Abstract
Background
Despite a number of studies comparing postoperative stability and function after anatomic double-bundle and single-bundle anterior cruciate ligament reconstruction (ACLR), it remains unclear whether double-bundle reconstruction improves stability or function.
Questions/purposes
We therefore asked whether patients having single- and double-bundle ACLR using semitendinosus (ST) alone differed with regard to (1) postoperative stability; (2) ROM; and (3) five functional scores.
Methods
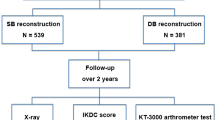
We prospectively followed 60 patients with an isolated anterior cruciate ligament (ACL) injury. Thirty patients underwent single-bundle and 30 patients underwent double-bundle ACL reconstruction. Clinically we assessed stability and range of motion (ROM); anteroposterior stability was assessed by Rolimeter and rotational stability by a pivot shift test. Function was assessed by IKDC, Noyes, Lysholm, Marx, and Tegner activity scales. The minimum followup was 36 months (mean, 46.2 months; range, 36–60 months).
Results
Residual anteroposterior laxity at 3 years postoperatively was similar in both groups: 1.4 ± 0.3 mm versus 1.4 ± 0.2 mm, respectively. We observed no difference in the pivot shift test. ROM was similar in both groups, although double-bundle patients required more physical therapy sessions to gain full ROM. IKDC, Noyes, Lysholm, Marx, and Tegner scores were similar at final followup.
Conclusion
Double-bundle reconstruction of the ACL did not improve function or stability compared with single-bundle reconstruction.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disruption of the ACL is among the most frequent musculoskeletal injuries affecting physically active men and women. According to an ongoing study in the United States, an estimated 200,000 ACL reconstructions (ACLR) are performed annually, and the incidence of ACL injury is roughly one in 3000 per year [32]. Anatomic studies have demonstrated the anterior cruciate ligament consists of two functional bundles: the anteromedial (AM) and the posterolateral (PL) bundle whose nomenclature is related to their insertion in the tibial plateau [2, 11, 36]. In cadaveric studies, the AM bundle tightens during knee flexion, whereas the PL bundle slackens; in contrast, the PL bundle tightens during knee extension, whereas the AM bundle slackens [2, 27, 36].
Some authors consider single-bundle ACLR the standard option to treat ACL lesions [5, 7, 17, 42]. However, recent studies suggesting the need for better rotational control have stimulated interest in a more anatomic reconstruction with double-bundle [6, 9, 21, 26, 38, 41, 49, 51]. Several studies suggest the anatomic double-bundle ACLR should improve pivot shift resistance and increase rotational knee control [8, 38, 46, 47, 49, 51] and should help preserve menisci and limit progression toward arthritis [8, 38, 46, 47, 49, 51]. A number of studies, however, reported no difference in terms of anteroposterior laxity, rotational stability, and/or any other clinical aspects at final followup between the two techniques [1, 4, 20, 23, 24, 30, 34, 43].
A recent systematic review [47] suggested that in most published studies on double-bundle ACLR have inadequate descriptions of the specific operative technique data. The authors found a low percentage of Level 1 and 2 studies (2.7% and 14.9%, respectively), whereas most of studies classified as Level 3 (23%) and Level 5 (mainly consisted of technical notes and expert opinions [60%]). Foremost among the concerns associated with this particularly complex procedure is the expertise required to perform the double-bundle technique properly; therefore, some theoretical advantages could be negated by the complexity of this procedure and steep learning curve. One recent study demonstrated a higher number of patients with tibial and femoral bone tunnel enlargement and double-tunnel communication in patients treated with the double-bundle technique [40]. Performing an anatomic double-bundle reconstruction entails the use of both the semitendinosus (ST) and the gracilis autografts, requiring the use of independent femoral and tibial fixations. Further, hamstring strength deficits in deep flexion and internal rotation resulting from the use of both tendons could represent a possible complication documented in various studies [12, 14, 31]. Therefore, we have used ST in this study to reduce the risk of this complication and none of the published studies compare single-bundle versus double-bundle techniques with the use only of ST. Furthermore, despite one review [47], it remains unclear whether there is any difference in stability or function after double-bundle or single-bundle reconstructions.
We therefore asked whether patients having single- and double-bundle ACLR using ST alone differed with regard to (1) postoperative stability; (2) ROM; and (3) five functional scores.
Patients and Methods
We prospectively followed 60 athletes who underwent ACLR from February 2004 until January 2007. During that same time, we treated 138 patients with either single- or double-bundle ACLR; from these patients, only 60 met the inclusion-exclusion criteria as mentioned. The patients were randomly assigned to two treatment groups: ST single-bundle (SB group; n = 30) and ST double-bundle (DB group; n = 30) ACLR group. The 60 patients were blinded to the specific type of reconstruction they would undergo. Each patient was allocated to the one treatment or the other depending on the order of arrival; researchers did not have the total sample of patients from the beginning and did not know in advance the specific characteristics of each patient.
The inclusion criteria for the study were: (1) primary ACLR with no associated Grade III ligament injury, PL rotatory instability, or fracture around knee, no previous knee ligament surgery (except diagnostic arthroscopy or partial meniscectomy), no arthritic changes or Grade III–IV chondral damage, no subtotal or total meniscectomy, no malalignment, and a normal contralateral knee; (2) ACL injury reported within 5 months; (3) consent for participation in this study; (4) willingness to followup at 3, 6, 12, 24, and 36 months or when asked for; and (5) compliance to a specific rehabilitation program. Patients were excluded from the study when the examination under anesthesia or intraoperative findings did not meet the previously mentioned inclusion criteria. Patients with a partially torn ACL were also excluded from the study.
In the SB group, the mean age of patients at surgery was 31.9 ± 1.9 years; 50% of patients were males and 50% females. In the DB group, the mean age of our patients at surgery was 28.9 ± 1.9 years; 60% of patients were males and 40% females (Table 1). We determined confidence intervals to compare the demographic factors (Table 2).
The injuries were all sports-related. Pivoting, while playing a sport (eg, skiing, soccer, karate), was the main mechanism of injury (82%), whereas a fall during sports participation (eg, motocross) accounted for only 18%. In our series, injuries while playing soccer made up 38%, skiing 33%, motocross 16%, tennis 10%, and karate 3%. All of the patients clinically presented with an ACL-deficient knee with a positive Lachman test [45] and pivot shift [22], both of which were confirmed with a complete ACL rupture on MRI. Associated knee injuries included first- and second-degree medial collateral ligament sprain in 11%, meniscal lesions in 30%, Grade I or II chondropathy in 7%, and a combined meniscal and chondropathy lesion in 15%. Concomitant injuries for both groups and confidence intervals are reported in Table 3. Pearson chi-square test was performed and showed that the two groups were homogeneous regarding concomitant injuries (p > 0.0476). The minimum followup was 36 months (mean, 46.2 months; range, 36–60 months). No patients were lost to followup.
The sample size of each group was determined beforehand by using statistical power analysis. Anteroposterior laxity (ΔLaxity), which was evaluated at 3 years postoperatively, was defined as the primary parameter. To detect a difference of 1.7 mm with a SD of 2.2 mm, 25 patients were required per group (power = 0.8 and p < 0.05). Therefore, we included 30 patients per group [50].
After the administration of either a spinal or general anesthesia, the patient was positioned supine on the operating table. A tourniquet was placed at the proximal aspect of the thigh with sufficient distance from the expected exit point of the Kirschner wire suture passer in the thigh’s lateral aspect. A lateral post for thigh support and a foot bar were then placed to enable the knee to be positioned at 90° flexion on the table during surgery. This setup also allowed intraoperative testing of full ROM. The preparation of the graft was similar irrespective of the surgeon performing a single- or double-bundle technique. Once standard prepping and draping were completed, the tourniquet was inflated to 300 mmHg only for graft harvesting and then deflated. A 3-cm vertical incision was then made centered approximately 5 cm below the medial joint line midway between the tibial tubercle (Gerdy’s tubercle) and the posteromedial aspect of the tibia. The sartorial fascia was incised and the ST tendon was dissected. The tendon was completely detached from its proximal attachment with an open tendon stripper. On its tibial end, the tendon’s length was maximized preserving as much length as possible by detaching the ST close to the bone. Ideally, a length of 28 cm or greater was desired. While the surgeon prepared the tunnels, the surgical assistant at the back table proceeded with the preparation of the graft. Once the graft was cleaned and devoid of excess tissues, measurement of the tendon followed. The minimum length needed was 28 cm to allow the possibility of cutting the graft in half with sufficient length to fold each half of the graft to a length of 7 cm. In such way, we had a 2-cm graft length for the femoral and tibial tunnels and 3 cm intra-articularly. The ends of the grafts were then whipstitched using OrthocordTM sutures (Orthocord; DePuy, Mitek, Raynham, MA) (Fig. 1A–B).
For the SB technique, using standard anterolateral and anteromedial portals, the knee was visualized and prepared for tunnel placements. The anatomic footprints of the native ACL on both the femoral and tibial sides were identified and not removed. We used remnants of torn ACL on femoral and tibial sides as landmarks for positioning of tunnels. Notchplasty was never performed in any patient in either group. We used the center of the ACL footprint on femoral and tibial sides as a landmark for placing tunnels for SB ACL reconstruction. The femoral tunnel position was first identified and drilled using a Kirschner wire in an anatomic position through the anteromedial portal with the knee flexed at 110° of flexion. After checking the proper positioning at 10 o’clock for the right knee, a 4.5-mm cannulated drill was used to create the femoral tunnel and with the specific instrument and the length of the tunnel was measured (Smith & Nephew Endoscopy, Andover, MA) [9, 20, 39]. Once the required graft size was assessed, the half tunnel was prepared using a drill and dilators to obtain a tunnel 0.5 mm in diameter smaller than the graft to have a good press-fit and avoid possible movement of the graft. Average diameter of femoral tunnel in the SB group was 9 mm and the length of the tunnel was 40 mm. The tibial tunnel was then prepared in an anatomic position at the ligament’s footprint using an endoscopic aimer adjusted to a 45° position in the coronal plane (Smith and Nephew tibial guide) [9, 20, 39]. The alignment on the sagittal plane should be at 70° with respect to the medial plateau [9, 20, 39]. Average diameter of tibial tunnel in the SB group was 9.5 mm and the length was 45 mm. After selecting the appropriate size of the EndoButton® CL (Smith & Nephew Endoscopy), the quadrupled ST tendon was inserted and fixed at the femoral end with an EndoButton. At this point, we suggest appropriate preconditioning of the graft with cyclic flexion and extension of the knee and finally the two strands were fixed at the tibial side under maximum manual tension (30 Newtons) using a new-generation biocomposite screw (average size, 9 × 30 mm).
For the DB technique, the PL femoral tunnel was initially prepared using an “outside-in” technique. To properly achieve this step, a customized PL tunnel guide was used (Smith &. Nephew Inc Endoscopy). This customized guide has a component arm designed to reach either the 9 o’clock or the 3 o’clock position. The arm of the PL guide was inserted in the anterolateral portal and positioned at either 9 o’clock or 3 o’clock on the medial wall of the lateral condyle while the handle was maneuvered at the area of the junction of the distal femur and lateral condyle to fix the entry point for the tunnel. We used the lateral intercondylar ridge and lateral bifurcate ridge, indicating the superior border of the femoral ACL insertion site and the border between the AM and PL bundle, respectively, as bony landmarks for placement of tunnels [8]. A guidewire was inserted from outside in, which was followed by a 4.5-mm cannulated drill to prepare the pilot hole. Once the length of this hole was measured, a 6-mm PL tunnel with its appropriate depth is drilled. Preparation of the 7-mm AM tunnel followed using standard techniques with the tunnel placed at the either the 11 o’clock or 1 o’clock position. At the end of this step, we have two divergent tunnels positioned anatomically. The tibial tunnels were prepared at an angle of 50° to 60° with the entry point separated by a distance of 1 to 1.5 cm. These tunnels converge on the ACL ligament’s footprint intraarticularly [6, 8, 9, 20, 47]. The appropriate size of the EndoButton® CL (Smith & Nephew Endoscopy) as determined by the AM and PL tunnel lengths was then attached at the end of each graft. The diameter of each bundle was then measured using 0.5-mm increment sizers to match the size of the femoral and tibial tunnels. With the tunnels ready, the PL bundle was positioned first followed by the AM bundle. Once in place, the femoral fixation was double-checked to determine if the EndoButton was securely anchored against the cortex. On the other hand, after pretensioning and preconditioning with cyclic flexion and extension, the tibial end of the graft was fixed using a single screw-post construct connected to the graft with a new-generation high-strength suture (Orthocord; DePuy; and Fiberwire; Arthrex, Naples, FL). The AM bundle was secured at 20° of flexion and the PL bundle fixed at full extension; the graft was then checked for impingement and the knee examined for ROM and stability with the Lachman test. The graft’s position was confirmed with the postoperative radiograms (Fig. 2A–B).
The postoperative rehabilitation protocol was identical for both groups. For the first 3 weeks, walking with crutches with partial weightbearing was allowed without any brace or splint. Patients were encouraged to restore full extension of the knee and strengthen the quadriceps muscle power. Four weeks after surgery, patients returned to performing activities of daily living. Noncontact sports were permitted after 3 months, and contact sports were permitted 1 year after surgery (Table 4).
Patients were followed at 3, 6, 12, 24, and 36 months postoperatively. A single orthopaedic surgeon (CS), not associated with the surgery and blinded to the surgical procedure evaluated all patients pre- and postoperatively. The type and level of sport participation were documented at preoperative and postoperative intervals. The major complications were defined as postoperative intra-articular infection, rerupture of graft, vascular complications, or fractures after ACL reconstruction. Minor complications were defined as donor site morbidity, stiffness, and hardware complications [35]. Anterior laxity was documented using the Rolimeter (Aircast, Boca Raton, FL) [3, 10] preoperatively and at the same intervals postoperatively. The integrity of the hamstring function of both knees was determined using the modified technique of Nakamura for range of movement [31]. We obtained standard IKDC [19, 48], Noyes [33], Lysholm [25], Marx [29], and Tegner [44] knee scores. To determine the patients’ psychologic profile, a specific questionnaire (Psychovitality) was administered preoperatively (SocratesTM Orthopaedic Outcomes Software, Ortholink, Australia). This test is a six-item questionnaire including psychologic factors such as patients’ expectations related to treatment outcome and motivation to resume preinjury activity levels. Scores can range from 3 to 18 points; a higher score would indicate better motivation on the part of the patient [15].
Continuous data were described as average means ± standard error of the mean. Nonparametric analysis was performed with Friedman’s test to compare the anteroposterior stability as measured with Rolimeter from preoperative to postoperative 6-, 12-, and 36-month evaluation. Nonparametric Friedman’s test was also performed to evaluate postoperative improvement in clinical evaluation scores for each group (IKDC, Noyes, Lysholm, Tegner, and Marx). We used the nonparametric Mann-Whitney U test for intergroup comparison with respect to anteroposterior stability as well as in clinical evaluation scores at preoperative to postoperative 6-, 12-, and 36-month followup. Spearman’s rho test was performed to analyze the correlation between preoperative psychovitality and Tegner score at last followup for both groups. Z-score and p values are provided for all the parameters evaluated. For statistical analysis, SPSS software was used (SPSS 17.0; SPSS, Chicago, IL).
Results
Patients in both groups improved (p < 0.001) in terms of anteroposterior laxity from preoperatively and at 6-, 12-, and 36-month followup (Table 5). We observed no difference in improvement between the two groups at final followup; both SB and DB group patients maintained postoperative stability at 3-year followup (Table 5). The lateral pivot shift test at 3-year followup was negative in 83.3% and Grade 1 in 16.7% in the SB group patients and was negative in 87% and Grade 1 in 13% in the DB group patients (Table 6).
At final followup, the ROM was similar in both groups: a mean of 135.5° ± 5.5° in the SB group and 134.5° ± 1.0° in the DB group (Table 6). The rehabilitation time involved 10% more physical therapy sessions in the DB group than the SB group to regain the same ROM.
All clinical scores (IKDC, Noyes, Lysholm, Tegner, and Marx) improved from preoperative evaluation to final followup for each group of patients; however, there was no difference between the two groups at final followup (Table 5). All patients returned to their previous sports activities. In the SB group, patients went back to competitive sports at an average of 7.4 months and in the DB group at an average of 8.2 months; we observed no difference in Tegner scores between preinjury and last followup for both groups (Fig. 3A–B). We observed a correlation between preoperative psychovitality score and Tegner score at last followup for both groups (Fig. 4A–B).
Box plots showing improvement in Tegner score (A) and laxity (B) from preoperative evaluation to 6-, 12-, and 36-month followup. We observed no difference in improvement between the two groups at the prospective followup; the Tegner score at last followup approached that of the preinjury value in the single-bundle group.
We observed no major postoperative complications and no reruptures. One year after surgery one of the patients in the SB group was involved in a motocross crash and sustained a tibial plateau fracture (Schatzker Type I). Diagnostic arthroscopy was performed and the ACL was intact. The patient was treated nonoperatively and returned to motocross after 3 months. Another patient in the DB group after 4.5 years sustained a tibial plateau fracture (Schatzker Type IV) after a trivial trauma while playing soccer and was managed operatively with open reduction and fixation with an LCP plate and screws.
Discussion
The ACL is composed of AM and PL bundles, each with its own characteristics. Many surgeons try to reconstruct each bundle of the ACL separately. For more successful reconstruction of the ACL, the ideal outcome would be restoration of the anatomy of the ACL, which means functional restoration of the ACL to its native dimensions, collagen orientation, and insertion sites to achieve better stability [8]. Many different techniques have been suggested for anatomic ACLR using different tunnels positions, fixation systems, and types of graft [16, 28, 37, 39]. A number of studies have been conducted to compare postoperative stability and function after anatomic DB and SB ACLR. Some authors consider SB ACLR as the standard option to treat ACL lesions [5, 7, 17, 42], whereas others suggest the anatomic DB ACLR should improve pivot shift resistance and increase rotational knee control and should help preserve menisci and limit progression toward arthritis [8, 38, 46, 47, 49, 51]. Many studies, however, found no difference in terms of anteroposterior laxity, rotational stability, and/or any other clinical aspects at final followup between the two techniques [1, 4, 20, 23, 24, 30, 34, 43]. Furthermore, it remains unclear if one has clear long-term advantages over the other. We therefore asked whether patients having single- and double-bundle ACLR using ST alone differed with regard to (1) postoperative stability; (2) ROM; and (3) five functional scores.
Our study has some limitations. First, it was not possible to objectively evaluate the pivot shift test in our patients with specific instruments because these instruments are not freely available and accessible. We used the pivot shift test, which is a subjective test commonly used during clinical examination. We believe it would be important to compare patients with Grade 3 pivot shift treated with SB and DB reconstruction using specific instruments. Second, we did not include patients with complex instability because we believed it would introduce confounding variables such as medial-lateral collateral instability and posterior ligament insufficiency. Further studies are required to compare patients with complex instability treated with SB and DB reconstruction.
Our findings confirm those of several previous studies [1, 34, 43] reporting no difference between ACL-deficient patients treated either with single- or double-bundle ACLR regarding postoperative stability, pivot shift grade, varus-valgus limb morphology, and type of sport (Table 7). Recent meta-analyses also found no difference in the chance of having a normal pivot shift between single- and double-bundle ACL reconstruction [23, 24, 30]. Our data also showed no difference between the two groups regarding postoperative stability as assessed with the Rolimeter and pivot shift test (Table 6). Although double-bundle ACLR reportedly produces better intraoperative stability than SB ACLR [43], the two modalities are similar in terms of clinical aspects evaluated such as Lysholm and Tegner scores as well as postoperative stability after a minimum of 2 years of followup [43]. The AP laxity as measured with the Rolimeter was also similar in the two groups at 3 years; furthermore, for each group, we found no difference in laxity from 1 to 3 years of followup. Our observations confirm recent studies suggesting stability was achieved and remained for both groups [34, 43].
Even with theoretical advantages of DB ACLR, there will still be room for the anatomic SB technique with its less complex preparation of tunnels. An increasing arsenal in the sports surgeon’s hands must now lead us to create an improved algorithm in treating ACL complete tears: what technique, graft type, and fixation for a specific patient should be used? This should be answered by an algorithm [46]. We must always plan our surgery according to the type of patient we are presented with; for instance, a double-bundle ACLR may be more appropriate for an athlete of high-contact or impact sport but certainly not for a skeletally immature patient or a patient with important lateral femoral condyle bone bruise [8, 38, 46, 47, 49, 51]. Furthermore, we must also consider the anthropometric anatomy; thus, a thin light female would not be a good candidate for DB ACLR [13]. There is a considerable learning curve associated with DB ACLR. A recent study [18] demonstrated most European and American surgeons performing ACLR do less than 10 cases per year; should these surgeons be addressed about the DB technique? The failure rate is approximately 10% to 20% of all ACLRs; this rate might increase if all surgeons were to perform this new technique.
We found no differences in ACLR using SB or DB. Based on our findings and those in the literature, we suggest that at present, the surgeon should use the most anatomic technique for ACLR with less complexity, easier fixation, a least invasive revision technique, and minor graft harvesting morbidity. Surgeons should be aware of the reported incidence of hamstring weakness when using both the ST and the gracilis tendons for this type of reconstruction; therefore, we emphasize harvesting only the ST tendon for ACLR [12, 14, 31]. Today ACLR cannot be a fixed menu in the clinics of sports surgeons; rather, we advise a “menu a la carte” with many options to choose from, including regenerative therapy (stem cells); different graft sources, autografts, and allografts; and different fixations and a variety of techniques, which would not limit the surgeon in doing what is best for the patient. Further studies in the future might demonstrate if the DB technique could offer better stability and clinical outcome than SB, especially in patients with complex instability and greater transverse plane rotational knee stress demands.
References
Adachi N, Ochi M, Uchio Y, Iwasa J, Kuriwaka M, Ito Y. Reconstruction of the anterior cruciate ligament: single- versus double-bundle multistranded hamstring tendons. J Bone Joint Surg Br. 2004;86:515–520.
Amis AA, Dawkins GP. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br. 1991;73:260–267.
Balasch H, Schiller M, Friebel H, Hoffmann F. Evaluation of anterior knee joint instability with the Rolimeter. A test in comparison with manual assessment and measuring with the KT-1000 arthrometer. Knee Surg Sports Traumatol Arthrosc. 1999;7:204–208.
Calvisi V, Lupparelli S, Rinonapoli G, Padua R. Single-bundle versus double bundle arthroscopic reconstruction of the anterior cruciate ligament: what does the available evidence suggest? J Orthop Traumatol. 2007;8:95–100.
Chen CH, Chuang TY, Wang KC, Chen WJ, Shih CH. Arthroscopic anterior cruciate ligament reconstruction with quadriceps tendon autograft: clinical outcome in 4–7 years. Knee Surg Sports Traumatol Arthrosc. 2006;14:1077–1085.
Crawford C, Nyland J, Landes S, Jackson R, Chang HC, Nawab A, Caborn DN. Anatomic double bundle ACL reconstruction: a literature review. Knee Surg Sports Traumatol Arthrosc. 2007;15:946–964.
Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31:2–11.
Fu FH, Karlsson J. A long journey to be anatomic. Knee Surg Sports Traumatol Arthrosc. 2010;18:1151–1153.
Gadikota HR, Seon JK, Kozanek M, Oh LS, Gill TJ, Montgomery KD, Li G. Biomechanical comparison of single-tunnel-double-bundle and single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2009;37:962–969.
Ganko A, Engebretsen L, Ozer H. The Rolimeter: a new arthrometer compared with the KT-1000. Knee Surg Sports Traumatol Arthrosc. 2000;8:36–39.
Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216–231.
Gobbi A. Single versus double hamstring tendon harvest for ACL reconstruction. Sports Med Arthrosc. 2010;18:15–19.
Gobbi A, Domzalski M, Pascual J. Comparison of anterior cruciate ligament reconstruction in male and female athletes using the patellar tendon and hamstring autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12:534–539.
Gobbi A, Domzalski M, Pascual J, Zanazzo M. Hamstring anterior cruciate ligament reconstruction: is it necessary to sacrifice the gracilis? Arthroscopy. 2005;21:275–280.
Gobbi A, Francisco R. Factors affecting return to sports after anterior cruciate ligament reconstruction with patellar tendon and hamstring graft: a prospective clinical investigation. Knee Surg Sports Traumatol Arthrosc. 2006;14:1021–1028.
Gobbi A, Mahajan S, Tuy B, Panuncialman I. Hamstring graft tibial fixation: biomechanical properties of different linkage systems. Knee Surg Sports Traumatol Arthrosc. 2002;10:330–334.
Gobbi A, Mahajan S, Zanazzo M, Tuy B. Patellar tendon versus quadrupled bone-semitendinosus anterior cruciate ligament reconstruction: a prospective clinical investigation in athletes. Arthroscopy. 2003;19:592–601.
Harner CD, Poehling GG. Double bundle or double trouble? Arthroscopy. 2004;20:1013–1014.
Irrgang JJ, Anderson AF, Boland AL, et al.: Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med 2001;29:600–613.
Kanaya A, Ochi M, Deie M, Adachi N, Nishimori M, Nakamae A. Intraoperative evaluation of anteroposterior and rotational stabilities in anterior cruciate ligament reconstruction: lower femoral tunnel placed single-bundle versus double-bundle reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17:907–913.
Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical comparisons of knee stability after anterior cruciate ligament reconstruction between 2 clinically available transtibial procedures: anatomic double bundle versus single bundle. Am J Sports Med. 2010;38:1349–1358.
Lane C, Warren R, Pearle A. D. The pivot shift. J Am Acad Orthop Surg. 2008;16:679–688.
Lewis PB, Parameswaran AD, Rue JP, Bach BR Jr. Systematic review of single-bundle anterior cruciate ligament reconstruction outcomes: a baseline assessment for consideration of double-bundle techniques. Am J Sports Med. 2008;36:2028–2036.
Longo UG, King JB, Denaro V, Maffulli N. Double-bundle arthroscopic reconstruction of the anterior cruciate ligament: does the evidence add up? J Bone Joint Surg Br. 2008;90:995–999.
Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154.
Mae T, Shino K, Matsumoto N, Nakata K, Nakamura N, Iwahashi T. Force sharing between two grafts in the anatomical two-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:505–509.
Mae T, Shino K, Miyama T, Shinjo H, Ochi T, Yoshikawa H, Fujie H. Single- versus two-femoral socket anterior cruciate ligament reconstruction technique: biomechanical analysis using a robotic simulator. Arthroscopy. 2001;17:708–716.
Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament-reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38:912–917.
Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–218.
Meredick RB, Vance KJ, Appleby D, Lubowitz JH. Outcome of single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a meta-analysis. Am J Sports Med. 2008;36:1414–1421.
Nakamura N, Horibe S, Sasaki S, Kitaguchi T, Tagami M, Mitsuoka T, Toritsuka Y, Hamada M, Shino K. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2002;18:598–602.
National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Vanderbilt University, United States. Prognosis and Predictors of ACL Reconstruction—A Multicenter Cohort Study. Available at: http://clinicaltrials.gov/ct2/show/NCT00463099. Accessed March 1, 2011.
Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res. 1989;246:238–249.
Park SJ, Jung YB, Jung HJ, Jung HJ, Shin HK, Kim E, Song KS, Kim GS, Cheon HY, Kim S. Outcome of arthroscopic single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a preliminary 2-year prospective study. Arthroscopy. 2010;26:630–636.
Prodromos C, Brown C, Fu FH, Georgoulis A, Gobbi A, Howell S, Johnson D, Paulos L, Shelbourne K. The Anterior Cruciate Ligament Reconstruction and Basic Science. Philadelphia: Saunders Elsevier Publications; 2008:551–614.
Radford WJ, Amis AA. Biomechanics of a double prosthetic ligament in the anterior cruciate ligament. J Bone Joint Surg Br. 1990;72:1038–1043.
Reinhardt KR, Hetsroni I, Marx RG. Graft selection for anterior cruciate ligament reconstruction: a level I systematic review comparing failure rates and functional outcomes. Orthop Clin North Am. 2010;41:249–262.
Shen W, Jordan S, Fu F. Review article: anatomic double bundle anterior cruciate ligament reconstruction. J Orthop Surg (Hong Kong). 2007;15:216–221.
Shino K, Nakata K, Nakamura N, Toritsuka Y, Horibe S, Nakagawa S, Suzuki T. Rectangular tunnel double-bundle anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft to mimic natural fiber arrangement. Arthroscopy. 2008;24:1178–1183.
Siebold R, Cafaltzis K. Differentiation between intraoperative and postoperative bone tunnel widening and communication in double-bundle anterior cruciate ligament reconstruction: a prospective study. Arthroscopy. 2010;26:1066–1073.
Siebold R, Dehler C, Ellert T. Prospective randomized comparison of double-bundle versus single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:137–145.
Siebold R, Webster KE, Feller JA, Sutherland AG, Elliott J. Anterior cruciate ligament reconstruction in females: a comparison of hamstring tendon and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2006;14:1070–1076.
Song EK, Oh LS, Gill TJ, Li G, Gadikota HR, Seon JK. Prospective comparative study of anterior cruciate ligament reconstruction using the double-bundle and single-bundle techniques. Am J Sports Med. 2009;37:1705–1711.
Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49.
Torg JS, Conrad W, Kalen V. Clinical diagnosis of anterior cruciate ligament instability in the athlete. Am J Sports Med. 1976;4:84–93.
Van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy. 2010;26:258–268.
Van Eck CF, Schreiber VM, Mejia HA, Samuelsson K, van Dijk CN, Karlsson J, Fu FH. ‘Anatomic’ anterior cruciate ligament reconstruction: a systematic review of surgical techniques and reporting of surgical data. Arthroscopy. 2010;26(Suppl):S2–12.
Wright RW. Knee injury outcomes measures. J Am Acad Orthop Surg. 2009;17:31–39.
Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660–666.
Yasuda K, Kondo E, Ichiyama H, Kitamura N, Tanabe Y, Tohyama H, Minami A. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy. 2004;20:1015–1025.
Zelle BA, Brucker PU, Feng MT, Fu FH. Anatomical double-bundle anterior cruciate ligament reconstruction. Sports Med. 2006;36:99–108.
Acknowledgments
We thank Dr Celeste Scotti for evaluating all patients preoperatively and at 3, 6, 12, 24, and 36 months postoperatively. We also thank Dr Somanna Malchira for his help in preparing the manuscript. We also thank Andrea Primo, professional statistician, for statistical analysis of our study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Gobbi, A., Mahajan, V., Karnatzikos, G. et al. Single- versus Double-bundle ACL Reconstruction: Is There Any Difference in Stability and Function at 3-year Followup?. Clin Orthop Relat Res 470, 824–834 (2012). https://doi.org/10.1007/s11999-011-1940-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-1940-9