Abstract
In the present study, we report a new process for modifying cellulosic fabrics by using ionic liquids. To this aim, 1-methylimidazolium chloride propyltriethoxysilane and 1-pyridinium chloride propyltriethoxysilane salts were synthesised. Then, the cotton fabrics were treated with sols containing these salts by the pad-dry-cure process. Finally, the treated fabrics were impregnated in a diluted solution of HPF6 to perform the metathesis reaction. The morphology and chemical composition of treated and untreated fabrics were analysed by scanning electron microscopy, elemental analysis and infrared spectroscopy. The droplet shape analysis confirmed that the water repellency of the fabrics was significant after surface modification. The flame retardancy was also enhanced by using the PF6 anion. Thermogravimetric analysis was used to assess the thermal stability of these treated fabrics in air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
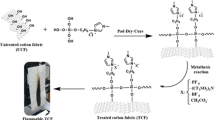
The ionic liquids (ILs) belong to diverse groups of salts that are liquid below 100 °C. They are typically constituted of a large organic cation and an inorganic polyatomic anion. There is virtually no limit to the number of possible ILs since there are many cations and anions that can be combined (Jiu-Ju et al. 2013). ILs have been the subject of increasing attention because of their unique physicochemical properties such as high thermal stability, high ionic conductivity as well as tremendous solvating capacity (Keskin et al. 2007). Non-volatility and non-flammability are their common characteristics, making them quite relevant materials for many applications. Their range of applications includes electrolytes in batteries, solvents and catalysis in synthesis (Böhm et al. 2014; Ferreira et al. 2014b; Jinmei et al. 2011; Le et al. 2014; Martín et al. 2014). They are also used as matrices for mass spectroscopy and as solvents to synthesise nanomaterials (Brisinski et al. 2014; Pratap et al. 2014; Ruiz et al. 2014; Zhaoxian et al. 2014). In textile industries, ILs are employed to remove textile dyes from aqueous solutions (Ferreira et al. 2014a; Gao et al. 2013; Hejun et al. 2013; de Menezes et al. 2012) and to impart antifungal properties to linen fabrics (Foksowicz-Flaczyk and Walentowska 2013), and a few patents have addressed the use of ionic liquids as “green” flame retardants for textile fabrics (Xu 2012). ILs may be introduced onto the fibers or fabrics using chemical post-treatment methods, for instance, during the dyeing (Price et al. 2006). In the current study, we have demonstrated that the immobilisation of ionic liquids onto textile matrices can be achieved by the sol–gel process. This synthesis method is currently attracting growing interest for textile functionalisation because of its simplicity and flexibility. As reported in the literature, ILs may play different roles in synthesis procedures. They have been shown to be able to act as chemical additives for drying control, catalysts, structure directing agents, solvents and even precursors for achieving homogeneous gel fibers and adsorbent hybrid materials (de Menezes et al. 2012; Tarkanovskaja et al. 2014). In our case, we have prepared organosilanes based on ILs to enhance flame stability and water-repellent properties of cotton fabrics. For this aim, 1-methylimidazolium chloride propyltriethoxysilane (MCPTS) and pyridinium chloridetriethoxysilane (PCPTS) salts were synthesised and characterised by means of 1H and 13C NMR, mass spectroscopy as well as infrared spectroscopy. Then, we prepared different sols with these salts, which were coated onto cotton fabrics by the pad-dry-cure process (Fig. 1). Afterwards, sol–gel modified textiles were impregnated in a diluted solution of HPF6 acid to carry out the metathesis reaction to replace the chloride anions with PF6 − (Fig. 2). Indeed, PF6 −-based ILs are expected to confer better flame stability to cotton fabrics. Thus, in order to assess the final properties of Il-coated cottons, water-repellency and flame-retardancy tests were carried out. Finally, the thermal stability of the sol–gel modified samples was investigated by thermogravimetric analysis.
Materials and methods
Materials
Cotton (CO) woven fabric weighing 168 g m−2 was used. The chloropropyltriethoxysilane (CPTS, Mw: 240.79 g mol−1), ethyl alcohol (EtOH, 99 %), HCl (37 %), 1-methylimidazole (99 %), pyridine (99 %) and hexafluorophosphoric acid (HPF6, 65 %) were purchased from Sigma-Aldrich Co. All the chemicals were analytically pure.
Synthesis of 1-methylimidazolium and pyridinium chloride propyltriethoxysilanes (MCPTS and PCPTS)
The schematic diagram of the synthetic procedure for the MCPTS and PCPTS salts is shown in Scheme 1. One eq 1-methylimidazole or pyridine and 1 eq CPTS were added to a round-bottom flask fitted to a reflux condenser and allowed to react at 100–115 °C for 18 h to obtain 1-methylimidazolium chloride propyltriethoxysilane (MCPTS) and pyridinium chloride propyltriethoxysilane (PCPTS) salts, respectively. An orangish viscous liquid was obtained for MCPTS salts and a brownish viscous liquid for PCPTS salts.
MCPTS
FTIR (cm −1 )
2973–2888 (C–H), 1570 (C=C stretching of the imidazole ring), 1389 (stretching vibrations of C–N of imidazole ring), 1166 (C–O), 1077 (Si–O) (Mahltig and Bottcher 2003).
1 H NMR (300 MHz, MeOD, δppm)
7.63 (s, 1H, N–CHN),7.06 (s, 1H, NCH=CHN), 6.94 (s, 1H, NCH=CHN), 4.20 (t, 2H, J = 7.20 Hz, NCH2), 3.86 (s, 3H, NCH3), 3.81(q, 6H, J = 6.9 Hz, O–CH2), 3.31 (t, 9H, J = 3.3 Hz, O–CH2–CH3), 1.2(quint, 2H, J = 5.1 Hz, CH2–CH2–CH2), 0.604 (t, 2H, J = 9 Hz, Si–CH2).
13 C (DMSO, 300 MHz, δppm)
147.52, 137.73, 129.86, 114.85, 76.39, 58.58, 52.62, 45.26, 40.53, 40.25, 39.97, 39.70, 39.42, and 12.15.
LC/MSESI: m/z
Calculated: 322.15; found: cation (287), ion corresponding to a cluster 2C + Cl (608.89).
PCPTS
FTIR (cm −1 )
1630 (C=C), 1385 (C–N), 1166 (C–O), 1077 (Si–O).
1 H NMR (300 MHz, MeOD, δppm)
8.14–8.19 (d, 2H), 7.73–7.78 (t, 1H), 7.32–7.37 (d, 2H), 4.66 (t, 3H, NCH3), 3.73 (q, 6H, J = 6.9 Hz, O–CH2), 3.4 (t, 9H, J = 7 Hz, O–CH2–CH3), 1.02 (q, 2H, J = 5.1 Hz, CH2–CH2–CH2), 0.53 (t, Si–CH2).
13 C (DMSO, 300 MHz, δppm):
62.97, 58.23, 56.37, 48.041, 25.52, 18.94, 18.57, 6.94, 124.38, 128.52, 136.71, 145.94, and 149.89.
LC/MSESI: m/z
Calculated: 319, 14; found: cation (284, 18), ion corresponding to a cluster 2C + Cl (602, 85).
Sol–gel treatment of textile
(MCPTS, PCPTS), distilled water, EtOH (99 %) and HCl (37 %) were mixed with a molar ratio (MCPTS, PCPTS)/HCl/EtOH/H2O of 5/0008/60/55. Then, the mixture was stirred for 3 h at 70 °C until a homogeneous solution was obtained. The textile samples were impregnated in this sol for 24 h and then padded to give 80 % weight pick-up. The dry and cure temperatures were respectively 80 and 120 °C for 1 h. For comparison, the sample without the hybrid coating was cured under the same condition. The coated fabrics were dipped rapidly in a solution of HPF6 acid (0.66 mol/l), washed with distilled water for several times and dried separately at 80 and 120 °C. The resulting hybrid coatings are named [MCPTS]PF6 and [PCPTS]PF6. For comparison, hybrid coatings with a CPTS precursor were prepared by the same procedure. The mechanism of the reaction between ionic liquid functionalised siloxanes and cellulose is the same as the one described in a previous paper (Boukhriss et al. 2015).
FI-IR spectra
The infrared spectra were recorded on a Nicolet iS10 FTIR-ATR spectrophotometer.
Mass spectroscopy
ESI-MS data were obtained on a Quattro II tandem quadripole mass spectrometer (Micromass, Manchester, UK) fitted with electrospray ionisation.
RMN
1H NMR and 13C NMR spectra were recorded on Bruker Avance (300 MHz) apparel using TMS as internal reference.
SEM
First, a gold coating was sputtered onto textile samples to make them conductive. The SEM micrographs were recorded using a ZEISS Supra 55VP scanning electron microscope operating under high vacuum at 3 kV and using a secondary electron detector (Everhart-Thornley detector). Qualitative analysis of chemical elements was performed using an Oxford Instruments Aztec Energy Dispersive X-ray Spectroscopy (EDX) system with an X-Max 50 Silicon Drift Detector.
Water-repellency test
The dip test was performed to investigate the water repellency under the customary conditions of the coated textiles. To this aim, the water uptake of the textile under full contact with water was determined. A textile sample of 10 cm × 10 cm was placed in 300 ml of distilled water for 1 min. The water uptake by the textile during placement under water was determined using a balance (Mahltig et al. 2005; Mahltig and Bottcher 2003).
Flame-retardancy test
The flammability test in vertical configuration was carried out by applying a butane flame of 4 cm for 20 s [sccording to the ISO 6940:2004(F) standard] at the bottom of a fabric sample (20 cm × 10 cm). The test was repeated twice for each formulation measuring the burning time, burning surfaces and final residue weight.
Stability to the washing test
The stability of hybrid materials coated onto cotton fabrics was investigated according to the ISO 105-C06:2010 standard. The samples (20 cm × 10 cm) were treated in a 400-ml bath of ECE (European Colour-fastness Establishment) standard detergent with pH = 9.7. The washing was carried out in a standard machine (WashTEC Roaches) at 40 °C for 30 min. The washing procedure was repeated for three cycles.
TG analysis
The thermal stability of the fabrics was evaluated by thermogravimetric (TG) analyses from ambient temperature to 1100 °C with a heating rate of 10 °C/min. A SETARAM SETSYS evolution analyser was used, placing the samples in an open alumina crucible in the presence of air atmosphere.
Result and discussion
Characterisation of cotton fabric
In order to assess the morphology of the coatings deposited on cotton fibers by the sol–gel process, SEM observations were made. The typical morphology of cotton fibers is reported in Fig. 3a; as expected, the surface of untreated cotton fibers exhibits a certain level of irregularity. After treatment with [MCPTS]PF6, slight differences are observed between the untreated and treated sample surfaces, which became smoother (Fig. 3b).
The chemical compositions of untreated and treated fabrics were determined by elemental analysis. Untreated fabrics do not contain the Si, F, P and N elements, while in the sol–gel-treated ones, the presence of these elements was evidenced, as shown in Fig. 4. In the FTIR spectra, gathered in Fig. 5, the vibration band at 835 cm−1 indicates the presence of PF6 on the surface of the cotton fabric and thus confirms that the metathesis reaction was successful. Moreover, the band located at 1083 cm−1 is likely due to the vibration of the Si–C bonds in the silica networks.
Water-repellent properties
An untreated CO textile is hydrophilic so that a water droplet placed onto its surface soaks completely through the textile as shown in Fig. 6a. However, after sol–gel treatment with IL-based organosilanes, the surface of the treated fabrics nearly supports the formation of spherical droplets (Fig. 6b). To evaluate the hydrophobic properties of fabric coated with [MCPTS]PF6 and [PCPTS]PF6, investigations on coatings were performed using dip tests on CO fabrics. With increasing drying temperature, the amount of water uptake by the textile decreases. After the dip test, the uncoated CO sample exhibits a weight increase of 340 %. Samples sol–gel coated with [MCPTS]PF6 and dried respectively at 80 and 120 °C lead to a lower water uptake percentage, i.e., 235 and 50 %, respectively (Fig. 7). This behaviour may be induced by the rise of the temperature, which promotes the siloxane network formation and thus the IL adhesion onto the textile. The same trends were observed for cotton treated with [PCPTS]PF6.
Flame retardancy and thermal stability
Several articles on flame-retardant cellulose have been published. Significant efforts have been made to improve the flame-retardant property of cotton textiles using various methods and chemical compounds that can react with the cellulosic fiber or form cross-linked structures on the fiber (Basak et al. 2015; Han et al. 2015; Nguyen et al. 2012; Waly et al. 2012; Yang and Yang 2005; Yang et al. 2012). In this work, as already demonstrated, the sol–gel process was a useful method to enhance the flame retardancy of synthetic and natural textiles. To this aim, metal alkoxides based on silicon, titanium and zirconium have already been used (Alongi et al. 2010, 2012b).
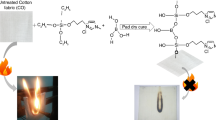
In the present work, ionic liquids combined with a silica precursor showed good resistance to a direct flame. Indeed, as shown in Fig. 8b, d, untreated and treated cotton with CPTS keeps burning for 45 and 67 s after the removal of the flame, respectively. The final residue after the test is 1 % (in weight) for untreated cotton and 15 % for the one treated with CPTS, and the burned area is 200 cm2 for both samples. However, cotton fabrics functionalised with [MCPTS]PF6 or [PCPTS]PF6 do not burn. As shown in Fig. 8e, f, treated cotton does not flame during and after the removal of the flame. The weight of the final residue is increased from 1 to 93 %, and the burned area decreased from 200 to 56 cm2 (see Table 1).
The results obtained with the ionic liquids are much more significant compared to those obtained with the pure silica, as previously reported by Alongi et al. (2012a). A final residue of 48 % was obtained for cotton treated with tetramethylorthosilicate (TMOS) after the application of a flame for 5 s. In our case, a highest final residue of 93 % was obtained after the application of a flame for 20 s. The comparison of these results allows concluding that ionic liquids can be considered as excellent flame retardants for cotton textiles.
To investigate the hybrid coating stability to washing, an ISO 105-C06:2010 standard test was performed. After three washing cycles, the infrared spectrum (Fig. 9) evidenced the departure of PF6 − anions, whereas the silica bands were still present. This departure of PF6 − anions may be justified by the weak interaction between these anions and pyridinium or imidazolium cations. The flammability test was carried out for washed samples. The results are gathered in Table 2. It can be noticed that when the PF6 − anions were removed after three washing cycles, the samples were almost completely burnt.
To study the thermal stability of cotton fabrics, thermogravimetric curves of the samples in air are plotted in Fig. 10. For the untreated cellulosic fibers, three steps can be distinguished as previously reported (Price et al. 1997). The first one occurs between 100 and 430 °C and involves two competitive pathways, which yield aliphatic chars and volatile products; the second step, between 430 and 530 °C, corresponds to the conversion of aliphatic chars into aromatics, producing carbon monoxide and carbon dioxide because of the simultaneous carbonisation. During the third and last decomposition step, which ends at 620 °C, the char is oxidised. Figure 9 shows the effect of [MCPTS]PF6 and [PCPTS]PF6 coatings on the thermal degradation of cotton fabrics. Based on thermogravimetric curves, there are several distinct thermal degradation behaviours. Treated samples clearly exhibit an improvement of the thermal stability even though they start to be decomposed earlier than the untreated sample. After 430 °C, the pure cotton sample exhibits a lower thermal stability in comparison with sol–gel-treated samples. At 620 °C, the untreated sample was completely decomposed, while the remaining residues (% weight) for cotton fabrics treated with [MCPTS]PF6 and [PCPTS]PF6 were respectively 31 and 16 %.
Conclusion
In this article, we have developed a very original methodology to synthesise two onium salts based on methylimidazole and pyridine. These salts were then grafted on cotton fabrics via organosilane-derived compounds. The treated fabrics were subsequently subjected to a metathesis reaction with HPF6 acid to obtain PF6 − anions on their surface.
Different characterisations have evidenced that the deposition of ionic liquids onto the textiles by the sol–gel process was effective. The results showed that these fabrics exhibit excellent water-repellent and flame-retardant properties. Indeed, untreated cottons keep burning for 45 s after removal of the flame, unlike those functionalised with [MCPTS]PF6 and [PCPTS]PF6, which did not burn. As a result, based on these relevant results, ionic liquids can be considered as excellent flame retardants for cotton textiles. Unfortunately, in order to retain their fire resistance, PF6 − anions have to be trapped into the cotton fabrics and not removed after the washing test. Future work will be devoted to this task to enhance the stability of PF6 − anions inside cotton fabrics upon the washing test.
In addition, the thermal study showed that the fabrics coated with hybrid materials based on ionic liquids are more thermally stable compared to the untreated ones.
References
Alongi J, Ciobanu M, Tata J, Carosio F, Malucelli G (2010) Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol–gel treatments. J Appl Polym Sci 119:1961–1969
Alongi J, Ciobanu M, Malucelli G (2012a) Sol–gel treatments on cotton fabrics for improving thermal and flame stability: effect of the structure of the alkoxysilane precursor. Carbohydr Polym 87:627–635
Alongi J, Ciobanu M, Malucelli G (2012b) Thermal stability, flame retardancy and mechanical properties of cotton fabrics treated with inorganic coatings synthesized through sol–gel processes. Carbohydr Polym 87:2093–2099
Alongi J, Colleoni C, Malucelli G, Rosace G (2012c) Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym Degrad Stab 97:1334–1344
Basak S, Samanta KK, Saxena S, Chattopadhyay SK, Narkar R, Mahangade R, Hadge GB (2015) Flame resistant cellulosic substrate using banana pseudostem sap. Pol J Chem Technol 17:123–133
Böhm M, Tietze AA, Heimer P, Chen M, Imhof D (2014) Ionic liquids as reaction media for oxidative folding and native chemical ligation of cysteine-containing peptides. J Mol Liq 192:67–70
Boukhriss A, Boyer D, Hannache H, Roblin JP, Mahiou R, Cherkaoui O, Therias S, Gmouh S (2015) Sol–gel based water repellent coatings for textiles. Cellulose 22:1415–1425
Brisinski V, Spitczok N, Oliver H, Frank E (2014) Plasma electrochemistry in ionic liquids: from silver to silicon nanoparticles. J Mol Liq 192:59–66
de Menezes EW, Lima EC, Royer B, de Souza FE, dos Santos BD, Gregorio JR, Costa TM, Gushikem Y, Benvenutti EV (2012) Ionic silica based hybrid material containing the pyridinium group used as an adsorbent for textile dye. J Colloid Interface Sci 378:10–20
Ferreira AM, Coutinho JAP, Fernandes AM, Freire MG (2014a) Complete removal of textile dyes from aqueous media using ionic-liquid-based aqueous two-phase systems. Sep Purif Technol 128:58–66
Ferreira AR, Freire MG, Ribeiro JC, Lopes FM, Crespo JG, Coutinho JAP (2014b) Ionic liquids for thiols desulfurization: experimental liquid–liquid equilibrium and COSMO-RS description. Fuel 128:314–329
Foksowicz-Flaczyk J, Walentowska J (2013) Antifungal activity of ionic liquid applied to linen fabric. Int Biodeterior Biodegrad 84:412–415
Gao H, Kan T, Zhao S, Qian Y, Cheng X, Wu W, Wang X, Zheng L (2013) Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer. J Hazard Mater 261:83–90
Han Y, Zhang X, Wu X, Lu C (2015) Flame retardant, heat insulating cellulose aerogels from waste cotton fabrics by in situ formation of magnesium hydroxide nanoparticles in cellulose gel nanostructures. ACS Sustain Chem Eng 3:1853–1859
Hejun G, Yun W, Liqiang Z (2013) Hydroxyl-functionalized ionic liquid-based cross-linked polymer as highly efficient adsorbent for anionic azo dyes removal. Chem Eng J 234:372–379
Jinmei M, Hui W, Guofeng G (2011) Synthesis of immobilized Brønsted acidic ionic liquid on silica gel as heterogeneous catalyst for esterification. Catal Commun 12:353–356
Jiu-Ju F, Zhang-Ying L, Su-Fang Q, Ao-Qi L, Yao F, Wang A-J (2013) N-methylimidazole-assisted electrodeposition of Au porous textile-like sheet arrays and its application to electrocatalysis. Electrochim Acta 102:312–318
Keskin S, Kayrak-Talay D, Akman U, Hortaçsu Ö (2007) A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids 43:150–180
Le KA, Rudaz C, Budtova T (2014) Phase diagram, solubility limit and hydrodynamic properties of cellulose in binary solvents with ionic liquid. Carbohydr Polym 105:237–243
Mahltig B, Bottcher H (2003) Modified silica sol coatings for water-repellent textiles. J Sol-Gel Sci Technol 27:43–52
Mahltig B, Audenaert F, Bottcher H (2005) Hydrophobic silica sol coatings on textiles—the influence of solvent and sol concentration. J Sol-Gel Sci Technol 34:103–109
Martín M, Darío R, Pablo A, Karin G (2014) Ionic liquids as solvents for liquid scintillation technology. Čerenkov counting with 1-Butyl-3-Methylimidazolium Chloride. Radiat Phys Chem 98:98–102
Nguyen T-MD, Chang S, Condon B, Uchimiya M, Fortier C (2012) Development of an environmentally friendly halogen-free phosphorus-nitrogen bond flame retardant for cotton fabrics. Polym Adv Technol 23:1555–1563
Pratap SM, Kumar SR, Suresh C (2014) Ionic liquids confined in porous matrices: physicochemical properties and applications. Prog Mater Sci 64:73–120
Price D, Horrocks AR, Akalinc M, Faroqd AA (1997) Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in air. J Anal Appl Pyrolysis 40–41:511–524
Price KN, Wang J, Washington NM, Hecht SE, Miracle GS, Scheibel JJ (2006) Process for modifying textiles using ionic liquids. WO 2006/050300 A2. https://www.google.ch/patents/WO2006050300A2?cl=en
Ruiz E, Ferro VR, de Riva J, Moreno D, Palomar J (2014) Evaluation of ionic liquids as absorbents for ammonia absorption refrigeration cycles using COSMO-based process simulations. Appl Energy 123:281–291
Tarkanovskaja M, Välbe R, Põhako-Esko K, Mäeorg U, Reedo V, Hoop A, Saal K, Krumme A, Kink I, Heinmaa I et al (2014) Novel homogeneous gel fibers and capillaries from blend of titanium tetrabutoxide and siloxane functionalized ionic liquid. Ceram Int 40:7729–7735
Waly AI, Elmaaty TMA, Abd El-Shakour SA (2012) Modification of cellulosic fabrics to impart flame retardancy properties. J Appl Polym Sci 123:2147–2153
Xu Y (2012) Ionic liquid flame retardants. WO 2012/021146A1
Yang H, Yang CQ (2005) Durable flame retardant finishing of the nylon/cotton blend fabric using a hydroxyl-functional organophosphorus oligomer. Polym Degrad Stab 88:363–370
Yang Z, Wang X, Lei D, Fei B, Xin JH (2012) A durable flame retardant for cellulosic fabrics. Polym Degrad Stab 97:2467–2472
Zhaoxian L, Teng Z, Lifang C, Yinmei Y, Kai S, Zhiwen Q (2014) Simulation based ionic liquid screening for benzene–cyclohexane extractive separation. Chem Eng Sci 113:45–53
Acknowledgments
The authors gratefully acknowledge Campus France for the financial support through PHC Toubkal project no. 32506VM.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Boukhriss, A., Gmouh, S., Hannach, H. et al. Treatment of cotton fabrics by ionic liquid with PF6 − anion for enhancing their flame retardancy and water repellency. Cellulose 23, 3355–3364 (2016). https://doi.org/10.1007/s10570-016-1016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1016-9