The effect of periodate oxidation on the molecular weight and fraction homogeneity of dextran dialdehydes and carboxymethylcellulose dialdehydes was studied using 13C NMR spectra and gel-permeation chromatography with dual detection. Oxidation of dextran formed homogeneous polymer fractions containing three types of oxidized residues. Oxidation of carboxymethylcellulose occurred more slowly, caused a sharp drop of molecular weight, and formed heterogeneous polymer fractions in the early reaction stages that became homogeneous at high conversions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
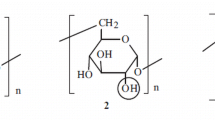
Polysaccharides are widely used as drug carriers, especially for peptides with a primary amine in their structures. However, most of them are chemically inert and require preliminary functionalization in order to react with physiologically active compounds. A simple and effective method for introducing reactive groups is Malaprade periodate oxidation [1–4] (Scheme 1). Polysaccharide dialdehydes prepared in this manner from dextran and carboxymethylcellulose (CMC) are widely used not only for protein conjugation but also for chemical modification of natural polyphenols and aromatic aldehydes [3–5].
An important aspect of polysaccharide dialdehyde preparation is the fractional heterogeneity. In several instances, a polymer in which the number of oxidized residues in the macromolecules depended on their molecular weight was formed by the oxidation. Conjugates with physiologically active substances exhibited erratic activity because different polymer fractions contained different specific amounts of drug [1, 2, 5, 6].
The goal of the present investigation was to determine the reasons for the fractional heterogeneity and to seek conditions allowing dextran dialdehyde (DDA) and carboxymethylcellulose dialdehyde (CMCDA) without these drawbacks to be prepared.
Compounds with hydroxyls on neighboring C atoms can undergo a Malaprade reaction. In the first step, a complex of periodate and vicinal hydroxyls forms. Then, the complex disproportionates to form the dialdehyde and iodate (Scheme 2).
Dextran was oxidized very rapidly. The reaction was completed in 3 h. It had two C–C bonds that were capable of being oxidized (Scheme 1). The reaction occurred with equally probable cleavage of the C-2–C-3 and C-3–C-4 bonds.
Therefore, an equimolar mixture of products containing oxidized residues (2 and 3) was formed in the first step with a periodate:100 anhydroglucose residues (AGR) ratio of up to 12 mol% (Scheme 1). A slight over-oxidation occurred if small amounts of periodate were used. A product containing a slightly greater number of oxidized residues than the number of moles of added periodate formed. Residues 2 and 3 were partially oxidized to product 4 with release of formic acid at IO4 – ratios from 20 to 100 mol%. The resulting copolymer contained all three types of residues. A larger amount of periodate in the reaction caused oxidation of practically all 2 and 3 residues to 4. Thus, only 4 contained dextran dialdehydes containing greater than one half oxidized residues (degree of oxidation γox > 50) (Table 1). The degree of oxidation depended little on the initial weight average molecular weight (Mw) and was determined only by the periodate:dextran ratio.
CMC has only one site capable of being oxidized, i.e., the C-2–C-3 bond. Thus, only one type of oxidized residue was formed (Scheme 2). It reacted much more slowly. Conversion of 85% (γox = 85%) could be achieved only by increasing the reaction time to 72 h (Table 2).
The slower oxidation rate than that of dextran was possibly due to the inactivating effect of the acid, which formed a H-bond to the C-3 OH of the AGR. This prevented the periodate complex from forming and hindered considerably the reaction (Scheme 2).
Polysaccharides do not contain substituents that absorb in the UV region. They can be detected in gel-permeation chromatography using a non-specific refractive-index detector (refractometric detector) that is sensitive to all polymer types regardless of the presence of chromophores in them. Generation of carbonyl substituents in the oxidized polymer changes its spectrum and allows a UV detector to be used to determine them. Thus, their distribution over fractions with different molecular weights could be studied by using a combination of a non-specific refractometric detector and a UV detector, which was sensitive only to oxidized polymer residues.
Periodate oxidation of dextran in aqueous solutions produced a fraction with a completely homogeneous product. Gel-permeation chromatograms obtained using a mass-specific refractometric detector and UV detector tuned to the wavelength of carbonyl absorption of the oxidized residues (~220 nm) were fully consistent. The Mw distributions were practically the same as those of the starting polymers regardless of their Mw for DDAs with degrees of oxidation less than 20. Increasing the degree of oxidation further decreased the Mw without changing significantly the polydispersity (Table 3).
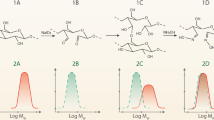
In contrast with dextran, CMC formed highly viscous solutions. Its oxidation at low conversions formed a fraction of heterogeneous products. The high Mw fractions contained significantly fewer oxidized residues. This shifted the maximum of the Mw distribution according to the UV detector toward lower masses (Fig. 1a). The heterogeneity was related to hindered diffusion of periodate ions into the swollen balls in the initial reaction step.
CMC with less than 65% carboxymethylation is poorly soluble in cold water. As a result, its oxidation formed a fraction of highly heterogeneous polymers. The Mw decreased and the heterogeneity was practically completely removed upon further oxidation (Fig. 1b).
Unsubstituted glycoside rings in the polymer were oxidized faster than carboxymethylated ones and were consumed first. They were practically absent from CMCDA samples with greater than 40% degree of oxidation (γox = 40). This was due to the fact that there were no carboxylic acids to deactivate them. Lightly substituted CMC was oxidized faster. The Mw did not decrease substantially during the process.
A side reaction in which polymer oxidized residues positioned randomly along the main chain were destroyed was initiated simultaneously with the oxidation. The hydrolysis, which by the law of chance was atypical of polysaccharides, caused a sharp drop of the Mw. The polydispersity trended toward the most probable. The destruction rate for rapidly oxidized dextran was significantly less than the periodate oxidation rate. Therefore, the Mw was not significantly reduced. The reaction for slowly oxidized CMC was associated with a significant decrease of Mw because of the reaction time more than the degree of oxidation.
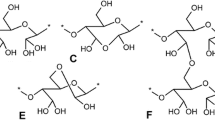
Oxidized residues of both polymers closed into hemiacetal rings. They were stable to hydrolysis in alkaline and neutral solution. However, they were quickly hydrolyzed at pH < 4.5. Two side reactions accompanied the oxidation. These were disproportionation of the dialdehyde to form hydroxyacid 10 and over-oxidation to a dibasic acid 9. Both reactions led to hemiacetal ring closure and subsequent quick hydrolysis of the unstable structures (Scheme 3).
The composition of the final product was affected greatly by process impurities in the CMC that were formed during carboxymethylation of starting cellulose by chloroacetic acid in alkaline solution and assembled into difficultly separated aggregates linked through H-bonds to the polymer main chain. The aggregates were destroyed and the impurities became detectable as separate peaks in the low-molecular-weight region as the ionic strength changed during gel-permeation chromatography.
We isolated the low-molecular-weight fraction that passed through a 600-Da dialysis membrane and determined its composition. As it turned out, the CMC contained three main impurities of glycolic acid, its dimeric lactone glycolide, and formic acid. The presence of these in the product should be considered when evaluating the physiological activity of polymeric drugs prepared from industrial samples of CMC that were not additionally purified by dialysis. The contents of the impurities were quantified and their structures were confirmed using 13C NMR and PMR spectroscopy.
Thus, periodate oxidation of dextran formed a fraction of homogeneous DDA containing three types of residues and did not change significantly the Mw characteristics of the polymer. CMC drug substance contained difficultly separable impurities of formic and glycolic acids and a cyclic dimeric glycolide. Periodate oxidation of it gave a product containing a small amount of oxidized residues (γox = 20) and formed a fraction of heterogeneous polymer of reduced Mw in which a large part of the oxidized residues was found in the low-molecular-weight fraction. Carboxylic-acid substituents slowed the rate of periodate oxidation of the polysaccharide glucose rings.
Experimental
13C NMR spectra were recorded in NaOH solution (1%) in D2O on a Bruker Avance 600 instrument at operating frequency 150.94 MHz and 297 K with full suppression of proton coupling. 13C NMR spectrum of the low-molecular-weight fraction containing formic and glycolic acids and the cyclic lactone glycolide (150.94 MHz, D2O, δ, ppm): 180.0 (s, glycolic acid carbonyl), 171.0 (s, glycolide carbonyl), 163.0 (s, formic acid carbonyl), 61.0 (s, glycolic acid CH2), 22.0 (s, glycolide).
Gel-permeation chromatography was performed on a Waters chromatograph equipped with a Waters 2998 PDA diode matrix multi-wavelength spectrophotometric detector and a Waters 2414 differential refractometric detector connected in series. Two columns, Ultrahydrogel 1000 and Ultrahydrogel 120 (Waters), connected in series and thermostatted at 30°C were used for the analysis. The eluent was aqueous borate buffer (pH 10) at flow rate 0.3 mL/min. UV spectra were recorded in the range 190–800 nm.
Standard polyethyleneglycols with Mw from 20.6 kDa to 106 Da and standard dextrans with number average Mn from 576 kDa to 7870 Da were used to construct a universal calibration curve. The resulting chromatograms were processed using the Breeze 2 program.
The rates of periodate oxidation of dextran and CMC were determined from the consumption rate of periodate, which was measured by UV spectroscopy from the change of optical density at 260 nm, the wavelength of periodate absorption.
The rate of formic acid release was also monitored by titration of the reaction mixture.
The number of aldehydes in the polymer was determined by reverse iodometric titration.
All polymers were purified by dialysis for 96 h with four changes of the solution followed by lyophilization. Lowmolecular-weight components in the CMC were also isolated by dialysis followed by lyophilization.
The hydrolysis rate of the oxidized polysaccharides was estimated from the change of Mw of the polymer dissolved in acetate buffer at pH 3.5 over time. The resistance to hydrolysis was determined from the change after 24 h of the Mw characteristics of the polymers in solutions at pH 3, 3.5, 4, 4.5, and 6.2.
References
V. I. Gumnikova, V. A. Dyatlov, T. A. Grebeneva, I. S. Kruppa, V. V. Kireev, and V. I. Bakhmutov, Plast. Massy, 44 (2013).
V. A. Dyatlov, V. I. Gumnikova, T. A. Grebeneva, I. S. Kruppa, I. R. Rustamov, and V. I. Maleev, Plast. Massy, 6 (2013).
H. Li, B. Wu, C. Mu, and W. Lin, Carbohydr. Polym., 84, 881 (2011).
V. N. Syutkin, A. G. Nikolaev, S. A. Sazhin, V. M. Popov, and A. A. Zamoryanskii, Khim. Rastit. Syr’ya, 91 (1999).
M. Strlic, J. Kolar, M. Zigon, and B. Pihlar, J. Chromatogr. A, 805, 93 (1988).
P. Calvini, G. Conio, E. Princi, S. Vicini, and E. Pedemonte, Cellulose, 13 (5), 571 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2014, pp. 845–849.
Rights and permissions
About this article
Cite this article
Dyatlov, V.A., Kruppa, I.S., Mamaeva, S.A. et al. Change of Polysaccharide Molecular-Weight Distribution and Fraction Homogeneity After Periodate Oxidation. Chem Nat Compd 50, 973–977 (2014). https://doi.org/10.1007/s10600-014-1139-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-1139-x