Abstract
This work aims to improve the thermal stability and flame retardancy of cotton fabrics treated just by a single SiO2, a single B2O3 and their binary composite sol–gel systems. Tetraethyl orthosilicate (TEOS) and tributyl borate were used as precursors. The sols were coated onto cotton fabrics via dipping-baking processes. Techniques including Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDS), thermogravimetric analysis (TGA), limiting oxygen index (LOI) and vertical burning test (VBT) were used to investigate the surface functional groups, elemental compositions, crystal structures, surface morphologies, elemental contents, thermal stability and flame retardancy levels of original and sols-treated cotton fabrics, respectively. The results show that the sols converted gel coatings are successfully deposited on the cotton fabric surfaces. SiO2-B2O3 sol finishing cotton fabric shows the best flame retardancy. It shows the highest char residue rate (43.82%), the highest LOI (25.7%) and ΔLOI/Δm (7.0%/g), the lowest after-flame time (10.0 s) and after-glow time (0.0 s without a smoldering process), a relatively complete and continuous char residue after a VBT. The SiO2 and B2O3 sol–gel systems play more synergistic effects than competitive effects in flame retarding a cotton fabric. A SiO2 gel shows effects of a high melting point, a structural support and dimensional stability, while a B2O3 gel shows a fusing capability and a longer pyrolysis process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most important natural textiles, cotton fabrics play vital roles in our daily lives. Due to their excellent inherent properties, such as comfort, softness, hydrophilicity, hygroscopicity and biodegradability, they are widely used in interior decorations, clothing productions, medical treatments, furniture and bedding end products [1, 2].
Nevertheless, cotton, as a natural cellulose-based fiber polymer material, has a low LOI value of usually 18.0% and a combustion temperature of 360.0–425.0 °C [3, 4]. Once ignited, it will burn rapidly and may cause fatal burns within 15 s after ignition [4]. Therefore, it is particularly important to impart flame retardancy capabilities to cotton fabrics.
Historically and presently, a variety of methods have been explored for flame retardant finishes (after-treatments) of cotton and other cellulose fabrics (like flax, viscose rayon, blends with cellulose fibers ≥ 50 wt%) [5], such as durable finishes [5], semi-durable treatments [5], back coating treatments [5], sol–gel treatments [6], ultraviolet curing [7], plasma deposition [8] and layer-by-layer (LBL) assembly [9], etc. As for commercialization, multiple factors need to be considered for successful flame retardant finishes including fabric constructions, fiber types, expected service performances (like durability to laundering, strength, abrasion resistance, dry cleaning, dyeability, hand-feel) [5], detrimental effects on fabrics and environment, manufacturing costs. Hereby, among the above methods, durable finishes [5] have been commercially realized based on N-methylol dialkyl phosphonopropionamides (trademark of Pyrovatex®), also tetrakis (hydroxymethyl) phosphonium chloride (THPC)/urea chemistry (trademarks of Proban® and Perform®). Semi-durable treatments [5] have been applied by pad-dry-cure processing based on ammonium polyphosphate and related soluble phosphorus-containing salts. Back-coating treatments [5] have been applied in resin matrixes based typically on organobromine/antimony trioxide formulations. Some nano-sized inorganic fillers like silica (silicon dioxide) prepared via a sol–gel process have not been commercially successful since they need conventional flame retardants as additives [10]. Ultrasonic cavitation (ultrasound technology) has been used in wet processes as a chemical processing technique of textiles, while it is not commercially that common in the textile finishing industry [11]. Plasma processing has been applied in textile pre-treatments on a commercial scale [12]. LBL assemblies can produce multilayer multifunctional films and may be commercialized once they overcome some issues like negative impacts on fabric properties, costs, poor durability to commercial washing.
Among them, a sol–gel process is regarded as a simple, convenient, mild, efficient and flexible surface modification technology in finishing fabrics and has been increasingly studied [13, 14]. By physically doping, chemically bonding or changing process parameters during a sol preparation stage [15, 16], the sol–gel process can modify a material bulk on its surface and offer it the required functions, such as antibiosis [17], anti-wrinkling [18], UV resistance [19], superhydrophobicity [20], dye fastness [21], self-clean [22, 23], flame retardancy [24], etc. For example, the precursor of a silica sol can form a three-dimensional polysilane network structure via polycondensations and fix functional compounds (organic or inorganic) inside it [25] on the fiber surface after baking (curing). Thereby, an inorganic or organic–inorganic hybrid coating with high molecular uniformity and certain expected properties [26, 27] like flame retardancy [25, 28] can be produced. However, proper flame retardants are important to reach reasonable formulations with as-prepared sols.
Halogenated compounds as traditional flame retardants are ever widely used in finishing or coating cotton fabrics. Although excellent in flame retardancy [28], they will produce toxic and harmful substances (like formaldehyde) and corrosive gases (like hydrogen halides) during the pyrolysis and combustion process [29,30,31]. In this sense, it is increasingly banned. In response to the requirements of health and environmental protection, it is particularly important to develop high-efficiency, non-toxic or low toxicity, non-smoke or low smoke, halogen-free, formaldehyde-free and environmentally friendly flame retardants [32]. In recent years, certain eco-friendly flame retardants containing boron, nitrogen, silicon and other elemental compounds have attracted more attentions in flame retarding fabrics [33]. Boron-containing flame retardant has excellent smoke suppression performance, low toxicity, good thermal stability [32, 34], anti-corrosion effect, neutral pH value [35] and other properties, and its reserves are rich, low cost, easy to obtain. Therefore, they have broad application prospects.
Early studies have found that when sol–gel processes are used for finishing fabrics, potential synergistic effects between boron-based precursors (functional additives) and other compounds (such as those containing silicon [20], nitrogen [36], and phosphorus [37]) can enhance thermal stability and flame retardancy capabilities [31, 38]. Qianghua Zhang et al. [39] prepared a series of boron-doped silica sol by a sol–gel method to improve the flame retardancy levels and thermal stabilities of wool fabrics. The flame retardant coatings show a good smoke suppression performance (65.2 g−1 at 680 s) and a flame retardancy level (LOI up to 29.9%). Qianghua Zhang et al. [40] used hydrolysis and condensation reactions between TEOS and boric acid (H3BO3) to prepare a binary silicon-boron hybrid nanosol flame retardant system, used 1,2,3,4-butanetetracarboxylic acid (BTCA) of hybrid silica sol for durable flame retardant finishing of silk fabrics. Results show that LOI values of the treated samples increase from about 25.0% to about 32.0%. The peak heat release rates (PHRRs) decrease from about 140 kW/m2 to about 90 kW/m2. Aziz Bentis et al. [15] used titanium-based sol and boric acid with different molar ratios as additives to prepare TiO2-boron-based sol coatings via sol–gel processes and applied them to modify the surfaces of cotton fabrics via pad-dry-cure processes. Results show that the coated fabrics have good flame retardancy (a higher Fire Performance Index value of around 45.46%) and water repellency levels. Li Gang et al. [41] prepared SiO2 sol and SiO2-KH570 sol system by sol–gel method, and hybridized with disodium octoborate tetrahydrate (DOT, Na2B8O13·4H2O) for flame retardant finishing of cotton fabrics. SiO2-KH570-DOT@Cotton shows the second-highest pyrolysis temperature difference (198 ℃) and the highest char residue rate (41.7%), the highest LOI (24.30%) and ΔLOI/Δm (6.0%/g) values, almost the most beneficial after flame time (14.8 s) and afterglow time (0.0 s), and the most complete, compactest and stablest char residues. Li Dan et al. [42] prepared a ternary sol–gel system mainly including silica sol, KH560 and ZB (zinc borate) via sol–gel method. The SiO2-KH560-ZB finishing of cotton fabrics has acceptable flame retardant properties, and the value of ΔLOI/Δm reaches the highest value of 0.37%/g. Meanwhile, the hybrid gel coating delays the initial pyrolysis of the cotton fabric, reduces PHRR by 26.9% and THR by 22.9%, reduces the amount of smoke generated, and improves the ability to form char.
The above studies provide instructive practices of sol–gel processes to offer wool, silk and cotton fabrics flame retardancy levels via combinations of silica sol and boron compounds. Whatever, when a flame retardant is introduced to a silica sol, there are inevitable issues of compatibility, complexity, applicability, end property and cost, etc. Therefore, it is a natural and positive attempt to just create a binary and simple SiO2-B2O3 sol–gel system and apply them to finish a cotton fabric with certain flame retardancy. Indeed, there are a few very early studies of such systems, but they are aiming at obtaining SiO2-B2O3 glasses [43,44,45]. Among them, triethyl borate B(OC2H5)3 and trimethyl borate (B(OCH3)3) are used as B2O3 sources (precursors). Notably, no research on flame retarding cotton fabrics with SiO2-B2O3 sol–gel systems has been reported. Therefore, in present work a single SiO2 sol, a single B2O3 sol and a composite SiO2-B2O3 sol were prepared through sol–gel processes. TEOS and tributyl borate were used as source precursors of SiO2 and B2O3. Then these sols were treated (finished) onto cotton fabrics through dipping-baking methods for flame retardant functions. DRIFTS, XPS, XRD, SEM and EDS techniques were used to investigate the surface functional groups, elemental compositions, crystal structures, surface morphologies and elemental contents of original and treated cotton fabrics. The pyrolysis and flame retardant properties of sol-treated cotton fabrics were evaluated by TG and LOI experiments and VBT. Then, the binding modes among sol–gel system components and those between sol–gel systems and cotton fabrics were discussed. Corresponding intrinsic flame retardant mechanisms were explored.
Materials and methods
Material
The pure cotton fabric (the warp of 133 yarn/cm and the weft of 72 yarn/cm) was obtained from Nanjing Qicaimei Textile Company (Nanjing, China). Tetraethyl orthosilicate (TEOS, AR, ≥ 35.0%) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China); Tributyl borate (CR, ≥ 98.0%) was supplied from Aladdin Reagent Co., Ltd. (Shanghai, China); Ethanol (EtOH, AR, ≥ 99.6%) was purchased from Wuxi Yasheng Chemical Co., Ltd. (Wuxi, China); the catalyst of hydrochloric acid (HCl, AR, 37.0%) was provided from Shanghai Pilot Chemical Company (Shanghai, China). The chemical structures of TEOS, tributyl borate and cotton fiber are shown in Fig. 1. All the reagents were analytically pure and used without any further purification.
Preparations of sols
Preparation of a single SiO2 sol
TEOS, anhydrous ethanol, and deionized water were added to a beaker (250 mL) with a molar ratio of n (TEOS): n (C2H5OH): n (H2O) = 1.0:2.0:6.0, and stirred at 60.0 ℃ by the Constant Temperature Heating Magnetic Agitator (78 HW-1, provided by Ronghua Instrument Manufacturing Co., Ltd., Changzhou, China). After about 30 min, a small amount of hydrochloric acid was added as a catalyst. A Portable Multi-parameter Measuring Device (SKOLL, provided by Beijing Xuxin Instrument and Equipment Company, Beijing, China) was used to control the pH between 5.0 and 6.0, the temperature was kept constant at 60.0 ℃. After stirring for 3 h, a stable SiO2 (molecular weight: 60.09; theoretical Si element content: 46.7%; theoretical O element content: 53.2%.) sol (basic sol) was prepared. The synthesis reaction of the SiO2 sol is shown in Scheme 1.
Preparation of a single B2O3 sol
Tributyl borate, ethanol, and deionized water were mixed in a beaker (250 mL) with a molar ratio of n (C12H27BO3): n (C2H5OH): n (H2O) = 1.00:8.30:0.85, and stirred at 60.0 ℃ by the Constant Temperature Heating Magnetic Agitator. After stirring for about 30 min, a small amount of hydrochloric acid was added. Also, the pH of the mixed solution was controlled to be between 5.0 and 6.0, the temperature was kept constant at 60.0 ℃. After stirring for 3 h, a stable B2O3 (molecular weight: 69.62; theoretical B element content: 31.0%; theoretical O element content: 68.9%.) sol was prepared. The synthesis reaction of the B2O3 sol is shown in Scheme 2.
Preparation of a composite SiO2-B2O3 sol
The pre-prepared SiO2 sol and B2O3 sol were added into the beaker (250 mL) in a mass ratio of 1.0:1.0, and stirred at 60.0 ℃ in the Constant Temperature Heating Magnetic Agitator. The pH value was controlled to be between 5.0 and 6.0. After stirring for 2 h, a stable, clear and transparent SiO2-B2O3 sol was prepared. The synthesis reaction of a SiO2-B2O3 sol is shown in Scheme 3.
Treatments of expected flame retardant cotton fabrics with sols
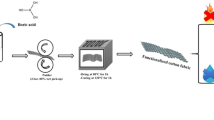
Before sol treatments, the cotton fabrics were completely immersed in deionized water at 100 ℃ for 5 min to remove dust and chemical residues on the surfaces, then rinsed with deionized water until the left solution was not turbid, and dried naturally in a well-ventilated area. The treated cotton fabrics were impregnated with the as-prepared sols for 2 h at room temperature, then they were taken out and the excess treatment liquid on the surfaces was squeezed out, following this they were dried at 90.0 ℃ for 50 min and cured at 100.0 ℃ for 5 min in an oven (PHG-9036A, provided by Shanghai Jinghong Experimental Equipment Co., Ltd). These treated cotton fabrics are numbered as A (original cotton fabric), B (SiO2 sol finishing cotton fabric), C (B2O3 sol finishing cotton fabric) and D (SiO2-B2O3 sol finishing cotton fabric). The processes of preparing a SiO2-B2O3 sol and finishing a cotton fabric are shown in Fig. 2.
In addition, the add-on values (W, weight gains) of the samples are calculated according to Eq. (1).
where wf and wi are the weights (g) of cotton fabrics before and after the sol finishing processes, respectively.
Characterization techniques for original and treated cotton fabrics
The DRIFTS spectra of original and treated cotton fabrifigcs were recorded at room temperature in the range of 400–4000 cm−1 with a resolution of 4.0 cm−1 by an IS5 FTIR spectrometer (Thermo Fisher Scientific Co., Ltd., Shanghai, China.). The diffuse reflectance sampling mode was adopted. KBr pellets made of finely cut and ground fabrics were used.
The XPS spectra of original and treated cotton fabrics were obtained by an X-ray photoelectron spectrometer (K-Alpha + , Thermo Fisher Scientific Co., Ltd., Waltham, USA). The solid surface (5 mm × 5 mm) was excited by an x-ray beam, and the kinetic energy of electron emission in the range of 1–10 nm from the surface of the analyzed material was measured.
XRD patterns of original and treated cotton fabrics (2 mm × 2 mm) were obtained by using a D8 advance diffractometer (Bruker Co., Ltd, Saarbrucken, German) with CuKa radiation generated at 36 kV and 20 mA at room temperature. The diffractograms were obtained at scattering angles from 10° to 80°.
Microscopic surface morphologies of original and treated cotton fabrics were investigated by a SU8010 SEM instrument (Hitachi Co., Ltd., Tokyo, Japan). A cotton fabric piece was cut with a size of 3.0 mm × 3.0 mm and fixed on black carbon conductive tape. All samples were processed by using a sputter coating machine (provided by Nanjing Bosheng Technology Co., Ltd., Nanjing, China). After platinum coating and gold evaporation, the surface morphology forms of the investigated samples were scanned. The element contents of selected areas for the cotton fabrics were inspected with an Xplore-30 energy dispersive X-Ray spectrometer (Oxford Instrument Technology Co., Ltd., Shanghai, China).
Thermal stability levels of original and treated cotton fabrics (10 mg) were investigated through the TGA/DSC3 + Thermogravimetric Analyzer (Mettler Toledo International Inc., Columbus, USA) from room temperature to 700.0 ℃ with a heating rate of 10.0 ℃/min in the N2 (a flow rate of 60.0 mL/min).
According to GB/T 5454–1997 "Textile Burning Performance Test Oxygen Index Method" standard, the LOI experiments of original and treated cotton fabrics were performed by using the ZY6155A Limit Oxygen Index Tester (Zhongnuo Quality Inspection Equipment Co., Ltd., Dongguan, China). The dimension of the sample was 150 mm (length) × 58 mm (width).
According to GB/T 5455–2014 "Textiles-Burning Behaviour-Determination of Damaged Length, After-glow Time and After-flame Time of Vertically Oriented Specimens" standard, the vertical burning tests of original and treated cotton fabrics were performed by using the CZF-5CD 50W vertical burning tester (Tianjin Xunyu Technology Co., Ltd., Tianjin, China). The dimension of the sample was 300 mm (length) × 89 mm (width).
Results and discussion
Internal chemical compositions of original and treated cotton fabrics
To confirm the existence states of compositions inside original and treated cotton fabrics by single and composite gels converted from sols, DRIFTS characterizations were performed. The normalized DRIFTS transmittance spectra of original and treated cotton fabrics are shown in Fig. 3.
For original cotton fabric (A), the wide absorption band at 3647–3020 cm−1 is caused by -OH stretching vibrations in cellulose. The absorption peak at 2994–2883 cm−1 is caused by CH- and CH2- (long alkyl chain) stretching vibrations [15, 46], and the absorption peak at 1626 cm−1 is derived from a certain amount of moisture in the cotton fibers [46]. The absorption peak at 1313 cm−1 is caused by -CH wagging or/and OH in-plane bending vibrations. These are typical absorption peaks of cotton fibers. In addition, the absorption peak at 1026 cm−1 corresponds to the stretching vibrations of the C–O–C bonds from the glycosidic bonds of the cellulosic structures [47]. The sol-finishing cotton fabrics still contain these groups, indicating that the sol-converted gel coatings after baking will not damage the original molecular structures of cotton fabrics.
For the cotton fabric finished by a SiO2 sol (B), besides the characteristic peaks of the original cotton fabric itself, its wide and weak (hidden) absorption peak at 3647–3020 cm−1 is caused by Si–OH bonds (Schemes 1, 3 and Fig. 2). Moreover, it has three new characteristic peaks of 517 cm−1, 795 cm−1 and 1160–1060 cm−1. The former two correspond to the vibration absorption peaks of the Si–O-Si bond [17] (Scheme 1). The third is caused by the asymmetric stretching vibrations of the Si–O bonds [18, 42] (Schemes 1, 3 and Fig. 2). Besides, the O-Si–O deformation vibration band [43, 44] is situated at 441 cm−1. These groups reflect the implementations of hydrolysis and polycondensation reactions of TEOS (Fig. 1 (a)). The appearances of such absorption peaks indicate that after baking a SiO2 gel is successfully coated on the cotton fabric.
For the cotton fabric finished by a B2O3 sol (C), besides the characteristic peaks of original cotton fabric itself, the absorption peak near 3200 cm−1 belongs to the stretching vibrations of the B-OH bonds [43, 44] (Schemes 2 and 3, Fig. 2). The absorption peaks near 1426 cm−1 come from the stretching vibrations of the B-O bonds [46, 47],and the absorption peak at 717 cm−1 is caused by the B-O-B deformation vibration (Schemes 2 and 3, Fig. 2). These groups reflect the implementations of hydrolysis and polycondensation reactions for tributyl borate (see Fig. 1 (b)). These facts indicate that a B2O3 gel is coated on the cotton fabric.
For the cotton fabric finished by a SiO2-B2O3 sol (D), besides the characteristic peaks of original cotton fabric itself, its wide and weak (hidden) absorption peaks at 3647–3020 cm−1 are caused by Si–OH and B-OH bonds (Schemes 1, 2, 3 and Fig. 2). In addition, it contains all the characteristic peaks of a SiO2 gel (Si–O bond, Si–O-Si bond). A new absorption peak appears near 661 cm−1 [44] and can be attributed to the stretching vibrations of the Si–O-B bonds [42], indicating that a B2O3 gel has been successfully incorporated into the three-dimensional network structure of a SiO2 gel (Scheme 3 and Fig. 2). Moreover, the absorption peaks near 1426 cm−1 and 717 cm−1 correspond to the stretching vibrations of the B-O bonds [43,44,45] (Schemes 2 and 3, Fig. 2). Due to the low content of boron element in the SiO2-B2O3 composite sol (lower W value of sample D (14.7%) than sample C (16.7%), later Table 1 and Table 3), its absorption peak intensity is relatively weaker than that of sample C. The appearances of Si–O-Si, B-O and Si–O-B bonds indicate that single and composite sols have been successfully hybridized and coated on the cotton fabric (Fig. 2).
Surface elements of original and treated cotton fabrics
To determine the surface element distributions of treated cotton fabrics, the samples were characterized by XPS. Figure 4 shows the global range and local high-resolution XPS spectra of cotton fabrics before and after sols finishing. The corresponding element distributions are shown in Table 1. However, the effective detection depth of a substrate in XPS is less than 10.0 nm (https://www.phi.com/surface-analysis-techniques/). Therefore, the signal only reveals the outermost surface compositions (elements) of a sample.
For original cotton fabric (A), two main elements of C1s (296.43 eV) and O1s (545.43 eV) are detected (Fig. 1(c)) and ascribed to C–O–C, C–OH, and C–C species in a cotton cellulose molecule, respectively.
For SiO2 sol finishing cotton fabric (B), besides C element, it shows new XPS peaks at 103.35 eV and 152.60 eV, which are attributed to the absorption peaks of Si2p and Si2s, respectively. As shown in Fig. 4(a), the high-resolution spectrum of Si2p presents two bands, the binding energy ranges of Si–O-C and Si–O-Si are 101–102 eV and 103–104 eV [23]. The peak at 102.9 eV is attributed to the Si–O-C bond, indicating that a covalent bond is formed between the cotton fabric and the SiO2 gel [31]. The existences of Si–O-Si bonds here confirm the evidence of DRIFTS spectrum of sample B.
For B2O3 sol finishing cotton fabric (C), besides C and O elements, the B1s absorption peak is identified at 193.32 eV and derived from B-O and B-OH bonds. This is consistent with the results of DRIFTS spectrum of sample C. Also, this indicates that the B2O3 gel has effectively bonded to the cotton fabric. The absorption peak of B1s is rather weak due to the limited loading amount of tributyl borate (Fig. 1(b), Table 1).
For SiO2-B2O3 composite sol finishing cotton fabric (D), four elements of C, O, Si and B are detected. Compared with the original cotton fabric, new characteristic peaks of Si and B, namely Si2p (103.35 eV), Si2s (152.60 eV), and B1s (193.32 eV), appear. Moreover, the high-resolution spectrum of Si2p (Fig. 4(c)) shows three peaks, the binding energy ranges of Si–O-C, Si–O-B and Si–O-Si correspond to 101–102 eV, 102–103 eV and 103–104 eV, respectively [16, 47]. The appearances of these three peaks indicate that the SiO2-B2O3 composite sol is successfully prepared (Scheme 3), and the two sols are internally bonded through Si–OH and B-OH by removing water molecules to form Si–O-B bonds. These bonds are also confirmed by DRIFTS spectrum of sample D. Also, the SiO2-B2O3 composite gel is bonded with the cotton fabric via hydrogen bonds between Si–OH and B-OH groups of the gel and -OH groups of the cotton fabric.
Overall, the composite sol has been successfully finished on the cotton fabric and formed a connection with the cotton fabric to a certain extent, which strongly proves the DRIFTS analyses.
Internal crystal structures of original and treated cotton fabrics
Figure 5 shows the XRD patterns of cotton fabrics before and after the sols finishing. For original cotton fabric, it shows three diffraction peaks at 14.9°, 16.5° and 34.3° corresponding to the amorphous structures and one diffraction peak at 22.8° corresponding to the crystal faces (1–10), (110), (200) and (004) of cellulose I [48].
For SiO2 sol finishing cotton fabric (B), its XRD pattern is not significantly different from that of original cotton fabric. It shows that deposited SiO2 gel coating converted from sol does not affect general crystal structure of original cotton fabric. In view of the fact that the diffraction peak strength of cotton fabric (22.8°) is much stronger than that of silica sol (22.0°) [49], the diffraction peak of the SiO2 gel can not be clearly displayed. Secondly, the thickness of the SiO2 gel, its chemical crosslinking on the surface of cotton fabric and the increased area of amorphous region (Fig. 5B) indicate that the SiO2 gel coating is also amorphous.
For B2O3 sol finishing cotton fabric (C), the diffraction peak strength at 14.9° is weakened, but the overall position and shape do not change significantly. Secondly, due to the low boron content in the material itself and low treatment temperature (drying at 90.0 ℃ and curing at 100.0 ℃, "Treatments of expected flame retardant cotton fabrics with sols" section) of the impregnation-drying finishing process, it does not show the obvious absorption peak of B2O3 at 28° [44]. In this sense, the B2O3 gel coating does not alter the crystal structures of original cotton fibers. These confirm that the boron gel coating is also amorphous.
For SiO2-B2O3 composite sol finishing cotton fabric (D), it shows stronger diffraction peak intensity in the non-crystalline region than that of original cotton fabric, but the overall position and shape do not change significantly. Combined with DRIFTS and XPS analyses, it can be seen that the chemical bonding forces between the SiO2-B2O3 sol and the cotton fabric mainly act on the non-crystalline regions of cotton fiber surfaces.
Microscopic morphologies and element distributions of original and treated cotton fabrics
Figure 6 shows the SEM and EDX images of original and treated cotton fabrics before VBTs. The detection depths of such techniques are around 1000–3000 nm (https://www.phi.com/surface-analysis-techniques/).
For original cotton fabric (A, Fig. 6(A)), there are natural gullies among cotton fibers and distinct fiber bundles with twisted structures. The EDX result shows that its surface contains of C (61.7%) and O (38.3%) elements. For treated cotton fabric samples (B, C and D), original cotton fiber gullies and fiber bundles (morphologies, directions) are hidden to certain extents due to the protective gel layers formed by sols after baking treatments at certain temperatures.
For SiO2 sol finishing cotton fabric (B, Fig. 6(B)), its surface is obviously rough, a large number of silica sol particles are deposited on surfaces of cotton fibers, but a single fiber bundle is still visible. This is due to the hollow internal structure of cotton fiber and its twisted porous surface that provide good carriers for absorbing fine particles [42]. EDX result shows that it has a Si element content of 7.3%, its C element content (51.7%) decreases by 10.0% and O element content (40.9%) increases by 2.6% as compared with those of original cotton fabric (61.7% for C and 38.3% for O). Firstly, a large amount of -OH groups on the surfaces of cotton fibers and from the SiO2 sol are dehydrated and condensed at a high temperature (drying at 90.0 ℃ and curing at 100.0 ℃) to form a stable three-dimensional Si–O-Si network structure (Scheme 1). This is consistent with the results of DRIFTS analyses since the absorption peaks of Si–O bonds and Si–O-Si bonds are detected on the cotton fabric. Secondly, theoretical C and O element contents of TEOS (36.3% and 48.4%, Fig. 1) are lower by 18.2% and 1.8% than those of cotton fiber (44.4% and 49.3%, Fig. 1). Also, theoretical O element content of SiO2 (53.2%) is higher by 7.9% than that of cotton fiber (49.3%, Fig. 1). Here, theoretical content of an element is simply calculated as the ratio of atomic weights and a molecular weight. Inevitably, there should be some SiO2 sol components that are not dehydrated and condensed. Current C and O elements mainly come from the remanent TEOS and a SiO2 gel coating. Thirdly, besides the chemical bonding effect, the fine (nano- and/or micro-scales) SiO2 sol particles can adhere to the cotton fiber surfaces through multiple physical effects like capillary forces, Van der Waals forces, surface tensions, diffusibility, etc. Fourthly, according to their surface contents, Si and O atoms (especially the latter with an increasing content) are arranged on outer surface of the gel coating due to inherent Si–O bond connection forms (Scheme 3 and Fig. 2) and general as-formed spacial configurations of SiO2 sol clusters (dehydrated and condensed sol components). The unreacted sol components are blended with the gel coating. These are consistent with the appearances of Si2s and Si2p peaks, lower peak strength of C1s and higher peak strength of O1s in Fig. 4 (XPS spectrum of sample B). In addition, XRD data also show that the chemical bonding forces between the SiO2 gel and the cotton fabric are mainly on the non-crystalline regions of the cotton fiber surfaces.
For B2O3 sol finishing cotton fabric (C, Fig. 6(C)), there are some obvious and uneven particles attach to the surfaces of the cotton fibers, and a single fiber can still be identified. Its B element (6.0%) is detected on the cotton fabric surface. Its C element content (50.2%) is reduced by 11.5% and O element content (43.8%) is increased by 5.5% as compared with those of original cotton fabric (61.7% for C and 38.3% for O). This is because the B-OH groups in the B2O3 sol can bond with -OH groups in cotton cellulose to form B-O-B bonds (Scheme 2 and Fig. 2) and produce water molecules. It is known that theoretical C and O element contents of tributyl borate (62.6% and 20.8%, Fig. 1) are higher by 41.0% and lower by 57.8% than those of a cotton fiber (44.4% and 49.3%, Fig. 1). In addition, theoretical O element content of B2O3 (68.9%) is higher by 39.7% than that of a cotton fiber (49.3%, Fig. 1). Inevitably, there should be some B2O3 sol components that are not dehydrated and condensed and are the source of current C elements. Current O elements mainly come from a B2O3 gel coating. Similarly, the coarser B2O3 sol particles can be adsorbed on the cotton fiber surfaces through physical effects like van der Waals force, surface tensions, diffusibility, etc. The absorption peaks of B-O bonds and B-OH bonds detected in DRIFTS spectrum of sample C can confirm the above analyses. In addition, the spatial structure formed by B and O atoms is on the outer surface of the gel coating due to inherent B-O angular connection bonds and general as-formed spacial configurations of B2O3 sol clusters (dehydrated and condensed sol components). The unreacted sol components are blended with the gel coating. These are consistent with the increase in the intensity of O1s peak in the XPS spectrum of sample C, the decrease in the intensity of C1s peak, and the weak absorption peak of B1s. XRD analyses also show that the bonding forces between boron sol and cotton fabric are mainly in the amorphous regions.
For SiO2-B2O3 composite sol finishing cotton fabric (D, Fig. 6(D)), its surface is smoother than those of both sample B and C. It is covered with a wider, evener and denser gel coating. The gaps between cotton fibers are almost completely filled with as-produced gels. Inevitably, there should be some SiO2 sol and B2O3 sol components that are not dehydrated and condensed. Also, there should be some remanent SiO2 sol and B2O3 sol that do not interact with each other. In view of this, C, O, Si and B elements are all detected on the surface of the fabric (sample D) with contents of 55.1%, 36.9%, 3.6% and 4.9%, respectively. Among them, C element content decreases by 6.6%, increases by 3.4% and 4.9% as compared with those of sample A (61.7% for C), sample B (51.7% for C) and sample C (50.2% for C). Suppose that the cotton fibers are covered with the SiO2-B2O3 gel (nano-micro-scale) coating completely, there should be none C elements detected on their surfaces. Hence, current C elements should come from the remanent SiO2 and B2O3 sol components. Theoretical C element contents of tributyl borate (62.6%, Fig. 1) and TEOS (36.3%, Fig. 1) are higher by 41.0% and lower by 18.2% than that of cotton fiber (44.4%, Fig. 1). This means that more B2O3 sol components are involved in a hybridization process with SiO2 sol components. Since tributyl borate has a higher theoretical C element content than that of TEOS, the remanent part of sample D should contain more SiO2 sol components and less B2O3 sol components. Therefore, C element content detected by EDX of sample D increases more for sample C (4.9%) than sample B (3.4%). As for O element content of sample D detected by EDX, it decreases by 1.4%, 4.0% and 6.9% as compared with those of sample A (38.3% for O), sample B (40.9% for O) and sample C (43.8% for O). Likewise, suppose cotton fibers of sample D are covered by the SiO2-B2O3 gel coating completely, current O element should come from SiO2 and B2O3 gels and the remanent part of SiO2 and B2O3 sol components. Theoretical O element contents of tributyl borate (20.8%, Fig. 1) and TEOS (48.4%, Fig. 1) are lower by 57.8% and 1.8% than that of cotton fiber (49.3%, Fig. 1). Moreover, theoretical O element content of B2O3 (68.9%) is higher by 29.5% than that of SiO2 (53.2%). This means more portions of SiO2 sol components and B2O3 gel coating contribute to the O element contents. Certainly, spacial arrangements of the SiO2-B2O3 network gel members (Fig. 2) also affect the detected elements. This is in agreement with the XPS results. As for Si element content, it decreases by 4.1% as compared with that of sample B. As for B element content, it decreases by 1.1% as compared with that of sample C. This means that SiO2 and B2O3 sol components are both physically embedded and chemically bonded in an as-produced gel coating although some of them are left. Theoretical Si element contents of TEOS and SiO2 are 10.6% and 46.7%, theoretical B element contents of tributyl borate and B2O3 are 4.7% and 31.0%. Therefore, current Si and B elements mainly come from the SiO2 and B2O3 gel coatings.
Similar to a single SiO2 sol and B2O3 sol, besides the chemical bonding effects (hydrogen bonds among SiO2 and B2O3 sol components and between them and cotton fibers), the fine SiO2 sol and coarse B2O3 particles can adhere to the cotton fiber surfaces through multiple physical effects like capillary forces, Van der Waals forces, surface tensions, diffusibility, etc. Apparently, it can fully conceal the characteristic shapes, orientations and gaps of cotton fibers (Fig. 2). The absorption peaks of Si–O-C, Si–O-B and Si–O-Si bonds on cotton fabrics are also detected in the DRIFTS spectrum, which confirms the above analyses. Generally, the three-dimensional structures formed by Si, O and B atoms are in exterior layer of the gel coating. This agrees well with the work of Villegas and Navarro [44] (“…the existence of mixed Si–O-B bonds, preferential located at the outside of the material particles”). Also, remained sol components are blended in the gel coating. These correspond with the change trends of C1s, O1s, B1s, Si2s and Si2p peaks in the XPS spectrum of sample D. Furthermore, XRD also shows that the composite gel and cotton fabric are bonded on the cotton fiber surfaces without affecting their internal structures.
By combing DRIFTS, XPS, XRD and SEM–EDX results, it is known that most of the sols have been successfully gelled and attached to cotton fabric surfaces without changing the original molecular structures. They can be chemically bonded with cotton fabrics through hydroxyl groups (Fig. 2). Also they are physically absorbed and cover cotton fibers through multiple forces as mentioned above. However, due to the incomplete dehydration/condensation reactions and inconsistent adsorption performances of different sols converted gels on cotton fabrics, in practical application, the proportions of elements on cotton fabrics can not reach the set molar ratios.
Pyrolysis characteristics of original and treated cotton fabrics
The pyrolysis characteristics of cotton fabrics have obvious influences on their flame retardancy performances. Figures 7 and 8 are the TG and DTG curves of original and treated cotton fabrics in nitrogen. Table 2 shows the primary pyrolysis parameters.
For original cotton fabric (A), its pyrolysis in nitrogen is roughly divided into three stages. Below 302.58 ℃ (Table 2) is the initial pyrolysis stage. The mass loss in this stage reaches about 5.0% and is mainly due to the evaporation behaviors of water molecules physically adsorbed in the cotton fabric as the temperature rises [31, 50]. Range of 302.58–384.29 ℃ (Table 2) is the main pyrolysis stage. As the temperature continues to rise, cotton cellulose is decomposed to produce L-glucose and combustible gases. Range of 384.29–700.00 ℃ is the decomposition and carbonization stage. The dehydration of cellulose into char is more obvious, and a large amount of glucose, carbon dioxide, and water molecules are generated, and the mass loss is about 10.0%, the char residue rate is 8.5%.
For SiO2 sol finishing cotton fabric (B), there are also three decomposition stages reflected by its DTG curve (Fig. 8). Tmax and char residue rate values are both higher by 4.34% and 268.23% than those of original fabric (Table 2). This is because when drying at 90.0 °C and curing at 100.0 °C, Si–OH groups (Schemes 1, 3 and Fig. 2) of SiO2 sol and -OH groups of cotton fiber surfaces are further dehydrated to release water molecules and condensed to form Si–O-C bonds (Fig. 2). Water molecules evaporate in the process. Then produced SiO2 gel is coated onto cotton fibers through multiple physical forces as mentioned before ("Microscopic morphologies and element distributions of original and treated cotton fabrics section", reflected by SEM–EDX results). Due to inherent chemical structures of SiO2 sol components, spacial configurations of functional groups like Si–O, Si–O-Si and Si–O-C (reflected by DRIFTS and XPS results) in a Si–O-Si three-dimensional network are formed. Some unreacted components are also embedded in such a structure. Due to these factors, much heat generated in reactions is consumed. Most importantly, the as-produced SiO2 gel coating does form a relatively complete inorganic insulation barrier (fine SiO2 particles and local networks, Fig. 6(B)) with acceptable thermal stability [39, 51]. It can protect bulk cotton fibers below the coating and block exchanges of aftermath heat, oxygen and pyrolysis process products gradually and effectively. However, its Tdif is a little lower by 3.78% than that of original cotton fabric. This is caused by initial pyrolysis of unreacted TEOS and later cracking behavior of a SiO2 gel.
For B2O3 sol finishing cotton fabric (C), its Ti, Tf, Tdif, Tmax and char residue rate values are also all higher by 5.54%, 10.11%, 27.02%, 2.57% and 388.94% than those of original cotton fabric. Similar to a SiO2 gel coating, the inorganic B2O3 gel coating can also consume released heat from reactions and form a rough and dense insulation barrier (Fig. 6(C)) onto cotton fibers [52] with certain spacial arrangements of interior functional groups (bonds) like B-O, B-O-B (DRIFTS and XPS results) and speculated B-O-C (Fig. 2). The innermost chemical structure of unreacted tributyl borate (Fig. 1) is more difficult to break. These effects improve its general thermal stability.
Ti and Tmax values of sample C are both a little lower by 2.23% and 1.69% than those of sample B, while Tf, Tdif and char residue rate values of sample C are all higher by 4.41%, 32.01% and 32.78% than those of sample B. This derives from chemical structure differences of unreacted tributyl borate and TEOS (Fig. 1). Tributyl borate shows three longer side alkyl chains (B-O-CH2-CH2-CH2-CH3) while TEOS shows four shorter side alky chains (Si–O-CH2-CH3). The side alkyl chains of tributyl borate are more easily broken and lost (lower Ti and Tmax) in a pyrolysis process. However, tributyl borate has a trigonal geometry and three B-O covalent bonds in inner layer of its molecule. According to XPS results (Fig. 4, "Surface elements of original and treated cotton fabrics section"), B-O bond has a higher binding energy of 193.12 eV than those of Si–O and Si–O-Si (103.00–104.00 eV). Therefore, it is relatively harder to destroy these bonds in the end pyrolysis stage. Also, the longer side chains of tributyl borate consume more time to break and produce more pieces in a pyrolysis process. Additionally and theoretically, B2O3 has a higher molecular weight (69.62 g/mol) than that of SiO2 (60.09 g/mol). Finally, a B2O3 gel can also chemically absorb to cotton fibers through dehydration. In view of these, more residues can be kept for tributyl borate after an elongated pyrolysis process (higher Tf and char residue rate).
For SiO2-B2O3 sol finishing cotton fabric (D), its Ti, Tf, Tdif, Tmax and char residue rate values are all higher by 7.23%, 12.06%, 29.92%, 4.07% and 415.53% and by 1.60%, 1.76%, 2.28%, 1.46% and 5.43% than those of original cotton fabric and sample C. Its Ti and Tmax values are a little lower by 0.67% and 0.25% than those of sample B, while its Tf, Tdif and char residue rate values are higher by 6.25%, 35.03% and 40.0% than those of sample B. As analyzed above, such facts are mainly ascribed to chemical structure changes of unreacted tributyl borate and as-produced B2O3 gel in a pyrolysis process. They also reflect the compensations of pyrolysis performances of unreacted tributyl borate and TEOS ("Microscopic morphologies and element distributions of original and treated cotton fabrics section"). Similar to a single SiO2 and B2O3 gel coating, the inorganic SiO2-B2O3 gel coating can best consume released heat from reactions, form the most continuous and densest insulation barrier (Fig. 6(D)) onto cotton fibers with certain spacial arrangements of interior functional bonds like Si–O, Si–O-Si, Si–O-C, B-O, B-O-B, Si–O-B and speculated B-O-C (DRIFTS and XPS results, also Scheme 3 and Fig. 2). These effects much improve general thermal stability of sample D. Certainly, these TG data indicate that a SiO2 gel plays more role in structural support (higher Ti and Tmax), while a B2O3 gel contributes more to a longer pyrolysis process and more char residues (higher Tf, Tdif and char residue rate). This reflects the good synergistic effect between a SiO2 and B2O3 gel in improving thermal stability levels. As for correlations of pyrolysis char residue rates and W values, the former increases while the latter decreases sequentially from sample B to sample D (Table 2). Such fact just proves that less loading amounts of components leads to better flame retardancy levels.
Flame retardant performances of original and treated cotton fabrics
The flame retardant performances of cotton fabrics before and after sols finishing are evaluated by LOI experiments and VBTs. A new concept (flame retardant efficiency) is applied here, namely ΔLOI/Δm, which is defined as the increase in LOI corresponding to the unit increase in weight of two fabric samples [42]. Table 3 shows the main parameters of two experiments.
A fabric is thought to be flammable and combustible when its LOI is below 21.0% and in 21.0–26.0% [18], respectively. As shown in Table 3, the flame retardancy levels of all treated cotton fabrics have changed from flammable to combustible.
For original cotton fabric (A), its LOI is only 18.0% and reflects its flammability nature. As can be seen from Table 3, when it is pyrolyzed, it has the second lowest Tdif of 81.71 °C and the lowest char residue rate of 8.50%. Its weak thermal stability leads to its flammability.
For SiO2 sol finishing cotton fabric (B), its LOI increases by 16.67% more than that original cotton fabric although it is combustible. Combining its TG parameters in Table 2 and comparing to original cotton fabric, its Tdif is lower by 3.78% although its Tmax and char residue rate are both enhanced by 4.34% and by 268.23%. This suggests that purity (without redundant and unreacted sol components), compactness (without or less observable pores and crevices), smoothness, thickness, rigidity and internal chemical structure configurations (spacial arrangements) of a SiO2 gel coating are important in protecting cotton fibers below when it has to expose to heat. It is known from XRD results that a SiO2 gel is amorphous (without long-range order) clusters with nano- and/or micro-scale voids and pores. These spaces may contain either liquid (like unreacted sol components) or gas (like air). Additionally, for a commercial SiO2 gel (CAS 112926–00-8), it has a melting point of 1200 °C (reported at https://www.americanelements.com). This means it is impossible to melt and pyrolyze at regular high temperatures below this. Thus it can provide strong structural support and keep dimensional stability. These facts are conducive to heat insulation effects. However, a single SiO2 compound has a linear triatomic shape with two oxygen atoms covalently connected to a Si atom by double bonds. As pyrolysis temperature increases, a SiO2 gel coating is prone to becoming brittle and cracking since multiple physical forces are acting and competing in the process. To sum up, the thermal stability level of sample B is restrained to a certain degree. As a result, its flame retardant effect is limited.
For B2O3 sol finishing cotton fabric (C), its LOI increases by 25.00% and 7.14% than those of original cotton fabric and sample B. Its ΔLOI/Δm increases by 36.36%/g than that of sample B. Combining its TG parameters in Table 2 and comparing to sample B, its Tdif and char residue rate values increase much by 32.01% and 32.78%. Similar to a SiO2 gel coating, the apparent (exterior) and interior qualities of a B2O3 gel coating are important to protect inside cotton fibers from heat. It is known that three B-O bonds in a form of a triangle (Schemes 2 and 3, Fig. 2) join together by sharing an oxygen bridge and thus they are distributed in space as networks of triangular rows with their planes oriented in different ways. Such networks naturally tend to adopt amorphous but not crystalline structures as reflected by XRD results. Hence, a B2O3 gel is a solid more glassy than crystalline. For B-O networks, instead of being arranged in a trigonal geometry, they end up linking together to create a boroxol ring (more stable than a linear Si–O atomic arrangement). Also, as mentioned above, the binding energy of a B-O bond is higher than that of a Si–O bond. Such facts improve thermal stability level of its treated cotton fabric. Naturally, its flame retardancy level is bettered than that of sample B.
For SiO2-B2O3 composite sol finishing cotton fabric (D), its LOI increases by 42.78%, 22.38% and 14.22% than those of original cotton fabric, sample B and sample C. Its ΔLOI/Δm increases by 112.12% and 55.56% than those of sample B and sample C. As discussed before, a SiO2 gel shows a high melting point and a good structural support effect, while a B2O3 gel shows a more stable network. Moreover, it is reported that B2O3 has a low melting point around 450.0 °C and is thus conducive to fusing components with high melting points like a SiO2 gel into slags [52]. According to SEM images, coarse particles of unreacted tributyl borate and B2O3 (Fig. 6(C)) disappear (Fig. 6(D)) and blend well with unreacted TEOS and fine SiO2 particles (Fig. 6(B)). Also, element ratio of B/Si is around 1.53 (Fig. 6, EDX image of sample D). This shows surface enrichment levels of B-O bonds (higher binding energy, harder breakage) and generally specific arrangements. In this way, when temperature rises over 450.0 °C, on the surface of sample D, B2O3 clusters melt and fuse with SiO2 clusters. The fused and glassy film-like mass then spreads over onto the latter and conceal possible cracks and pores induced by a SiO2 gel at high temperature. Besides the heat taken away by H2O molecules in dehydration processes and multiple physical forces, such produced SiO2-B2O3 composite gel coating can further form into chars (slags) on the cotton fiber surfaces and block heat, oxygen and gaseous products. In this sense, overall thermal stability and corresponding flame retardancy level is further enhanced than those of sample B and sample C. These facts just reflect that a SiO2 gel and a B2O3 gel show generally additive and synergistic effects on flame retardancy levels of the cotton fabric (sample D). In this case, four flame retardant mechanisms are shown, that is, condensed phase barrier (glassy B2O3 film and SiO2 char), cooling action (dehydration processes to release H2O molecules), noncombustible gas (H2O molecules) and synergistic actions (SiO2 and B2O3 protective gel layer both physically and chemically combined). Certainly, these performances come from existence conditions, chemical structures and natures, spacial configurations of components in such a coating.
Moreover, based on the above analyses, it is known the ΔLOI/Δm index can abandon the influences of material properties and is more reasonable than LOI value solely. Moreover, the dimensionless ΔLOI/W keeps the same trends with those of ΔLOI/Δm.
Original and treated cotton fabrics were also subjected to VBTs to visually reflect char residues and evaluate flame retardant properties as shown in Fig. 9, and the flammability data are shown in Table 3.
For original cotton fabric (A), it has almost no char residues left after combustion, and its after-flame time (23.0 s) and after-glow time (35.4 s) are the highest. Natural cotton fabrics are easily ignited and keep burning with flames until they extinguish with a period of smoldering behaviors (no flames). Their combustion products are mainly H2O, CO2 and a small amount of ash. Such inflammability nature of cotton fabric is well reflected by foregoing TG and LOI values.
For SiO2 sol finishing cotton fabric (B), its after-flame time (16.0 s) and after-glow time (13.0 s) are reduced by 30.43% and 63.28% than those of original cotton fabric. Its char residue is relatively continuous and complete (Fig. 9(B), a length of 30.0 cm). However, a long crevice appears in the left part of the char residue. As analyzed above in detail (TG and LOI results), amorphous and fine SiO2 particles form a network gel coating with certain rigidity, structural stability and a high melting point at ambient conditions, but it easily and continuously cracks due to multiple physical forces at high temperatures around 360.0 °C (Table 2, Tmax). A smoldering process occurs for some time after flames disappear. This is due to some cotton fibers exposed to intense heat again when there are some cracks and incomplete coverage of the SiO2 gel coating. Its flame retardancy capability is limited to some extent.
For B2O3 sol finishing cotton fabric (C), its after-flame time (15.5 s) and after-glow time (5.0 s) are reduced by (32.60% and 85.88%) and (3.13% and 61.54%) than those of original cotton fabric and sample B. Its char residue is also relatively continuous and complete (Fig. 9(C), a length of 30.0 cm). However, there are also some notable and dispersive crevices different from those of sample B. As analyzed above in detail (TG and LOI results), amorphous and coarse B2O3 particles also form a network gel coating with boroxol rings inside and a low melting point. The B2O3 gel coating also shows certain structural stability but also cracks due to multiple physical forces at high temperatures around 350.0 °C (Table 2, Tmax). However, it fuses and spreads over cotton fibers in a form of glassy film at temperatures higher than 450.0 °C. This helps to make up broken gel surfaces and maintain barrier effects to some extent. The pyrolysis process of the B2O3 gel is somewhat alleviated. A smoldering process also occurs with a shorter duration than that of sample B after flames disappear. Some cotton fibers are exposed to intense heat again due to some cracks and incomplete coverage of the B2O3 gel coating. Its flame retardancy capability is further improved comparing to sample B.
For SiO2-B2O3 composite sol finishing cotton fabric (D), its after-flame time (10.0 s) and after-glow time (0.0 s) are reduced by (56.52% and 100.00%), (37.50% and 100.00%) and (35.48% and 100.00%) than those of original cotton fabric, sample B and sample C. Its char reside is also relatively continuous and complete (Fig. 9(D), a length of 30.0 cm). However, there are also a long notable crevice that is very similar to that of sample B. This is affected more by a SiO2 gel. As analyzed above in detail (TG and LOI results), a SiO2 gel and a B2O3 gel both expose their competing disadvantages like cracking in a burning process. Fortunately, the higher melting point and better dimensional stability of SiO2, the higher thermal stability and fusing capability of B2O3, and their char formation performances jointly overcome their disadvantages generally. The flame retardancy level of sample D reaches the optimal effect. A smoldering process disappears. This again confirms additive and synergistic effects of two gels. Certainly, such flame retardancy is still restrained by inherent chemical structures and natures, physical properties and inorganic natures of SiO2 and B2O3 gels. This urges more and new designs of inorganic–organic flame retardant formulations containing such gels.
Microscopic morphologies of char residues for original and treated cotton fabrics after VBTs
Figure 10 shows the microscopic morphologies of char residues for original and treated cotton fabrics after VBTs.
For original cotton fabric (A), the cotton fibers are almost burnt (Fig. 9(A)). Unlike microscopic appearances of cotton fibers before a VBT (Fig. 6(A)), a few residual chars of cotton fibers (Fig. 10(A)) are thinned, separated, fragmented, compressed and twisted in their natural directions with many holes and ashes on the surfaces. It is known that cotton cellulose, as a kind of polysaccharide, generates a large amount of glucose, carbon dioxide and water during a pyrolysis and combustion process [26, 28]. Cotton fiber structures and components are almost destroyed, lost or converted. This is consistent with the low char rates as shown by TG, LOI and VBT data.
For SiO2 sol finishing cotton fabric (B), its char residues with a long crevice are relatively continuous, complete and black (Fig. 9(B)). Microscopic appearances of its char residues (Fig. 10(B)) are quite different from those of original cotton fabric after a VBT and sample B before a VBT (Fig. 6(B)). Present char residues show broken and flat SiO2 gel blocks that still cover inside cotton fibers. Unlike microscopic appearances of sample B before a VBT, orientations of cotton fibers are not that observable. This shows the reasonable structural support and barrier effects of a SiO2 gel. However, as analyzed in the former parts, the inorganic SiO2 gel is easy to crack due to dehydrations of -OH bonds and multiple physical forces as temperature rises (359.50 °C, Table 2). Crevices are induced and interior cotton fibers are exposed to heat. More cotton fibers are gradually pyrolyzed and charred with a few residues. Main char residues of sample B are mainly from the SiO2 gel and left components of a SiO2 sol. This is consistent with TG, LOI and VBT results.
For B2O3 sol finishing cotton fabric (C), its char residues with dispersive crevices are relatively continuous, complete, compact and dark gray (Fig. 9(C)). Similar to microscopic appearances of sample C before a VBT (Fig. 6(C)), present char resides still contain observable cotton fibers with natural shapes and orientations (Fig. 10(B)). However, particles (unreacted B2O3 sol components and some B2O3 gel clusters) on the surfaces of cotton fibers in Fig. 6(C) disappear. In addition, coverings on cotton fiber surfaces become wider. Also, there are areas of clear melted traces (bottom left part, Fig. 10(C)). This well reflects the fusing capability of a B2O3 gel. It melts and flows over cotton fiber surfaces at a temperature over 450.0 °C. However, a B2O3 gel also cracks due to dehydration processes and multiple physical forces as temperature rises (353.39 °C, Table 2). The produced crevices promote gradual and continuous pyrolyses of interior cotton fibers. Final char residues mainly come from the B2O3 gel and left components of a B2O3 sol. This is consistent with TG, LOI and VBT results.
For SiO2-B2O3 composite sol finishing cotton fabric (D), its char resides with a long crevice that is relatively continuous, complete and black (Fig. 9(B)). Apparent appearances of sample D in Fig. 9(B) are very similar to those of sample B but also show a minor difference, that is, an improved integrity level. They are quite different from those of sample C. On micro-scales, unlike microscopic appearances of sample D before a VBT (Fig. 6(D)), present char residues (Fig. 10(D)) show a few broken blocks and more observable cotton fibers. The former is derived from stronger cracking behaviors of a SiO2 gel at high temperatures, but it is compensated by fusing behaviors of a B2O3 gel. Additionally, two gels can chemically combined through S–O-B bonds and coated onto cotton fibers together through Si–O-C and B-O-C bonds (DRIFTS and XPS results). This again shows synergistic effects between two gels. Inevitably, crevices still occur due to further dehydration processes and multiple physical forces for the composite gel as temperature rises (358.58 °C, Table 2). The produced crevices promote gradual and continuous pyrolyzes of interior cotton fibers. Final char residues are further increased and mainly from two gels and left components of SiO2 and B2O3 sols. This is consistent with TG, LOI and VBT results. Certainly, based on all the results, for sample D, there are multiple aspects of flame retardancy performances and mechanisms as discussed in "Flame retardant performances of original and treated cotton fabrics section".
Flame retardant mechanisms of SiO2 and B2O3 composite sol–gel systems
According to the comprehensive results of DRIFTS, XPS, XRD, SEM–EDX, TG-DTG, LOI, and VBT of original and treated cotton fabrics, the flame retardant mechanisms of treated cotton fabrics are proposed as shown in Fig. 11.
A cotton fabric itself, as a kind of polysaccharide, is rich in -OH groups (chemical structure in Figs. 1(c) and 2, DRIFTS spectrum in Fig. 3 and XPS spectrum in Fig. 4) and has both crystalline and amorphous regions (XRD pattern in Fig. 5). When it is exposed to a fire, it burns almost completely (LOI value of only 18.0% in Table 3, few ashes left in a VBT) and long (an after-flame time of 23.0 s in Table 3) with clear smoldering behaviors (an after-glow time of 35.4 s in Table 3). It dehydrates (producing H2O), pyrolyzes (producing L-glucose and others including combustible intermediate products), forms chars (producing glucose and others, a char residue rate of 8.50% in a TG experiment performed in N2) and produces H2O, CO2 and a small number of ashes (VBT, Fig. 9). Cotton fiber structures and components are almost destroyed, lost or converted after a burning process.
When the SiO2-B2O3 composite sol is finished to cotton fiber surfaces, interior sol clusters are combining with cotton fibers through both chemical bonding effects (hydrogen bonds among SiO2 and B2O3 sol components and between them and cotton fibers) and multiple physical effects like capillary forces, Van der Waals forces, surface tensions, diffusibility. It is converted into a wider, evener and denser gel coating and almost completely fills up gaps between cotton fibers after drying at 90.0 ℃ and curing at 100.0 ℃.
The apparent and interior quality of the gel coating, i.e., purity, compactness, smoothness, thickness, rigidity and internal chemical structure configurations, are important in protecting cotton fibers below when they are exposed to heat. Fortunately, the SiO2-B2O3 gel coating reaches the optimal level mainly and inherently derived from linear triatomic shapes (trigonal geometries) of Si–O-Si bonds and boroxol rings (triangular rows with planes oriented in different ways) of B-O-B bonds in microscopic networks of SiO2 and B2O3 gels, dehydration and condensation reactions among a SiO2 sol / a B2O3 sol /cotton fibers, a high melting point of 1200 °C of a SiO2 gel network with strong structural support and dimensional stability, a low melting point around 450.0 °C of a B2O3 gel network with conducive fusing capability. As a result, the inorganic SiO2-B2O3 gel coating forms the most continuous and densest insulation barrier (Fig. 6(D)) onto cotton fibers with certain spacial arrangements of interior functional bonds like Si–O, Si–O-Si, Si–O-C, B-O, B-O-B, Si–O-B and speculated B-O-C (DRIFTS and XPS results, also Scheme 3 and Fig. 2). However, both a SiO2 gel and a B2O3 gel crack due to dehydration processes and multiple physical forces as temperature rises to 359.50 °C (Table 2) and 353.39 °C (Table 2). Hereby, a SiO2-B2O3 composite gel also cracks due to similar reasons at a temperature of 358.58 °C (Table 2).
On one hand, a SiO2 gel plays more role in structural support and dimensional stability (higher Ti and Tmax), while a B2O3 gel contributes more to a longer pyrolysis process and more char residues (higher Tf, Tdif and char residue rate). Moreover, the fused and glassy film-like mass spreads over and conceal possible cracks and pores induced by a SiO2 gel at high temperature. Besides the heat taken away by H2O molecules in dehydration processes and multiple physical forces during pyrolysis and combustion reactions, such produced SiO2-B2O3 composite gel coating can further form into chars (slags) on the cotton fiber surfaces and block heat, oxygen and gaseous products. Smoldering behaviors disappear in the burning processes. Overall thermal stability and corresponding flame retardancy level is further enhanced. On the other hand, the produced crevices promote gradual and continuous pyrolyses and burning behaviors of interior cotton fibers. Certainly, such flame retardancy is still restrained by inherent chemical structures and natures, physical properties and inorganic natures of SiO2 and B2O3 gels.
To sum up, a SiO2 sol–gel system and a B2O3 sol–gel system play both competitive and synergistic effects in flame retarding a cotton fabric. Generally, additive and synergistic effects of them on flame retardancy levels of the cotton fabric (sample D) win. In this case, four flame retardant mechanisms are shown, that is, condensed phase barrier (glassy B2O3 film and SiO2 char), cooling action (dehydration processes to release H2O molecules), noncombustible gas (H2O molecules) and synergistic actions (SiO2 and B2O3 protective gel layer both physically and chemically combined). These performances come from existence conditions, chemical structures and natures, spacial configurations of components in such gel coatings.
Conclusion
In this paper, by using TEOS and tributyl borate as precursors, single SiO2 sol, B2O3 sol, and SiO2-B2O3 composite sol were prepared by sol–gel methods. The results show that the sols do not change the crystal structures of cotton fibers and usually adhered to the non-crystalline areas of cotton fiber surfaces through both chemical bonding effects (hydrogen bonds among SiO2 and B2O3 sol components and between them and cotton fibers) and multiple physical effects (capillary forces, Van der Waals forces, surface tensions, diffusibility…). The inorganic SiO2-B2O3 gel coating forms the most continuous and densest insulation barrier onto the cotton fabric with special spacial arrangements of interior functional bonds including Si–O, Si–O-Si, Si–O-C, B-O, B-O-B, Si–O-B and speculated B-O-C. However, a SiO2-B2O3 composite gel usually cracks due to dehydration processes and multiple physical forces as temperature rises to 358.58 °C. Thanks to the high melting point, structural support and dimensional stability effects of a SiO2 gel, the fusing capability and longer pyrolysis process of a B2O3 gel, char formation capabilities of both gels, releases of noncombustible gases like H2O molecules and cooling effects of dehydration and multiple physical forces, general thermal stability and corresponding flame retardancy level of SiO2-B2O3 composite sol finishing cotton fabric is further enhanced. Results of various characterization techniques including DRIFTS, XPS, XRD, SEM–EDX, TGA, LOI and VBTs confirm that the SiO2-B2O3 composite sol finishing cotton fabric shows the greatest thermal stability and flame retardancy. It shows the highest Tf (430.62 ℃), Tdif (106.16 ℃) and char residue rate (43.82%) and second highest Ti (324.46 ℃) and Tmax (358.58 ℃) in a pyrolysis process, the highest LOI (25.7%) and ΔLOI/Δm (7.0%/g), the lowest after-flame time (10.0 s) and after-glow time (0.0 s, no smoldering process). Its char residue after a VBT is relatively complete and continuous. Clearly, a SiO2 sol–gel and a B2O3 sol–gel play more synergistic effects than competitive effects in flame retarding a cotton fabric.
In the future, in order to overcome the shortcomings of silica and boron trioxide gels and consider the washing resistances of finished cotton fabrics, it is necessary to design more inorganic–organic flame retardant formulations containing these gels or related chemical modifications of these gels, test and improve their service performances like washing resistances, mechanical and aging properties.
Data availability
No data was used for the research described in the article.
References
Gao WW, Zhang GX, Zhang FX (2015) Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose 22:2787–2796. https://doi.org/10.1007/s10570-015-0641-z
Zhang DQ, Williams BL, Shrestha SB, Nasir Z, Becher EM, Lofink BJ, Santos VH, Patel H, Peng XH, Sun LY (2017) Flame retardant and hydrophobic coatings on cotton fabrics via sol-gel and self-assembly techniques. J Colloid Interface Sci 505:892–899. https://doi.org/10.1016/j.jcis.2017.06.087
Silva-Santos MC, Oliveira MS, Giacomin AM, Laktim MC, Baruque-Ramos J (2017) Flammability on textile of business uniforms: use of natural fibers. Procedia Eng 200:148–154. https://doi.org/10.1016/j.proeng.2017.07.022
Li YC, Schulz J, Mannen S, Delhom C, Condon B, Chang SC, Zammarano M, Grunlan JC (2010) Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 4(6):3325–3337. https://doi.org/10.1021/nn100467e
Horrocks AR (2017) Flame Retardant finishes. In Textiles: Current Developments and Future Trends; Bahners T, Mittal K, Eds; Beverly: Scrivener Publishing LLC, MA, USA, 69–127. https://doi.org/10.1002/9781119426790.ch2
Alongi J, Carosio F, Malucelli G (2014) Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym Degrad Stab 106:138–149. https://doi.org/10.1016/j.polymdegradstab.2013.07.012
Periolatto M, Ferrero F (2015) Cotton and polyester surface modification by methacrylic silane and fluorinated alkoxysilane via sol-gel and UV-curing coupled process. Surf Coat Technol 271:165–173. https://doi.org/10.1016/j.surfcoat.2014.12.048
Caschera D, Toro RG, Federici F, Riccucci C, Ingo GM, Gigli G, Cellulose BC (2015) Flame retardant properties of plasma pre-treated/diamond-like carbon (DLC) coated cotton fabrics. Cellulose 22:2797–2809. https://doi.org/10.1007/s10570-015-0661-8
Davesne AL, Jimenez M, Samyn F, Bourbigot S (2021) Thin coatings for fire protection: An overview of the existing strategies, with an emphasis on layer-by-layer surface treatments and promising new solutions. Prog Org Coat 154. https://doi.org/10.1016/j.porgcoat.2021.106217
Gaan S, Salimova V, Rupperet P, Ritter A (2011) Flame retardant functional textiles, in: Functional Textiles for Improved Performance, Protection and Health, N. Pan and G. Sun Eds, Cambridge: Woodhead Publishing Limited, UK, 98–130. https://doi.org/10.1533/9780857092878.98
Perincek S, Uzgur AE, Duran K, Doğan A, Körlü AE, Bahtiyari IM (2009) Design parameter investigation of industrial size ultrasound textile treatment bath. Ultrason Sonochem 16(1):184–189. https://doi.org/10.1016/j.ultsonch.2008.06.003
Jelil RA (2015) A review of low-temperature plasma treatment of textile materials. J Mater Sci 50:5913–5943. https://doi.org/10.1007/s10853-015-9152-4
Carosioa F, Ghanadpour M, Alongi J, Wågberg L (2018) Layer-by-layer-assembled chitosan/phosphorylated cellulose nanofibrils as a bio-based and flame protecting nano-exoskeleton on PU foams. Appl Sci Pub 202(15):479–487. https://doi.org/10.1016/j.carbpol.2018.09.005
Viscusi G, Liparoti S, Pantani R, Barra G, Gorrasi G (2022) A layer-by-layer approach based on APTES/Cloisite to produce novel and sustainable high performances materials based on hemp fiberboards. Polym Degrad Stab 198. https://doi.org/10.1016/j.polymdegradstab.2022.109892
Bentis A, Boukhriss A, Gmouh S (2020) Flame-retardant and water-repellent coating on cotton fabric by titania-boron sol-gel method. J Sol-Gel Sci Technol 94:719–730. https://doi.org/10.1007/s10971-020-05224-z
Ismail WNW (2016) Sol-gel technology for innovative fabric finishing-A Review. J Sol-Gel Sci Technol 78:698–707. https://doi.org/10.1007/s10971-016-4027-y
Poli R, Colleoni C, Calvimontes A, Polášková H, Dutschk V, Rosace G (2015) Innovative sol-gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J Sol-Gel Sci Technol 74:151–160. https://doi.org/10.1007/s10971-014-3589-9
Huang KS, Nien YH, Hsiao KC, Chang YS (2006) Application of DMEU/SiO2 gel solution in the antiwrinkle finishing of cotton fabrics. J Appl Polym Sci 102:4136–4143. https://doi.org/10.1002/app.24246
Yuzer B, Guida M, Ciner F, Aktan B, Aydin MI, Meric S, Selcuk H (2016) A multifaceted aggregation and toxicity assessment study of sol–gel-based TiO2 nanoparticles during textile wastewater treatment. Desalin Water Treat 57(11):4966–4973. https://doi.org/10.1080/19443994.2014.1000387
Cheng XW, Liang CX, Guan JP, Yang XH, Tang RC (2018) Flame retardant and hydrophobic properties of novel sol-gel derived phytic acid/silica hybrid organic-inorganic coatings for silk fabric. Appl Surf Sci 427:69–80. https://doi.org/10.1016/j.apsusc.2017.08.021
Mahltig B, Textor T (2006) Combination of silica sol and dyes on textiles. J Sol-Gel Sci Technol 39:111–118. https://doi.org/10.1007/s10971-006-7744-9
Vasiljević J, Gorjanc M, Tomšič B, Orel B, Jerman I, Mozetič M, Vesel A, Simončič B (2013) The surface modification of cellulose fibers to create super-hydrophobic, oleophobic and self-cleaning properties. Cellulose 20:277–289. https://doi.org/10.1007/s10570-012-9812-3
Nozari B, Montazer M, Mahmoudi Rad M (2021) Stable ZnO/SiO2 nano coating on polyester for anti-bacterial, self-cleaning and flame retardant applications. Mater Chem Phys 267. https://doi.org/10.1016/j.matchemphys.2021.124674
Alongi J, Ciobanu M, Malucelli G (2012) Sol-gel treatments on cotton fabrics for improving thermal and flame stability: Effect of the structure of the alkoxysilane precursor. Carbohyd Polym 87:627–635. https://doi.org/10.1016/j.carbpol.2011.08.036
Alongi J, Malucelli G (2012) State of the art and perspectives on sol-gel derived hybrid architectures for flame retardancy of textiles. J Mater Chem 22:21805–21809. https://doi.org/10.1039/C2JM32513F
Alongi J, Ciobanu M, Tata J, Carosio F, Malucelli G (2011) Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol-gel treatments. J Appl Polym Sci 119:1961–1969. https://doi.org/10.1002/app.32954
Ferrero F, Periolatto M (2013) Application of fluorinated compounds to cotton fabrics via sol-gel. Appl Surf Sci 275:201–207. https://doi.org/10.1016/j.apsusc.2013.01.001
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym Degrad Stab 96(3):377–392. https://doi.org/10.1016/j.polymdegradstab.2010.03.036
Brancatelli G, Colleoni C, Massafra MR, Rosace G (2011) Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym Degrad Stab 96:483–490. https://doi.org/10.1016/j.polymdegradstab.2011.01.013
Fang YC, Sun WH, Li JW, Liu HL, Liu XH (2021) Eco-friendly flame retardant and dripping-resistant of polyester/cotton blend fabrics through layer-by-layer assembly fully bio-based chitosan/phytic acid coating. Int J Biol Macromol 175:140–146. https://doi.org/10.1016/j.ijbiomac.2021.02.023
Zhou L, Liang ZS, Li R, Huang D, Ren XH (2016) Flame-retardant treatment of cotton fabric with organophosphorus derivative containing nitrogen and silicon. J Therm Anal Calorim 128(2):653–660. https://doi.org/10.1007/s10973-016-5949-x
Zhu WJ, Hao SS, Yang MY, Cheng BW, Zhang JM (2020) A synergistic flame retardant of glycosyl cross-linking boron acid and ammonium salt of phytic acid to enhance durable flame retardancy of cotton fabrics. Cellulose 27:9699–9710. https://doi.org/10.1007/s10570-020-03417-x
Xie KL, Gao AQ, Zhang YS (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohyd Polym 98(1):706–710. https://doi.org/10.1016/j.carbpol.2013.06.014
Cromwell B, Levenson A, Levine M (2020) Thermogravimetric analysis of aromatic boronic acids for potential flame retardant applications. Thermochim Acta 683. https://doi.org/10.1016/j.tca.2019.178476
LeVan SL, Tran HC (1990) The role of boron in flame-retardant treatments. First International Conference on Wood Protection With Diffusible Preservatives 1990.https://srs.fs.usda.gov/pubs/5831
Chan SY, Si L, Lee KI, Ng PF, Chen L, Yu B, Hu Y, Yuen RK (2018) A novel boron-nitrogen intumescent flame retardant coating on cotton with improved washing durability. Cellulose 25(1):843–857. https://doi.org/10.1007/s10570-017-1577-2
Yang S, Zhang QX, Hu YF (2016) Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym Degrad Stab 133:358–366. https://doi.org/10.1016/j.polymdegradstab.2016.09.023
Cheng XW, Wu YX, Huang YT, Jiang JR, Xu JT, Guan JP (2020) Synthesis of a reactive boron-based flame retardant to enhance the flame retardancy of silk. React Funct Polym 156. https://doi.org/10.1016/j.reactfunctpolym.2020.104731
Zhang QH, Zhang W, Huang JY, Lai YK, Xing TL, Chen GQ (2015) Flame retardance and thermal stability of wool fabric treated by boron containing silica sols. Mater Des 85:796–799. https://doi.org/10.1016/j.matdes.2015.07.163
Zhang QH, Chen GQ, Xing TL (2017) Silk flame retardant finish by ternary silica sol containing boron and nitrogen. Appl Surf Sci 421:52–60. https://doi.org/10.1016/j.apsusc.2017.01.283
Li G, You F, Zhou ST, Wang ZH, Li D, Zhang XF, Zhou C, Zhuang CH, Zhao YP (2022) Preparations, characterizations, thermal and flame retardant properties of cotton fabrics finished by boron-silica sol-gel coatings. Polym Degrad Stab 202. https://doi.org/10.1016/j.polymdegradstab.2022.110011
Li D, Wang Z, Zhu Y, You F, Zhou S, Li G, Zhang XF, Zhou C (2022) Synergistically improved flame retardancy of the cotton fabric finished by silica-coupling agent-zinc borate hybrid sol. J Ind Text 51(5S):8297S-8322S. https://doi.org/10.1177/15280837211028800
Kumar B (1984) Sol-gel processing of SiO2-B2O3 glasses. Mater Res Bull 19(3):331–338. https://doi.org/10.1016/0025-5408(84)90175-2
Villegas MA, Navarro JM (1988) Characterization of B2O3-SiO2 glasses prepared via sol-gel. J Mater Sci 23(7):2464–2478. https://doi.org/10.1007/BF01111904
Villegas MA, Aparicio M, Durán A (1997) Thick sol-gel coatings based on the B2O3-SiO2 system. J Non-Cryst Solids 218:146–150. https://doi.org/10.1016/S0022-3093(97)00073-2
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohyd Polym 58(4):417–420. https://doi.org/10.1016/j.carbpol.2004.08.005
Zhang QH, Gu JL, Chen GQ, Xing TL (2016) Durable flame retardant finish for silk fabric using boron hybrid silica sol. Appl Surf Sci 387:446–453. https://doi.org/10.1016/j.apsusc.2016.06.119
Moharram MA, Abou El Nasr TZ, Hakeem NA (1981) X-Ray diffraction and infrared studies on the effect of thermal treatments on cotton celluloses I and II. J Polym Sci Polym Lett Edition 19(4):183–187. https://doi.org/10.1002/pol.1981.130190405
Przekop R, Kirszensztejn P (2014) Porous xerogel systems B2O3-Al2O3 obtained by the sol-gel method. J Non-Cryst Solids 402:128–134. https://doi.org/10.1016/j.jnoncrysol.2014.05.027
Li SQ, Tang RC, Yu CB (2022) Flame retardant treatment of jute fabric with chitosan and sodium alginate. Polym Degrad Stab 196. https://doi.org/10.1016/j.polymdegradstab.2022.109826
Xu WZ, Zhong D, Chen R, Cheng ZH, Qiao MX (2021) Boron phenolic resin/silica sol coating gives rigid polyurethane foam excellent and long-lasting flame-retardant properties. Polym Adv Technol 32(10):4029–4040. https://doi.org/10.1002/pat.5408
Ma J, Li W, Fu GQ, Zhu MY (2021) Effect of B2O3 on the melting temperature and viscosity of CaO-SiO2-MgO-Al2O3-TiO2-Cr2O3 slag. J Sustain Metallurgy 7:1190–1199. https://doi.org/10.1007/s40831-021-00413-8
Acknowledgements
The study is financially supported by the National Natural Science Foundation of China (Grant No. 51376089 and 50906039) and the 2019 Key Project of The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJA520007, A Class).
Funding
National Natural Science Foundation of China, No. 51376089, Fei YOU, No. 50906039, Fei YOU.
Author information
Authors and Affiliations
Contributions
Chang Zhou: Conceptualization, Methodology, Formal analysis, Writing-original draft. Fei You: Supervision, Project administration, Funding acquisition. Songtao Zhou: Conceptualization, Methodology, Writing-review & editing. Zhenhua Wang: Investigation, Software, Data curation. Dan Li: Investigation, Software, Data curation. Gang Li: Resources, Visualization. Xuefeng Zhang: Resources, Visualization. Yu Pan: Investigation, Validation. Junqi Wang: Investigation, Validation. Jing Ma: Investigation, Validation.
Corresponding author
Ethics declarations
Conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, C., Zhou, S., You, F. et al. Effectively improving flame retardancy levels of finished cotton fabrics only by simple binary silicon-boron oxide sols. J Polym Res 30, 437 (2023). https://doi.org/10.1007/s10965-023-03812-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03812-5