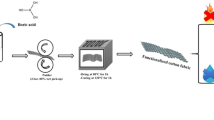
Abstract
Cotton (CO) fabrics with flame retardant properties based on ionic liquids: 1-methyl-3-(-((triethoxysilyl)oxy)propyl)-1H-imidazol-3-ium chloride (MCPTS) and 1-(3-(triethoxysilyl)propyl)pyridine-1-ium chloride (PCPTS) with boron from boric acid are successfully obtained via a sol-gel process. Fourier transform infrared spectroscopy (FTIR), optical microscopy analysis, and scanning electron microscopy (SEM) images, were first carried out to characterize the chemical composition and the morphology of the treated and untreated cotton fabrics, respectively. The investigation of flame resistance was evaluated by the vertical burning test. It was observed that the treated cotton fabrics exhibited good flame-retardant properties and did not burn even after 10 s flame application duration and the rate of flame spread was inhibited compared to the pristine cotton fabric. Furthermore, the thermal comportment of cotton fabrics was analyzed by thermogravimetric (TG) analysis and differential scanning calorimeter (DSC). Moreover, the tensile strength of the treated textiles is mostly reserved. In this work, we prove that the sol-gel method using ionic liquids and boron could be used as an effective flame retardant to develop finishing cotton fabrics for textile fireproof applications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Even as diverse novel fibers have appeared in the textile market, cotton fibers remain the most extensively used, thanks to their superb properties, namely: breathability, softness, comfort and wide availability. All these benefits make cotton material essential in various fields such as clothing, automotive, and decorations.1 Recently, several cotton products have appeared by adding some functionalities for example electrical conduction,2,3,4,5 oil-water separation6,7,8 water repellency,5,9,10,11,12,13 and antimicrobial finishing.14,15,16 However, cotton is more combustible and its inflammability is one of its largest drawbacks. It ignites easily, is less thermally stable, and degrades during ignition with the formation of highly combustible volatile compounds, mainly levoglucosan, with the spread of fire causing injury and death in fire accidents. Thus, the demand for flame retardant cotton fabrics has grown rapidly and has become a fundamental requirement for the production of high-performance and fire protection textile products. Therefore many researchers have been focused on the development of flame retardant treatment,17,18,19,20,21,22,23 for example, flame-retardant finishing on cotton fabric by deposition of a polyethyleneimine, ammonium polyphosphate, and fluorinated-octyl polyhedral oligomeric silsesquioxane,24 the deposition of phosphorus-rich hybrid organic-inorganic silica coating25 phosphorus, nitrogen and silicon,20 TiO2 nanoparticles and chitosan phosphate26 and the sol system containing silica, boron, and nitrogen.17,27 These flame retardants can react in two modes of action: chemical and physical action.28 Concerning the chemical reactions, the flame retardant interferes with the gas phase and condensed combustion processes. In the gas phase, the flame retardants inhibit radical reactions (trapping the H· and HO· radicals) responsible for flame propagation and are responsible for slowing down the combustion reaction and reducing the heat released. In the condensed phase, flame retardants promote the formation of a carbonaceous layer (char) that insulates the material from flame and oxygen and slows down the emission of flammable gases resulting from the degradation of the textile material (barrier effect).29 The ionic liquids get the increasing attention of researchers.30,31 They are defined as salts with melting points under 100°C. They are useful in different applications namely: as electro-deposition of metals32 and as solvents for polymerization methods.33 Additionally, in the textile domain, ionic liquids are employed in the process of removing textile dyes from aqueous solutions,34,35 improving antifungal activity for linen fabric36 and as flame retardants for cotton fabric. In addition, the ionic liquids have a fireproof performance due to a dilution of combustible gases by the emission of degradation products with the endothermic effect.37 In this regards, Xu et al.38,39 studied the use of ionic liquid derivatives of hydroxymethylimidazolium and the phosphinates such as 1-methyl-3- (oxiran-2-ylmethyl)-1H-imidazol-3-ium dimethyl phosphinate and dimethyl phosphinate of 1-allyl-3-methylimidazolium in the flame retardant finish of cotton fabrics. Recently, Boukhriss et al.40 developed a new eco-friendly finishing method which consists of treating cotton fabrics with 1-methyl-3-chloride (triethoxysilylpropyl) imidazolium and 1-methyl-3 chloride (triethoxysilylpropyl) pyridinium. The treated fabrics have shown effective flame retardant properties. With the same finishing method, Bentis et al.37 developed a flame retardant applied to cotton fabrics based on methylimidazolium and pyridinium cations combined with Cl–, PF6–, (CF3SO2)2N–, BF4–, and CH3CO2–.
Currently, various techniques of functionalization of cotton textile have been used such as electrostatic layer by layer assembly,41 surface grafting,42 and sol-gel.43,44 The latter method is carried out at a low temperature, considered as an evolving flexible surface modification, which can generate a hybrid material with homogeneity dispersion on the fabric’s surface. In this context, Hribernik et al.45 have used tetraethylorthosilicate (TEOS) coatings to reduce the flammability of viscose fibers. Continuing this research, Malucelli23 and colleagues studied the fire resistance and the burning behavior of cotton, polyester, and cotton/polyester blends treated with the same TEOS coating. Other work has been designed for the preparation of hybrid flame retardants by the sol-gel method. Kappes et al.46 designed a new coating formulation derived from sol-gel, based on the combination of (3-trimethoxysilylpropyl) diethylenetriamine and phenyl phosphonic acid, to have flame-retardant characteristics for different fabrics. Recent work has successfully incorporated nitrogen and phosphorus into a siloxane precursor for flame retardant sol-gel coatings.47,48
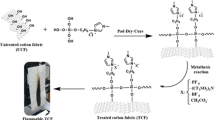
In this work, we have prepared a mixture solution based on ionic liquids and boric acid. For this purpose, 1-methyl-3-(-((triethoxysilyl)oxy)propyl)-1H-imidazol-3-ium chloride (MCPTS) and 1-(3-(triethoxysilyl)propyl)pyridine-1-ium chloride (PCPTS) were firstly synthesized. Then, the sol-gel reaction was achieved by adding boric acid on the MCPTS and PCPTS separately. The coating on the cotton fabric surface was carried out using the pad-dry-cure process. The treated samples were characterized by Fourier transform infrared spectroscopy (FTIR). After that, optical microscopy analysis and scanning electron microscopy (SEM) was carried out. The burning test by vertical flame test was performed to evaluate the flame retardancy of untreated and treated samples. The thermal stability was evaluated by thermogravimetric analysis (TGA) and differential scanning calorimeter (DSC). Finally, the mechanical test was performed to assess the tensile strength of all samples.
Experimental
Material
Pure cotton fabric weighing 122.7 g.m–2 was used. The following reagents were obtained from Sigma-Aldrich: 1-methylimidazole (99%), chloropropyltriethoxysilane (CPTS), pyridine (99%), ethanol (99%), and boric acid (H3BO3, 99.5%). All reagents were analytically pure.
Synthesis of MCPTS and PCPTS salts
The preparation of the salts was achieved according to our previous work.40 In the round-bottom flask, one eq 1-methylimidazole or pyridine and one eq chloropropyltriethoxysilane (CPTS) were added. The mixed product was fitted to a reflux condenser for 18 h at 100–115°C to obtain 1-methyl-3-(-((triethoxysilyl)oxy)propyl)-1H-imidazol-3-ium chloride (MCPTS) and 1-(3-(triethoxysilyl)propyl)pyridine-1-ium chloride (PCPTS).
Sols synthesis based on MCPTS doped boron
To achieve the insertion of boron in the silica system. The MCPTS and PCPTS were utilized as precursors, and the boric acid was added to mixture compounds according to the sol-gel reaction (Scheme 1).
Treatment of cotton fabric
The treatment of CO was achieved by the sol-gel process using MCPTS and PCPTS salts. At first, PCPTS or MCPTS, H3BO3, distilled water, and EtOH were mixed respecting a molar ratio of 5/2.5/55/60 (PCPTS or MCPTS/ H3BO3/EtOH/H2O), and the mixture was stirred for 3 h at 70°C until a homogeneous solution was achieved. After this, the CO samples were impregnated in this solution followed by padding. The obtained samples were dried at 80 and 120°C for 1 h each the samples’ codes were given in Table 1.
FTIR analysis
Fourier transform infrared (FTIR) spectra of the studied samples were recorded from 4000 to 400 cm–1 with a 4 cm–1 resolution along with an accumulation of 32 scans, using Nicolet IS10 FTIR-ATR spectrophotometer associated with the golden gate single attenuated total reflection (ATR) accessory.
Morphological analysis
The morphology of samples was observed by SEM Hirox SH-4000M microscope with an acceleration voltage of 15 kV. The samples were mounted on a conductive adhesive tape and coated with carbon before testing to make the textile material conductive.
Vertical flame test
The vertical combustion test of the pristine and treated cotton was carried out according to ISO 6940:2004 standard. A butane flame of 4 cm was applied at the bottom of the fabric samples for 10 s. At the end of the test, the burning time and residue weight was determined for each sample. The rate of flame spread was calculated as reported in the following49,50
where X: is flame application duration (s).
Thermogravimetric analysis
The thermal stability of the textile samples was evaluated by Versa Therm. The approximate weight of 10 mg was placed in a quartz crucible. Thermogravimetric (TG) analysis was performed in the temperature range of 25–600°C under air (25 mL/min) with a heating rate of 10°C/min.
Differential scanning calorimeter (DSC)
The differential scanning calorimeter (DSC) experiments were achieved with differential scanning calorimetry (SETRAM DSC Evo 131) instruments at a heating rate of 10°C/min. For this, 20 mg of each sample was analyzed under a nitrogen atmosphere (30 mL/min). The runs were performed over a temperature range from 40 to 600°C.
Mechanical properties measurements
Fabric tensile and strain measurements of the pristine and treated cotton fabrics were tested using an electronic fabric tensile tester (LUDWIC mpk) referring to the ISO 13934-1:2013 standard. The results given are the average of the triple test with their standard deviation.
The washing test
The washing fastness of the cotton fibers was carried out to ISO 105-C06:2010. The treated samples were immersed in 150 mL of a phosphate ECE (European Colour Fastness Establishment) detergent solution with a pH = 9.7. The test was achieved for 30 min at 40°C.51
Results and discussion
FTIR analysis
The chemical composition of the untreated and treated cotton fabrics was assessed by FTIR analyses as presented in the spectra in Fig. 1.
For untreated cotton fabrics, the feature peaks of cellulose have occurred. The wideband around 3300 cm−1 is attributed to the vibration of the O–H groups linked by hydrogen bonds. The C–H vibration has occurred at 2900 cm–1. The C–O–C bond vibration of the cellulosic structure is observed in the region 1200–1000 cm−1.51
The bands attributed to Si–O–Si are located at 1000 and 1110 cm–1 for Si–O–C.37 The peaks occurring at 1430 and 920 cm–1 are assigned to B–O and Si–O–B, respectively,27 which shows that the insertion of boron in the silica network was well achieved. The peak region 1700–1490 cm–1 corresponds to the amino groups given by the ionic liquids precursors.52
Morphological analysis
To investigate the surface morphologies of CO, CO–[MCPTS]–B and CO–[PCPTS]–B the SEM observations were carried out. As shown in Fig. 2a, the CO sample presents irregularity levels due to natural growth. All the treated samples reveal a continuous hybrid film.
SEM images (Figs. 2d and 2e) show that the residue maintains the shape of the original fibers and its weave structure, and many bubbles were observed on the surface of the fiber: these findings are attributed to the intumescent effect and expansion of CO modified. It is possible to see big bubbles on the surfaces of the burned layer of flame-retardant CO textile. These bubbles prevent the release of flammable gases from the degradation of the cellulosic material, and also the transmission of heat to the substrate. The intumescent keeps the fibers from extra burning, preserving the structure of the fibers, which is responsible for the self-extinguishing of the flame observed in a vertical burning test of cotton.20
Vertical flame test
The vertical flame test was carried out to investigate the impact of the sol-gel treatment on the cotton fabrics. Table 2 lists the flame retardant test results of CO fabrics treated with the ionic liquids and boron prepared via the sol-gel process. Figure 3 shows the images taken after the flammability test for untreated and treated cotton textiles. The obtained results indicate that the untreated cotton fabrics burn quickly for 18 s after the flame exclusion with a residue of 0.5% and the surface was burned totally. For the CO–[MCPTS]–B and CO–[MCPTS]–B, they remained a char length of 5 cm and 6 cm. Contrarily, the pristine CO fabrics were completely burned. In addition to the char length, from Table 2, the rate of flame spread was decreased for CO–[MCPTS]–B compared with CO–[PCPTS]–B. Concerning the treated samples, their flammability is lower than that of the control cotton as illustrated in Figs. 3a–3c. CO–[MCPTS]–B and CO–[PCPTS]–B did not entirely ignite after the flame was eliminated remaining a final residue of 97.5% and 82.4% respectively. The flame retardant based on the combination of Si, N generated by organosilicon ionic liquids and boron-based flame retardant generated by boric acid can lead to synergy effects.
The results demonstrate that the solution system, containing boron according to the synergistic effect of Si–O–B, shows an effective flame retardancy.22,53
Thermogravimetric analysis
The thermal stability of cotton fabric finishing by MCPTS and PCPTS containing boron was studied by thermogravimetric analysis, which is a crucial test to evaluate thermal degradation.23 The mechanism of stability was investigated by measuring the percentage mass loss in the variant temperature.18,54 Figure 4 illustrates the TG and DTG curves of control cotton and treated cotton fabric, while Table 3 presents the thermogravimetric information. T (20%) is the temperature needed to lose 20% of initial mass, and Tmax(°C) is the temperature corresponding to the maximum mass loss. The residue at 600°C is the percentage of the mass found at the end of TG analysis which allows us to judge thermal stability and the resistivity of samples against the high applicable temperature.
From Fig. 4, the degradation of CO has been assessed at 246.1°C which occurred the maximum of mass loss.55 The second step of CO fabric thermal degradation (depolymerization), was observed at 372.1°C, due to the high flammable behavior of control cotton. According to the curves presented in Fig. 4 the thermal stability of the treated samples is much more important than untreated which is confirmed by the results shown in Table 3 with the CO–[MCPTS]–B and CO–[PCPTS]–B showing a T (20%) at 235.6 and 222.2°C. However, the recorded temperature for untreated (CO) at T(20%) was 238.2°C. The residues at Tmax1 and Tmax2 were 94.2%, 72% and 80.3%, 23.5% respectively. Although the residue indicated at Tmax1 and Tmax2 for CO fabrics is of 60.3% and 8.4%.
It can be observed that the first rapid weight loss temperature of the finished cotton fabric CO–[MCPTS]–B and CO–[PCPTS]–B is at 101.7 and 232.5°C. It can be explained by the dehydration reaction of boric acid on the treated cotton fabric.56 Moreover, at 600°C the residue of CO–[MCPTS]–B and CO–[PCPTS]–B is higher (37.2% and 16.18 %) compared to CO fabrics 3.4%, which demonstrates that the treatment has a good impact on thermal stability. The results of TG analysis prove that the hybrid mixture using MCPTS and PCPTS as ionic liquids containing boron has flame barring, and it promotes the formation of carbonization during the combustion process thanks to the coating based on ionic liquids combined boron. The total thermal degradation of untreated cotton fabrics was terminated at 400°C, while in the treated samples, the thermal degradation was reached about 600°C.
Differential scanning calorimeter (DSC)
The differential scanning calorimeter of cotton fabric treated by MCPTS and PCPTS containing boron was studied and presented in Fig. 5.
DSC curves obtained in Fig. 5 show that all samples have a dehydration phenomenon with an endothermic peak around 100 and 160°C. As reported in Table 4, ΔH is about 155.19, 125.48, and 113.08 J/g for CO, CO–[MCPTS]–B and CO–[PCPTS]–B. The intensity of this peak and their temperature increased from untreated CO to treated CO fabrics due to the impact of the treatment applied.
Concerning the modified cotton fabric, an exothermic peak occurred about 250°C. These peaks are probably due to a high temperature of crosslinking reaction. The decomposition of cotton leading to the formation of levoglucosan was recorded at 380°C.
The results already mentioned in the TG analysis are in agreement with the DSC tests that the boron improves the creation of the thermal barrier with ionic liquids and favors the intumescence phenomenon.
Mechanical properties measurements
The tensile strength test is one of the crucial physical parameters to control the performance of cotton fabric after treatment.57 Herein, the mechanical analyses were carried out to CO and treated CO fabrics with a sol-gel process based on ionic liquids containing boron. The curves plotted of the tensile strength as a function of the strain of untreated and treated CO fabrics shown in Fig. 6a prove that the various curves are practically the same. As well as presented, the maximum value of tensile strength for CO, CO–[MCPTS]–B and CO–[PCPTS]–B (Fig. 6b) did not change radically. These results are in agreement with the literature.58
The washing test
The obtained properties for treated samples were investigated after the washing fastness. The results reveal that the functionalized fibers burned entirely after five washing cycles.
Conclusions
A flame retardant cotton fabric was developed through a sol-gel method using ionic liquids MCPTS and PCPTS containing boron. The FTIR, optical microscopy and SEM analysis prove that the deposition of the targeted coating on the surface of cotton fabrics was well performed. The vertical flame test demonstrates that finishing cotton fabrics have the best flame retardant properties. The TG and DSC analyses also proved that the treated samples present thermal stability. Finally, the mechanical analysis indicates that the coated samples by the sol-gel method using ionic liquids containing boron do not affect their mechanical properties. Because of the excellent flame retardant function based on ionic liquid containing boron, the method presented in this work is believed to have favorable potential in textile industries.
Data availability
Not applicable.
References
Khanzada, H, Khan, MQ, Kayani, S, “Cotton Based Clothing.” In: Wang, H, Memon, H (eds.) Cotton Science and Processing Technology: Gene, Ginning, Garment and Green Recycling, pp. 377–391. Springer, Singapore (2020)
Molina, J, “Graphene-Based Fabrics and their Applications: A Review.” RSC Adv., 6 68261–68291 (2016)
Yetisen, AK, Qu, H, Manbachi, A, Butt, H, Dokmeci, MR, Hinestroza, JP, Skorobogatiy, M, Khademhosseini, A, Yun, SH, “Nanotechnology in Textiles.” ACS Nano, 10 3042–3068 (2016)
Hansora, DP, Shimpi, NG, Mishra, S, “Performance of Hybrid Nanostructured Conductive Cotton Materials as Wearable Devices: An Overview of Materials, Fabrication, Properties and Applications.” RSC Adv., 5 107716–107770 (2015)
Zhou, X, Zhang, Z, Xu, X, Guo, F, Zhu, X, Men, X, Ge, B, “Robust and Durable Superhydrophobic Cotton Fabrics for Oil/Water Separation.” ACS Appl. Mater. Interfaces, 5 7208–7214 (2013)
Cheng, Q-Y, Guan, C-S, Wang, M, Li, Y-D, Zeng, J-B, “Cellulose Nanocrystal Coated Cotton Fabric with Superhydrophobicity for Efficient Oil/Water Separation.” Carbohydr. Polym., 199 390–396 (2018)
Lei, S, Shi, Z, Ou, J, Wang, F, Xue, M, Li, W, Qiao, G, Guan, X, Zhang, J, “Durable Superhydrophobic Cotton Fabric for Oil/Water Separation.” Colloids Surf. Physicochem. Eng. Asp., 533 249–254 (2017)
Li, Y, Feng, Z, He, Y, Fan, Y, Ma, J, Yin, X, “Facile Way in Fabricating a Cotton Fabric Membrane for Switchable Oil/Water Separation and Water Purification.” Appl. Surf. Sci., 441 500–507 (2018)
Xu, J, Jiang, S, Peng, L, Wang, Y, Shang, S, Miao, D, Guo, R, “AgNps-PVA–Coated Woven Cotton Fabric: Preparation, Water Repellency, Shielding Properties and Antibacterial Activity.” J. Ind. Text., 48 1545–1565 (2019)
Ma, Y, Zhu, D, Si, Y, Sun, G, “Fabricating Durable, Fluoride-Free, Water Repellency Cotton Fabrics with CPDMS.” J. Appl. Polym. Sci., 135 46396 (2018)
Liu, L, Huang, Z, Pan, Y, Wang, X, Song, L, Hu, Y, “Finishing of Cotton Fabrics by Multi-Layered Coatings to Improve their Flame Retardancy and Water Repellency.” Cellulose, 25 4791–4803 (2018)
El Messoudi, M, Boukhriss, A, Cherkaoui, O, El Kouali, M, Gmouh, S, “Adsorption–Desorption Kinetics of Silica Coated on Textile Fabrics by the Sol–Gel Process.” J. Coat. Technol. Res, 17 371–380 (2019)
Boukhriss, A, Boyer, D, Hannache, H, Roblin, J-P, Mahiou, R, Cherkaoui, O, Therias, S, Gmouh, S, “Sol–Gel Based Water Repellent Coatings for Textiles.” Cellulose, 22 1415–1425 (2015)
Xu, Q, Wu, Y, Zhang, Y, Fu, F, Liu, X, “Durable Antibacterial Cotton Modified by Silver Nanoparticles and Chitosan Derivative Binder.” Fibers Polym., 17 1782–1789 (2016)
Zhang, Y, Xu, Q, Fu, F, Liu, X, “Durable Antimicrobial Cotton Textiles Modified with Inorganic Nanoparticles.” Cellulose, 23 2791–2808 (2016)
Xu, Q, Ke, X, Cai, D, Zhang, Y, Fu, F, Endo, T, Liu, X, “Silver-Based, Single-Sided Antibacterial Cotton Fabrics with Improved Durability via an l-Cysteine Binding Effect.” Cellulose, 25 2129–2141 (2018)
Chan, SY, Si, L, Lee, KI, Ng, PF, Chen, L, Yu, B, Hu, Y, Yuen, RKK, Xin, JH, Fei, B, “A Novel Boron–Nitrogen Intumescent Flame Retardant Coating on Cotton with Improved Washing Durability.” Cellulose, 25 843–857 (2018)
Singh, MK, Zafar, S, “Disulfide-Containing Polyamidoamines with Remarkable Flame Retardant Activity for Cotton Fabrics.” Polym. Degrad. Stab., 156 1–13 (2018)
Nie, S, Jin, D, Yang, J, Dai, G, Luo, Y, “Fabrication of Environmentally-Benign Flame Retardant Cotton Fabrics with Hydrophobicity by a Facile Chemical Modification.” Cellulose, 26 5147–5158 (2019)
Castellano, A, Colleoni, C, Iacono, G, Mezzi, A, Plutino, MR, Malucelli, G, Rosace, G, “Synthesis and Characterization of a Phosphorous/Nitrogen Based Sol-Gel Coating as a Novel Halogen-and Formaldehyde-Free Flame Retardant Finishing for Cotton Fabric.” Polym. Degrad. Stab., 162 148–159 (2019)
Rosace, G, Castellano, A, Trovato, V, Iacono, G, Malucelli, G, “Thermal and Flame Retardant Behaviour of Cotton Fabrics Treated with a Novel Nitrogen-Containing Carboxyl-Functionalized Organophosphorus System.” Carbohydr. Polym., 196 348–358 (2018)
Zhang, Q, Gu, J, Chen, G, Xing, T, “Durable Flame Retardant Finish for Silk Fabric Using Boron Hybrid Silica Sol.” Appl. Surf. Sci., 387 446–453 (2016)
Alongi, J, Ciobanu, M, Tata, J, Carosio, F, Malucelli, GJ, “Thermal Stability and Flame Retardancy of Polyester, Cotton, and Relative Blend Textile Fabrics Subjected to Sol–Gel Treatments.” J. Appl. Polym. Sci., 119 1961–1969 (2011)
Chen, S, Li, X, Li, Y, Sun, J, “Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric.” ACS Nano, 9 4070–4076 (2015)
El Messoudi, M, Boukhriss, A, Sadallah, L, Sajid, L, El Kouali, M, Gmouh, S, “Deposition of Phosphate Nanoparticles onto Textile Fabrics via Sol-gel Method and Their Kinetics Desorption Studies.” Chem. Res. Chin. Univ., 36 877–884 (2020)
El-Shafei, A, ElShemy, M, Abou-Okeil, A, “Eco-Friendly Finishing Agent for Cotton Fabrics to Improve Flame Retardant and Antibacterial Properties.” Carbohydr. Polym., 118 83–90 (2015)
Zhang, Q, Chen, G, Xing, T, “Silk Flame Retardant Finish by Ternary Silica Sol Containing Boron and Nitrogen.” Appl. Surf. Sci., 421 52–60 (2017)
Nine, MJ, Tran, DNH, Tung, TT, Kabiri, S, Losic, D, “Graphene-Borate as an Efficient Fire Retardant for Cellulosic Materials with Multiple and Synergetic Modes of Action.” ACS Appl. Mater. Interfaces, 9 10160–10168 (2017)
Salmeia, K, Gaan, S, Malucelli, G, “Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry.” Polymers, 8 319 (2016)
Hejazifar, M, Lanaridi, O, Bica-Schröder, K, “Ionic Liquid Based Microemulsions: A Review.” J. Mol. Liq., 303 112264 (2020)
Yavir, K, Marcinkowski, Ł, Marcinkowska, R, Namieśnik, J, Kloskowski, A, “Analytical Applications and Physicochemical Properties of Ionic Liquid-Based Hybrid Materials: A Review.” Anal. Chim. Acta, 1054 1–16 (2019)
Abbott, AP, McKenzie, KJ, “Application of Ionic Liquids to the Electrodeposition of Metals.” Phys. Chem. Chem. Phys., 8 4265–4279 (2006)
Kubisa, P, “Application of Ionic Liquids as Solvents for Polymerization Processes.” Prog. Polym. Sci., 29 3–12 (2004)
Rizzo, C, Marullo, S, Campodonico, PR, Pibiri, I, Dintcheva, N. Tz., Noto, R, Millan, D, D’Anna, F, “Self-Sustaining Supramolecular Ionic Liquid Gels for Dye Adsorption.” ACS Sustain Chem. Eng., 6 12453–12462 (2018)
Ren, Y, Guo, J, Lu, Q, Xu, D, Qin, J, Yan, F, “Polypropylene Nonwoven Fabric@Poly(ionic liquid)s for Switchable Oil/Water Separation, Dye Absorption, and Antibacterial Applications.” ChemSusChem, 11 1092–1098 (2018)
Foksowicz-Flaczyk, J, Walentowska, J, “Antifungal Activity of Ionic Liquid Applied to Linen Fabric.” Int. Biodeterior. Biodegrad., 84 412–415 (2013)
Bentis, A, Boukhriss, A, Grancaric, AM, El Bouchti, M, El Achaby, M, Gmouh, S, “Flammability and Combustion Behavior of Cotton Fabrics Treated by the Sol Gel Method Using Ionic Liquids Combined with Different Anions.” Cellulose, 26 2139–2153 (2019)
Xu, Y, Ionic Liquid Flame Retardant. U.S. Patent, US20110073331A1 (2011)
Xu, Y, Phosphinate Ionic Liquid Compositions and Methods of Use. World Intellectual Property Organization, WO2014120488A1 (2014)
Boukhriss, A, Gmouh, S, Hannach, H, Roblin, J-P, Cherkaoui, O, Boyer, D, “Treatment of Cotton Fabrics by Ionic Liquid with PF6 − Anion for Enhancing their Flame Retardancy and Water Repellency.” Cellulose, 23 3355–3364 (2016)
Li, S, Ding, F, Lin, X, Li, Z, Ren, X, “Layer-by-Layer Self-Assembly of Organic-Inorganic Hybrid Intumescent Flame Retardant on Cotton Fabrics.” Fibers Polym., 20 538–544 (2019)
Jiang, P, Zhang, S, Bourbigot, S, Chen, Z, Duquesne, S, Casetta, M, “Surface Grafting of Sepiolite with a Phosphaphenanthrene Derivative and its Flame-Retardant Mechanism on PLA Nanocomposites.” Polym. Degrad. Stab., 165 68–79 (2019)
Lin, D, Zeng, X, Li, H, Lai, X, Wu, T, “One-Pot Fabrication of Superhydrophobic and Flame-Retardant Coatings on Cotton Fabrics via Sol-Gel Reaction.” J. Colloid Interface Sci., 533 198–206 (2019)
Boukhriss, A, El messoudi, M, Roblin, J-P, Aaboub, T, Boyer, D, Gmouh, S, “Luminescent Hybrid Coatings Prepared by a Sol–Gel Process for a Textile-Based pH Sensor.” Mater. Adv., 1 918–925 (2020)
Hribernik, S, Smole, MS, Kleinschek, KS, Bele, M, Jamnik, J, Gaberscek, M, “Flame Retardant Activity of SiO2-Coated Regenerated Cellulose Fibres.” Polym. Degrad. Stab., 92 1957–1965 (2007)
Kappes, RS, Urbainczyk, T, Artz, U, Textor, T, Gutmann, JS, “Flame Retardants Based on Amino Silanes and Phenylphosphonic Acid.” Polym. Degrad. Stab., 129 168–179 (2016)
Brancatelli, G, Colleoni, C, Massafra, MR, Rosace, G, “Effect of Hybrid Phosphorus-Doped Silica Thin Films Produced by Sol-Gel Method on the Thermal Behavior of Cotton Fabrics.” Polym. Degrad. Stab., 96 483–490 (2011)
Vasiljević, J, Hadžić, S, Jerman, I, Černe, L, Tomšič, B, Medved, J, Godec, M, Orel, B, Simončič, B, “Study of Flame-Retardant Finishing of Cellulose Fibres: Organic–Inorganic Hybrid Versus Conventional Organophosphonate.” Polym. Degrad. Stab., 98 2602–2608 (2013)
Zhao, B, Liu, Y-T, Zhang, C-Y, Liu, D-Y, Li, F, Liu, Y-Q, “A Novel Phosphoramidate and its Application on Cotton Fabrics: Synthesis, Flammability and Thermal Degradation.” J. Anal. Appl. Pyrolysis, 125 109–116 (2017)
Muralidhara, KS, Sreenivasan, S, “Thermal Degradation and Burning Behaviour of Cellulose Based and Cellulose-Silk Blended Upholstery Fabrics.” JSIR, 69 879–885 (2010)
Bentis, A, Boukhriss, A, Gmouh, S, “Flame-Retardant and Water-Repellent Coating on Cotton Fabric by Titania–Boron Sol–Gel Method.” J. Sol-Gel Sci. Technol., 94 719–730 (2020)
Tseng, DY, Edelman, ER, “Flame Retardance and Thermal Stability of Wool Fabric Treated by Boron Containing Silica Sols.” J. Biomed. Mater. Res., 42 188–198 (1998)
Zhang, Q, Zhang, W, Huang, J, Lai, Y, Xing, T, Chen, G, Jin, W, Liu, H, Sun, B, “Flame Retardance and Thermal Stability of Wool Fabric Treated by Boron Containing Silica Sols.” Mater. Des., 85 796–799 (2015)
Ali, SM, “Synthesis, Characterization and Applications of Nano-Structured Sol-Gel Coatings.” In: Saleem, H, Imtiaz, AC (eds.) Encyclopedia of Renewable and Sustainable Materials, pp. 596–604. Elsevier, Amsterdam (2020)
Dahiya, JB, “Flame Retardant Coating on Cotton Fabric with Phosphorus Containing Polymeric Film by Admicellar Polymerization.” Indian J. Fibre Text Res., 43 457–463 (2018)
Xie, K, Gao, A, Zhang, Y, “Flame Retardant Finishing of Cotton Fabric Based on Synergistic Compounds Containing Boron and Nitrogen.” Carbohydr. Polym., 98 706–710 (2013)
Plutino, MR, Colleoni, C, Donelli, I, Freddi, G, Guido, E, Maschi, O, Mezzi, A, Rosace, G, “Sol-gel 3 -Glycidoxypropyltriethoxysilane Finishing on Different Fabrics: The Role of Precursor Concentration and Catalyst on the Textile Performances and Cytotoxic Activity.” J. Colloid Interface Sci., 506 504–517 (2017)
Colleoni, C, Donelli, I, Freddi, G, Guido, E, Migani, V, Rosace, G, “A Novel Sol-Gel Multi-Layer Approach for Cotton Fabric Finishing by Tetraethoxysilane Precursor.” Surf. Coat. Technol., 235 192–203 (2013)
Acknowledgments
The authors are grateful to the Higher School of Textiles and Clothing Industries (ESITH) for supporting this work. This work is done through the support of the CNRST under the Program Research Excellence Scholarships.
Author information
Authors and Affiliations
Contributions
Mohamed El Messoudi: Conceptualization, Methodology, Formal analysis, Writing - original draft. Aicha Boukhriss: Resources, Writing - review & editing. Aziz Bendis: Visualization, Writing - review & editing. Mehdi El Bouchti: Investigation, Software, Resources. Mohamed Ait Chaoui: Resources, Data curation, review & editing. Mohammed El Kouali: Conceptualization, Writing - review & editing. Said Gmouh: Supervision, Project administration, Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Not applicable.
Consent for publication
All authors agree to this publication.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Messoudi, M., Boukhriss, A., Bentis, A. et al. Flame retardant finishing of cotton fabric based on ionic liquid compounds containing boron prepared with the sol-gel method. J Coat Technol Res 19, 1609–1619 (2022). https://doi.org/10.1007/s11998-022-00633-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00633-x