal of Molecular Biology, 229, 153–172.
Malherbe, D. F., Du Toit, M., Cordero, R. R., Otero, P., van Rensburg, I., & Pretorius, S. (2003). Applied Microbiology and Biotechnology, 61, 502–511.
Wei, Y., Feng, X., Chen, X., Hou, W., & Zhu, J. J. (2008). Biosensors and Bioelectronics, 23, 925–931.
Zhu, M., Jiang, Z., & Jing, W. (2005). Sensors and Actuators B, 110, 382–389.
Topcu Sulak, M., Gokdogan, O., Gulce, A., & Gulce, H. (2006). Biosensors and Bioelectronics, 21, 1719–1726.
Bullen, R. A., Arnot, T. C., & Lakeman, J. B. (2006). Biosensors and Bioelectronics, 21, 2015–2045.
Sacco, D., Bonneaux, F., & Dellacherie, E. (1988). Journal of Biological Macromolecules, 10, 305–310.
Deshpande, S. S., & Rocco, R. M. (1994). Food Technology, 8, 146–150.
Park, E. H., Shin, Y. M., Lim, Y. Y., Kwon, T. H., Kim, D. H., & Yang, M. S. (2000). Journal of Biotechnology, 81, 35–44.
Dai, G., Li, J., & Jiang, L. (2002). Biointerfaces, 24, 171–176.
Ye, H. (2006). Analytical Biochemistry, 356, 76–85.
Dilgimen, A. S., Mustafaeva, Z., Demchenco, M., Kaneko, T., Osada, Y., & Mustafaev, M. (2001). Biomaterials, 22, 2383–2392.
Mislovicova, D., Masarova, J., Bucko, M., & Gemeiner, P. (2006). Enzyme and Microbial Technology, 39, 579–585.
Haouz, A., Twist, C., Zentz, C., de Kersabiec, A.-M., Pin, S., & Alpert, B. (1998). Chemical Physics Letters, 294, 197–203.
Khatun Haq, S., Ahmad, F., & Hasan Khan, R. (2003). Biochemical and Biophysical Research Communications, 303, 685–692.
Yoshimoto, M., Sato, M., Wang, S., Fukunaga, K., & Nakao, K. (2006). Biochemical Engineering Journal, 30, 158–163.
Acknowledgments
This research was supported by grants from T.R. Prime Ministry State Planning Organization (project number 25-DPT-07-04-01) and Yıldız Technical University BAPK (The Production of Medical Biosensors, project number 27-07-04-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altikatoglu, M., Basaran, Y., Arioz, C. et al. Glucose Oxidase-dextran Conjugates with Enhanced Stabilities Against Temperature and pH. Appl Biochem Biotechnol 160, 2187–2197 (2010). https://doi.org/10.1007/s12010-009-8812-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8812-8