Abstract
Convective drying of strawberries was performed with or without ethanol pre-treatment (ET) in their fresh state or enriched with isomaltulose osmotic dehydration (OD) or by pulsed vacuum osmotic dehydration (PVOD). Both osmotic processes were executed by the immersion of 10 mm strawberry cubes in the osmotic solution for 300 min. The PVOD was done at reduced pressure (160 mbar) during the first 20 min. The ET was conducted by immersion of the samples in 95% ethanol for 2 min. Convective drying (60 °C and 1 m s−1) was performed until a final moisture content of 11.50%w.b.. The convective drying required from 195 to 300 min. The non-osmotically dehydrated dried sample required a shorter drying time and produced samples with lower water activity, hardness and color change, and higher total anthocyanin content (TAC), total phenolic content (TPC), and antioxidant capacity (AC). In addition to promoting enrichment with isomaltulose, both osmotic processes reduced shrinkage and hygroscopicity compared to non-osmotically dehydrated dried samples. ET reduced drying time by up to 30%, reduced hygroscopicity, and contributed to TAC, TPC, and AC preservation, showing as an interesting procedure to be applied in the convective drying of strawberries. However, the ET increased the shrinkage of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration (OD) is considered a unitary operation of mass transfer between the food and the osmotic solution where the food is immersed. In this process, the two main flows are solid gain and water loss. The solid gain flow represents the incorporation of the solute from the osmotic solution to the food (González-Pérez et al., 2021; Ramya & Jain, 2017). This flow makes OD an interesting technology to enrich fruits, especially when the solute is beneficial to human health, such as isomaltulose (also known as palatinose), which is a low glycemic and cariogenic index carbohydrate (Lazou et al., 2020; Sawale et al., 2017).
The other flow refers to the diffusion of water from the food to the osmotic solution. A third flow also occurs, which is the leaching of compounds, such as bioactive and aromatic compounds. This flow is quantitatively negligible, but it can result in significant changes qualitatively (Ramya & Jain, 2017). To maximize solid gain and water loss flows, the vacuum application at the beginning of the osmotic process promotes the replacement of the occluded air by osmotic solution, increasing the area of contact with the osmotic solution (González-Pérez et al., 2021).
OD reduces the moisture content and water activity of the food, but not enough to ensure stability and significantly extend the shelf life of the product (Ramya & Jain, 2017). Therefore, convective drying is commonly applied after OD (Kroehnke et al., 2021; Macedo et al., 2021b; Mendonça et al., 2017; Ramya & Jain, 2017; Turkiewicz et al., 2020), where heated air is used to complete the removal of water from the food. This is a low-cost, simple structure and convenient operation process (Liu et al., 2020; Wang et al., 2019). However, drying can influence the quality parameters of the food, causing loss of heat-sensitive nutrients, such as bioactive compounds, present in fruits (Macedo et al., 2020; Mendonça et al., 2017; Omolola et al., 2017).
Strawberries are non-climacteric fruits of high sensory appreciation, due to their attractive color and their characteristic aroma and flavor. Furthermore, the strawberry has high levels of antioxidant compounds, such as phenolics and anthocyanins. These compounds are beneficial to health, as they are associated with reduced risk of several diseases, such as cancer, cardiovascular disorders, neurodegenerative, and other chronic diseases (Basu et al., 2014). However, strawberries are very perishable, with a shelf life of a few days postharvest. Therefore, the use of preservation methods, such as convective drying, is essential for this fruit. However, studies have shown that convective drying has resulted in significant losses in the content of anthocyanins, phenolics, and antioxidant activity in strawberries (Karam et al., 2016; López-Ortiz et al., 2020; Méndez-Lagunas et al., 2017).
Treatments applied before drying fruits have been studied, aiming to accelerate the process of convective drying to reduce the time of exposure of the product to heated air (Wang et al., 2019). The addition of low-boiling organic solvents, such as ethanol, acetic acid, and isopropanol, is a pre-treatment that reduces the boiling point of water, due to the mixture of solvent + water. Furthermore, the solvent increases cell wall permeability, aiding the removal of water (Corrêa et al., 2012). As a low-cost and non-toxic solvent, ethanol has been the most used (Tatemoto et al., 2015). Studies have reported beneficial effects of ethanol pre-treatment on the drying of apple (Rojas et al., 2020a), banana (Corrêa et al., 2012), guaco leaves (Silva et al., 2018), melon (da Cunha et al., 2020), pineapple (Braga et al., 2009, 2010; Santos & Silva, 2009), pitaya (Araújo et al., 2020; Macedo et al., 2021a), potato (Rojas et al., 2019; Rojas & Augusto, 2018a), pumpkin (Carvalho et al., 2020; Rojas et al., 2020b; Rojas & Augusto, 2018c), and scallion (Wang et al., 2019).
This work aimed to evaluate the influence of (i) osmotic dehydration, using isomaltulose as a solute, with or without pulsed vacuum, and (ii) ethanol pre-treatment on the drying kinetics and quality parameters, such as moisture content, water activity, shrinkage, hygroscopicity, color, total anthocyanins, total phenolics, and antioxidant capacity of strawberry.
Material and Methods
Raw Material
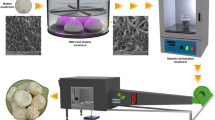
Fresh strawberries fruits, cultivar “Camino Real,” were purchased from the local market, selected for color (predominantly red skin), integrity (no injury), weight (19.41 ± 5.41 g), and maturation stage (8.28 ± 0.63°Brix). The strawberries were then washed in running water, sanitized with chlorinated water (200 ppm for 10 min), rinsed, drained with absorbent paper, and stored at 4 °C for up to 2 days. The fruit skin was removed and the inside was cut into 10 mm cubes (Fig. 1).
OD and PVOD
The osmotic solution of 35% (w w−1) of isomaltulose (Beneo-Palatinit, Mannheim, Germany) was prepared and used for OD and pulsed vacuum osmotic dehydration (PVOD) of strawberry cubes, by immersing the cubes in the osmotic solution (Fig. 1), in the proportion of 1:20 (w w−1), at 25 °C, for 300 min. OD was performed at atmospheric pressure throughout the process. PVOD was performed with vacuum application (160 mbar, absolute pressure) during the first 20 min. Then, atmospheric pressure was resumed. After the osmotic processes, the samples were immersed in an ice bath for 10s to interrupt the mass flow. The sample surfaces were drained with absorbent paper (Corrêa et al., 2016).
Ethanol Pre-treatment
Fresh and osmotic dehydrated by OD or PVOD samples were ethanol pre-treated (Fig. 1). The ethanol pre-treatment was performed by immersing the sample in 95% ethanol (v v−1), in the proportion of 1:5 (w v−1), for 2 min, at 25 °C.
Convective Drying
Drying Operation
Samples, fresh and dehydrated by OD or PVOD, non- or ethanol pre-treated were dried in a tunnel dryer (Eco Engenharia Educacional, MD018 model, Brazil) (Fig. 1), with the parallel flow at 60 °C and 1.0 m s−1. For each drying, 60.38 ± 0.44 g of sample was used. The sample was weighed during drying, at 15-min intervals, with the aid of an analytical balance (Marte Científica, AD33000 model, Brazil) (accuracy ± 0.01 g) coupled to the sample holder. Drying was carried out until the samples had a moisture content of 11.50 ± 0.96%w.b.. This value was established so that the samples of all treatments had water activity below 0.6 (Jay et al., 2005).
Moisture Ratio
The moisture ratio (MR) of the samples during drying was calculated according to Eq. (1). The moisture content at equilibrium (Xe) was determined by drying the samples for 1440 min.
where X0, Xt, and Xe represent the dry basis moisture (kg water kg sampled.b.−1) at the initial, time t, and the equilibrium stage, respectively.
Page Model
The Page model (Page, 1949), represented by Eq. (2), has been successfully fitted to represent the drying behavior (Rojas et al., 2020b; Rojas & Augusto, 2018c; Simpson et al., 2017).
where MR is the moisture ratio; t is the time (min); k is the drying rate parameter (min−1); n is the dimensionless drying parameter.
Peleg Model
The two-parameter model proposed by Peleg (1988) (Eq. 3) was also used to predict the drying kinetics.
where MR is the moisture ratio; MR0 is initial moisture ratio (equal to 1); t is the time (min); k1 is the Peleg rate parameter (min−1), and k2 is the Peleg capacity parameter.
DR
The drying rate (DR) was determined according to the moisture content on a dry basis, according to Eq. (4) (Macedo et al., 2020).
where DR is the drying rate, Xt and Xt+∆t are the moisture content (kg water kg dry solid−1) at t and t + ∆t, respectively, t is the time (min), and ∆t is the time difference (min).
Quality Analysis
The fresh and dried samples were characterized in terms of moisture content, water activity, shrinkage, hardness, hygroscopicity, color, total anthocyanins, total phenolics, and antioxidant capacity.
Moisture Content
The moisture content was determined by the gravimetric method established by method 934.06 of AOAC (2010), wherein the samples were placed in an oven at 70 °C, under vacuum.
aw
The water activity (aw) of the samples was determined on an electronic hygrometer (Aqualab, series 3TE, Washington, USA), at 25 °C (Corrêa et al., 2016).
Shrinkage
Sample volumes were determined using a toluene displacement method (Gamboa-Santos et al., 2014). The volumetric shrinkage of the samples was calculated according to Eq. (5).
where V and V0 are the volumes (m3) of dried and fresh samples, respectively.
Hardness
The hardness of the samples was measured using a texture analyzer (Stable Micro Systems, TA-X2T, Surrey, England), equipped with a 50 kg load cell, a 6 mm diameter probe, test speed of 2 mm s−1, and penetration distance of 3 mm. The hardness was expressed in Newton (N).
Hygroscopicity
The hygroscopicity of the dried samples was determined by placing approximately 1.0 g of sample in a desiccator with saturated sodium chloride solution (75% of moisture content) at 25 °C, for 7 days. Afterward, the samples were weighed and the hygroscopicity was calculated by the difference in weight of the sample, expressed in % (g of absorbed moisture per 100 g dry solids) (Sette et al., 2016).
Color
The colorimetric parameters of the samples were obtained by direct reading on a colorimeter (Konica Minolta, model CR-10, Osaka, Japan), with illuminant D65, using color scale CIELab.
The L* parameter indicates the lightness, ranging from 0 (black) to 100 (white). C* represents chromaticity or color saturation, in which the higher the C* values, the higher the perceived color intensity. The °h indicates the hue of the sample, where the angles of 0° or 360°, 90°, 180°, and 270° represent red, yellow, green, and blue angles, respectively. The total color difference (∆E) of dried strawberries was calculated according to Eq. (6) taking as reference the fresh strawberry, whose parameters are represented by the subscript “0” (Macedo et al., 2019).
where L*, a*, and b* are CIE lightness coordinate, CIE red( +)/green( −) color attribute and CIE yellow( +)/blue( −) color attribute, respectively.
TAC
The preparation of the extract for the quantification of total anthocyanin content (TAC) was performed by mixing 2 g in 20 mL of ethanol acidified with HCl (0.1%). The mixture was centrifuged at 25,400 × g, for 15 min. TAC was quantified by the differential pH method, according to Eq. (7) (Giusti & Wrolstad, 2001).
where MW is the molecular weight of pelargonidin-3-glucoside (433.0 g mol−1); DF is the dilution factor; ε is the molar absorptivity (22,400 mol−1); L is the path length in centimeters.
TPC
The extract for quantification of total phenolic content (TPC) was prepared by mixing 5 g in 40 mL of 50% methanol (v v−1). The homogenized mixture was left to stand for 1h, protected from light, at room temperature. The mixture was centrifuged at 25,400 × g for 15 min, and the supernatant was collected. The residue from the centrifugation was mixed with 40 mL of 70% acetone (v v−1) and left to stand for 1h, protected from light, at room temperature. The mixture was centrifuged at 25,400 × g for 15 min. The supernatant was collected and mixed with the first. The final volume was completed with deionized water up to 100 mL (Rufino et al., 2010).
TPC was quantified using the Folin–Ciocalteu method (Waterhouse, 2003), and the response was expressed in mg of gallic acid per 100 g sampled.b..
AC
The same extract prepared for the TPC analysis was used for the antioxidant capacity (AC) analysis (Rufino et al., 2010). AC was quantified by the ferric reducing antioxidant power capacity (FRAP) and 2.2-diphenyl-2-picryl-hydrazyl (DPPH) methods. The responses were expressed in μmol of ferrous sulfate per g of sampled.b. and g sampled.b. per g of DPPH (Rufino et al., 2010).
Statistical Analysis
The experiment was conducted in a completely randomized design, in a factorial scheme (2 × 3) (Table 1) and three repetitions. The data of the quality analyses were submitted to analysis of variance (ANOVA), followed by the Tukey test. The Dunnett test was used to compare the fresh sample with each treatment individually.
A multivariate analysis was conducted by means of principal component analysis (PCA) on the drying time, water activity, shrinkage, hardness, hygroscopicity, colorimetric parameters, total anthocyanin content, total phenolic content, and antioxidant capacity by FRAP and DPPH methods responses to analyze their particular interrelationships as influenced by osmotic process (none, OD, and PVOD) and ethanol pre-treatment.
Statistical analyzes were carried out at the level of 5% probability of error, with the aid of software Statistica (StatSoft Inc., Tulsa, OK, USA).
Results and Discussion
Drying Kinetics
The drying times and drying kinetics of non-osmotically dehydrated and subjected to OD or PVOD strawberries, non- or ethanol pre-treated, are shown in Fig. 2. The time required to finish the drying was from 195 to 300 min (Fig. 2). Figure 2a indicates the percentages of increasing drying time due to the inclusion of each osmotic process compared to the respective drying of fresh strawberry (CD or ET + CD). Figure 2b presents the percentages of reduction of drying time due to the application of the ethanol pre-treatment compared to non-ethanol pre-treated drying.
The osmotically dehydrated (by OD or PVOD) samples required a longer time to complete the drying in relation to the drying of the fresh (non-osmotically dehydrated) samples showing longer drying time by up to 33% (Fig. 2a). Fresh strawberry had a higher moisture content than both osmotically dehydrated strawberries. However, the water present in the fresh strawberry is mostly free, which facilitates its removal, requiring less time to complete the convective drying process.
Between osmotically dehydrated non-ethanol pre-treated samples (OD + CD and PVOD + CD), the process in which vacuum was applied (PVOD) required a longer drying time compared to OD. Although PVOD has resulted in higher water loss during the osmotic process, PVOD provides greater incorporation of solute than OD (González-Pérez et al., 2021). Macedo et al. (2020) also observed that samples in which there was greater incorporation of solids showed a trend towards a demand for longer drying time. This is due to the increased incorporation of solute during osmotic processes increasing the amount and strength of water bonds with the material, making it difficult to get out of the water from the material (Macedo et al., 2021b). However, between osmotically dehydrated ethanol pre-treated samples (OD + ET + CD and PVOD + ET + CD), the OD process required a longer drying time (Fig. 2a).
Compared to their respective non-ethanol pre-treated samples, the ethanol pre-treatment (ET) reduced the drying time of fresh samples and those submitted to OD and PVOD (Fig. 2b). ET reduced drying time by up to 30%. This reduction corresponds to the drying of the sample subjected to PVOD, in which the sample ethanol pre-treated (PVOD + ET + CD) required 210 min to complete the drying, while the non-ethanol pre-treated sample (PVOD + CD) required 300 min. The fresh and OD-submitted samples had their drying times reduced by 13 and 11%, respectively. Similar reductions were observed in other studies (Carvalho et al., 2020; Macedo et al., 2021a). It is possible to observe that ET is simple and low-cost processing, with a good capacity to reduce the time and cost of the drying process (Rojas et al., 2020b).
The added ethanol can reduce the drying time because the ethanol mixes with water, reducing the vaporization heat of the mixture (water + ethanol). Furthermore, the evaporation of ethanol (or the water + ethanol mixture) present in the surface layers of the material opens pores, facilitates the evaporation of water present in the innermost layers of the material. A third consequence of added ethanol is the Marangoni effect. This effect is due to the gradient of surface tension between water and ethanol, causing ethanol to promote water displacement, facilitating drying (Macedo et al., 2021a; Rojas et al., 2020a, 2020b).
The moisture content of the samples reduced exponentially during drying (Fig. 2c). Initially, fresh and pre-dehydrated by OD and PVOD strawberries presented moisture content of 90.59, 75.95, and 72.12%w.b., respectively. It is possible to observe an abrupt reduction in the moisture content of both non-osmotically dehydrated samples (CD and ET + CD) during the first minutes of drying. In just 120 min, the moisture content of samples submitted to CD and ET + CD treatments was approximately 50.71%w.b. and 42.62%w.b., respectively. This rapid reduction is due to the large proportion of free water present in the fresh strawberry initially, which facilitates water removal. On the other hand, the reduction in the moisture content of the osmotically dehydrated (both OD and PVOD) samples was mild, both for the non- and ethanol pre-treated samples.
Page Model
The values of the Page model parameters are presented in Table 2. Page’s model fitted very well to the data, presenting high R2 values and low SE values (Table 2), indicating excellent similarity between the predicted and experimental data, as observed in other studies (Macedo et al., 2021b; Rojas et al., 2020a; Rojas & Augusto, 2018c). The goodness of fit of Page’s model to the data justifies the great success of this equation when representing the migration of water during the drying of food (Simpson et al., 2017).
According to Table 2, the osmotically pre-dehydrated samples presented a higher value of the k parameter in relation to the control sample (not pre-dehydrated). There were no significant differences among the values of the k parameter between the osmotic processes, whether pre-treated or not, with ethanol. Furthermore, the ET did not significantly influence the value of the k parameter. According to Simpson et al. (2017), parameter k may be associated with the diffusion coefficient and sample geometry.
Osmotic processes reduced n values in relation to the values of the non-osmotically dehydrated samples (CD and ET + CD) (Table 2). A significant difference was not observed between both osmotic processes. Parameter n is related to the type of diffusion and microstructure of the material, where values above 1 indicate super-diffusion and below 1 indicate sub-diffusion (Macedo et al., 2021b; Rojas & Augusto, 2018c; Simpson et al., 2017). Rojas et al. (2020b) stated that when the value of n is different from 1, other mechanisms than diffusion are important. The drying of the samples submitted to the treatments OD + CD, OD + ET + CD, and PVOD + ET + CD showed n < 1, and samples submitted to the treatment PVOD + CD showed n > 1. However, these values were very close to 1 (Table 2). On the other hand, the n values of the non-osmotically dehydrated samples (CD and ET + CD) were higher than 1, indicating super-diffusion, associated with an important influence of another mechanism, such as capillarity, as reported in other studies (Rojas, et al., 2020b; Rojas & Augusto, 2018b, 2018c).
Peleg Model
Peleg model is used to describe moisture sorption curves. This model fitted well with the experimental data of the moisture ratio kinetics during the convective drying of strawberries, presenting high values of R2 and low values of SE (Table 3), as also reported in other studies (Aydar, 2020; Cruz et al., 2016).
As shown in Table 3, there was a trend of lower k1 with the ET. The exception was the OD + CD treatment, in which the non and submitted to ET samples did not differ from each other (p > 0.05). Cruz et al. (2016) observed that dryings that required less drying time had a lower value of parameter k1. Among the samples not submitted to ET, the non-osmotically dehydrated (CD) sample had the highest k1. The OD + CD sample had the lowest. On the other hand, no significant difference was observed in relation to the value of k1 among samples submitted to ET.
The k2 parameter showed a significant difference only for those osmotically dehydrated with vacuum application between non (PVOD + CD) and pre-treated ethanol (PVOD + CD + ET) samples. In the other treatments, no significant differences were observed between non- and submitted to ET samples. Furthermore, non-dehydrated strawberries had lower k2 values than osmotically pre-treated ones. According to Cruz et al. (2016), the k2 parameter is related to the characteristics of the material and does not depend on the drying conditions. This can be seen from the fact that the value of the k2 parameter was mainly influenced by the use of osmotic processes, which causes mass exchanges between the osmotic solution and the food, changing the composition of the material in relation to fresh one (not osmotically dehydrated).
DR
The DR during sample dryings is shown in Fig. 3. At the beginning of drying, the strawberries (especially the non-osmotically dehydrated) present high moisture content, aiding the removal of water, which results in high DR values.
During drying, the DR reduced, as the amount of water to be removed reduces throughout the process, making it increasingly difficult to remove the water.
Non-osmotically dehydrated dried strawberries (CD and ET + CD) showed higher DR compared to osmotically dehydrated samples (Fig. 3a), as also observed by Tylewicz et al. (2019). This is due to the amount of free water for removal being larger in fresh material compared to samples that were previously dehydrated by osmotic processes. Furthermore, the incorporation of solids during osmotic processes increases the interactions of water with the material, hindering the outflow of water, reducing the DR (Macedo et al., 2021b).
The DR values of the osmotically dehydrated samples are shown separately in Fig. 3b to facilitate the visualization of data behavior. Among osmotically dehydrated strawberries, the DR values were close throughout the dryings, except at the beginning of the drying process, when the OD process resulted in higher DR during drying (Fig. 3b). This is due to the PVOD process which results in higher water loss during the osmotic process, resulting in a sample with lower moisture content at the beginning of convective drying. Furthermore, the PVOD process provides higher solid gain during the osmotic process (González-Pérez et al., 2021). Therefore, in the samples submitted to PVOD, the amount of water to be removed is lower and the interactions of water with the constituents of the material are higher, by increasing dry matter.
ET showed a trend towards a higher DR compared to non-ethanol pre-treated samples, especially for non-osmotically dehydrated strawberries. This is due to the effects of the addition of ethanol in favor of facilitating the removal of water, as mentioned above.
Periods with constant or near-constant DR were rare or even non-existent. The same was observed by Gamboa-Santos et al. (2014) in convective drying of strawberries at different air temperatures.
aw
The fresh material presented a high value of aw, 0.986 ± 0.004 (Fig. 4a), as is the characteristic of strawberry (Dermesonlouoglou et al., 2016; Tylewicz et al., 2020). The dryings significantly (p < 0.05) reduced the aw of the non- and osmotically dehydrated samples. After drying, dried strawberries presented aw between 0.423 and 0.585. Similar results were observed by Amami et al. (2017) when performing convective drying of strawberries to reduce the moisture content by 90%.
Water activity (a), shrinkage (b), hardness (c), and hygroscopicity (d) of the samples. Values are mean ± standard deviation, n = 3. The same uppercase letter indicates no significant difference between non and ethanol pre-treated samples, and the same lowercase letter indicates that there is no significant difference among non-osmotically dehydrated, submitted to OD and PVOD dried samples, by the Tukey test (p < 0.05). The asterisk indicates a significant difference between each treatment and the fresh sample, by the Dunnett test (p < 0.05)
The non-osmotically dehydrated dried samples (CD and ET + CD) presented lower aw values compared to samples pre-dehydrated by OD or PVOD (Fig. 4a). During the osmotic processes, there was incorporation of solids (isomaltulose), changing the composition and structure of the food, which makes the water interact differently with the material, influencing the fugacity of the water and consequently the aw (Damodaran, 2017).
Among the osmotic processes, the application of the vacuum at the beginning of the osmotic process (PVOD) resulted in a lower aw value in dried strawberry (Fig. 4a). The same behavior was observed by Deng and Zhao (2008). This behavior can be explained by the lower aw value of the sample submitted to PVOD than to OD, before convective drying (data not shown). In addition, the application of vacuum during the osmotic process promotes changes in the structure of the food matrix. Therefore, among the samples submitted to OD and PVOD, OD caused less change in the food, resulting in higher aw.
The ethanol pre-treated samples did not present a significant difference in aw in relation to their respective non-pre-treated ethanol samples (Fig. 4a).
The aw is an important parameter in food preservation and quality. According to Jay et al. (2005), microorganisms and enzymes need water to become active. Drying is a preservation method by reducing the moisture content to the point of inhibiting the activity of microorganisms that deteriorate food and cause food poisoning (Jay et al., 2005). The range of the aw values of dried strawberries indicates that they are microbiologically stable and safe since they presented a value under 0.6 (value represented by the dotted line in Fig. 4a).
Shrinkage
Dried strawberries showed shrinkage from 55.56 to 71.49% (Fig. 4b). During drying, water is removed. The viscoelastic matrix moves towards space previously occupied by water, causing the material to shrink (Dehghannya et al., 2018). In processes where there is a high removal of water, as in the present study (90.59 to 11.50%w.b.), shrinkage occurs in a high proportion (Nahimana et al., 2011). Gamboa-Santos et al. (2014) found that shrinkage has a strong correlation with moisture ratio in strawberry drying.
OD and PVOD contributed to the reduction of shrinkage in relation to non-osmotically dehydrated strawberries (Fig. 4b). The incorporation of solids that occurs during osmotic processes reduces the mobility of the solid matrix of the food, giving the material an elastic behavior typical of the vitreous state, which reduces shrinkage along convective drying (Nahimana et al., 2011).
The ET use increased the shrinkage, both for non-osmotically dehydrated and pre-dehydrated by OD or PVOD samples (Fig. 4b). The same was observed in other studies (Silva et al., 2018). According to Silva et al. (2018), the rapid evaporation of water during the drying with ET causes greater damage to the material structure and consequently more shrinkage.
Hardness
Hardness can be defined physically as the force necessary to cause a deformation in the material. Sensorially, hardness represents the force necessary to compress a solid food between the molar teeth. Therefore, it is an important parameter of dried food texture (Macedo et al., 2021b; Szczesniak, 2002).
The hardness of the strawberries is shown in Fig. 4c. Similar values were found in other studies (Deng & Zhao, 2008; Khubber et al., 2020; Li et al., 2019; Schwieterman et al., 2014; Tylewicz et al., 2019). The dried samples showed higher hardness than the fresh strawberry, as also observed by Tylewicz et al. (2019). This is due to physical changes, such as shrinkage and distortion, which occur due to the moisture content reduction during the drying of the strawberry, resulting in increased hardness.
Among the dried strawberries, the osmotic processes increased the hardness of the dried strawberries. Some studies have observed that the inclusion of the osmotic process prior to convective drying can cause softening of the dried strawberry (Tylewicz et al., 2019). Khubber et al. (2020) studied different solutes for carrot osmotic dehydration, noting that the solute used influences the reduction or increase in the hardness of the dried product. In the present study, it was observed that the incorporation of isomaltulose resulted in dried strawberries with higher hardness (Fig. 4c). This same effect was observed by Lyu et al. (2017). Between both osmotic processes (OD and PVOD), significant differences were not observed, indicating that the vacuum application did not affect the hardness of the osmotically dehydrated dried strawberries.
ET had no significant influence on the hardness of dried strawberries, compared to the respective non-pretreated samples (Fig. 4c).
Hygroscopicity
The hygroscopicity of the samples was influenced by the treatments studied. Osmotic processes, especially PVOD, reduced dried strawberry hygroscopicity compared to non-osmotically dehydrated dried samples (Fig. 4d). Isomaltulose is a sugar of very low hygroscopicity, giving this characteristic to the product in which it is added (Sawale et al., 2017). Thus, the samples that incorporate more isomaltulose, that is, those submitted to PVOD, resulted in lower hygroscopicity.
ET contributed to the reduction of hygroscopicity of osmotically dehydrated by OD and PVOD samples. However, ET had no significant influence on non-osmotically dehydrated dried samples (Fig. 4d).
Hygroscopicity is an important property in dehydrated foods. It is the capacity of the product to absorb moisture from the air. Foods with high hygroscopicity can have their stability reduced quickly, due to increased water absorption from air, increasing their water activity, reducing the shelf life of the product (Sette et al., 2016). Therefore, in the case of the present study, it is desirable that the dried strawberry has a low hygroscopicity.
Color
Color is an important attribute of the quality of a product since it is the first parameter evaluated by the consumer and directly influences their choices and preferences. In the case of fruits, color is an attribute that can be associated with the degree of maturation and freshness of the fruit, influencing consumer acceptance. In strawberries, the consumer expects the color to be predominantly red (Pathare et al., 2013).
The influence of the treatments studied in relation to the colorimetric parameters of the samples is presented in Fig. 5.
Colorimetric parameters of the samples. Values are mean ± standard deviation, n = 3. The same uppercase letter indicates no significant difference between non- and ethanol pre-treated samples, and the same lowercase letter indicates that there is no significant difference among non-osmotically dehydrated, submitted to OD and PVOD dried samples, by the Tukey test (p < 0.05). The asterisk indicates a significant difference between each treatment and the fresh sample, by the Dunnett test (p < 0.05)
Non-osmotically dehydrated dried samples (CD and ET + CD) showed L* statistically equal to L* of fresh strawberry. The samples submitted to OD and PVOD showed high L*. This may be due to the pigments leaching during osmotic processes. The ET influenced the L* only in the PVOD treatment sample, in which the ET resulted in a higher value of this parameter (Fig. 5a).
The dried samples presented higher C* than fresh strawberries (Fig. 5b). The drying process causes a concentration of the constituents of the material, due to the reduction of moisture content, resulting in increased color saturation. The sample submitted to the OD + CD treatment presented the highest C* value. The ET influenced only the sample submitted to OD (Fig. 5b).
The dried samples showed a higher value of °h than fresh strawberries, indicating that the red hue was lower in the dried samples (Fig. 5c). It is due to the leaching of anthocyanins during osmotic processes and the degradation of anthocyanins during CD since anthocyanins are the pigments that confer red coloration to strawberries. ET did not influence non-osmotically dehydrated dried strawberry °h but influenced dried samples that were osmotically dehydrated (Fig. 5c).
The ∆E is used to express the magnitude of the color difference between the samples and a control sample (fresh strawberry). The non-osmotically dehydrated dried samples (CD and ET + CD) showed the lowest ∆E values. The ET had influence only in the treatment of PVOD, increasing the ∆E (Fig. 5d). Differences in perceptible color can be classified as very distinct (ΔE > 3), distinct (1.5 < ΔE < 3), and small differences (1.5 < ΔE) (Pathare et al., 2013). Based on this classification, the color difference between the dried samples and the fresh strawberry was very distinct.
Exposure to heated air can result in chemical reactions responsible for changing the color of the food, whether related to discoloration, due to degradation of heat-sensitive pigments, such as anthocyanins in strawberries; to non-enzymatic browning; to enzymatic browning by polyphenol oxidase (PPO) activity (López-Ortiz et al., 2020; Omolola et al., 2017). However, it can be observed that the dried samples showed equal or higher L*, and higher C* than the fresh strawberry, indicating that the browning reactions (enzymatic or non-enzymatic) did not occur excessively (Mierzwa & Kowalski, 2016).
TAC, TPC, and AC
The TAC, TPC, and AC by the FRAP and DPPH methods are presented in Fig. 6a, b, c, and d, respectively. Fresh strawberries presented as a good source of bioactive and antioxidant compounds (Fig. 6a-d). These compounds contribute to the strawberry being classified as a functional food with various preventive and therapeutic health benefits, with positive effects in various diseases, such as hypertension, inflammation, cancer, cardiovascular and neurodegenerative diseases (Basu et al., 2014).
Total anthocyanin content (a), total phenolic content (b), and antioxidant capacity by FRAP (c) and DPPH (d) methods of the samples. Values are mean ± standard deviation, n = 3. TAC total anthocyanin content TPC total phenolic content. The same uppercase letter indicates no significant difference between non- and ethanol pre-treated samples, and the same lowercase letter indicates that there is no significant difference among non-osmotically dehydrated, submitted to OD and PVOD dried samples, by the Tukey test (p < 0.05). The asterisk indicates a significant difference between each treatment and the fresh sample, by the Dunnett test (p < 0.05)
Fresh strawberries presented higher TAC, TPC, and AC than dried strawberries (Fig. 6a-d), since convective drying causes degradation of these compounds. During drying, the reduction of moisture content and physical changes favor oxidative reactions, which degrade the bioactive and antioxidant compounds of strawberry (Méndez-Lagunas et al., 2017).
The TAC was the group of compounds that suffered the greatest degradation. This is due to the loss of anthocyanins by leaching during osmotic processes since these compounds are very water soluble. Furthermore, anthocyanins are very sensitive, suffering high losses during exposure to drying air temperature, due to the change in the structure of anthocyanins, in addition to oxidative processes (Méndez-Lagunas et al., 2017). According to Méndez-Lagunas et al. (2017), anthocyanins can be degraded enzymatically by polyphenol oxidase, as they are relatively heat-stable enzymes. Méndez-Lagunas et al. (2017) found a significant reduction in TAC after convective drying of strawberries, mainly at the beginning of drying.
Osmotic processes and convective drying also significantly reduced the TPC and CA of the samples (Fig. 6a-d). Méndez-Lagunas et al. (2017) and Tylewicz et al. (2019) also observed a reduction in TPC and AC after strawberry convective drying. According to Méndez-Lagunas et al. (2017), during drying, thermal degradation of phenolic compounds occurs. Furthermore, bound phenolic compounds are released and partial degradation of lignin occurs, promoting the release of phenolic acid derivatives, increasing the thermal degradation of these compounds (Méndez-Lagunas et al., 2017).
The ET had a beneficial effect on the preservation of TAC, TPC, and AC (Fig. 6a-d). The effect of ethanol on the preservation of bioactive and antioxidant compounds may be related to the reduction of drying time, reducing the exposure time of the product to hot air (Araújo et al., 2020). Some studies report that ET reduces the content of some bioactive compounds. However, this effect may be related to the long time of the ET process, with the extraction of these compounds (da Cunha et al., 2020).
Principal Component Analysis
The principal component analysis (PCA) was used to investigate the influence of both studied factors on drying time, water activity, shrinkage, hardness, hygroscopicity, color parameters, total anthocyanin content, total phenolic content, and antioxidant capacity by FRAP and DPPH methods responses, as illustrated in Fig. 7a and b.
Principal component analysis of the dried strawberries responses in effect of osmotic processes and ethanol pre-treatment: a score plot and b loading plot. S shrinkage, TAC total anthocyanin content, TPC total phenolic content, ACFRAP antioxidant capacity by FRAP method, ACDPPH antioxidant capacity by DPPH method
The first (PC1) and second (PC2) axes were responsible for 71.78% and 12.11%, respectively, giving a total of 83.89% of the explained variance. As seen in the scores plot (Fig. 7a), the osmotically dehydrated dried samples were on the negative side of the PC1 axis, while the non-osmotically dehydrated dried samples (CD and ET + CD) were on the positive side of the PC1 axis, evidencing the differences between the properties of non- and osmotically dehydrated samples after drying process.
The shrinkage, TAC, TPC, and ACFRAP responses were observed to positively correlated (Fig. 7b). It can be observed that the highest values of these responses were associated with the ET + CD treatment (Fig. 7a and b). As expected, ACDPPH was opposite to TAC, TPC, and ACFRAP responses, as low ACDPPH response values indicate high AC. High values of anthocyanic, phenolic, and antioxidant compounds were obtained when drying time was short, indicating that longer drying times resulted in more degradation of these compounds. (Fig. 7b).
In general, the osmotically dehydrated samples (by OD or PVOD, non- or ethanol pre-treated) were associated with high values of drying time, aw, hardness, L*, C*, ∆E, and ACDPPH. Therefore, when searching for strawberries with maximum content of bioactive and antioxidant compounds, the choice should be made for the ethanol pre-treated dried fruit, non-osmotically dehydrated. However, if the main interest is in a dried strawberry enriched with isomaltulose, the inclusion of the osmotic process previously convective drying is recommended.
Conclusion
Osmotic dehydration, with or without vacuum application, enriched strawberries with isomaltulose and contributed to the reduction of shrinkage and hygroscopicity of the dried product. However, osmotic processes increased drying time, water activity, hardness and caused higher color change in relation to non-osmotically dehydrated strawberry, besides resulting in samples with lower total anthocyanin and total phenolic contents and antioxidant capacity.
Ethanol pre-treatment proved to be an interesting procedure to be applied in the convective drying of strawberries, as it was efficient to reduce drying time, contributed to the reduction of hygroscopicity, and minimized the degradation of total anthocyanin and total phenolic contents and antioxidant capacity. However, the use of ethanol caused higher shrinkage of the samples.
Availability of Data and Material
The authors chose not to present the data and material.
Code Availability
Not applicable.
References
Amami, E., Khezami, W., Mezrigui, S., Badwaik, L. S., Bejar, A. K., Perez, C. T., & Kechaou, N. (2017). Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrasonics Sonochemistry, 36, 286–300. https://doi.org/10.1016/j.ultsonch.2016.12.007
AOAC. (2010). Official methods of analysis (18th ed.). AOAC Internacional Association of Official Analytical Chemists.
da Araújo, C., Corrêa, J. L. G., Dev, S., Macedo, L. L., Vimercati, W. C., Rodrigues de Oliveira, C., & Pio, L. A. S. (2020). Influence of pretreatment with ethanol and drying temperature on physicochemical and antioxidant properties of white and red pulp pitayas dried in foam mat. Drying Technology, 38, 1–10. https://doi.org/10.1080/07373937.2020.1809446.
Aydar, A. Y. (2020). Ultrason Önişlemi Uygulanmış Mikrodalgada Kurutulan Zeytin Dilimlerinin Peleg Modeli Kullanılarak Kuruma ve Rehidrasyon Kinetikleri. Harran Tarım ve Gıda Bilimleri Dergisi, 24(4), 401–408. https://doi.org/10.29050/harranziraat.644838
Basu, A., Nguyen, A., Betts, N. M., & Lyons, T. J. (2014). Strawberry as a functional food: An evidence-based review. Critical Reviews in Food Science and Nutrition, 54(6), 790–806. https://doi.org/10.1080/10408398.2011.608174
Braga, A. M. P., Pedroso, M. P., Augusto, F., & Silva, M. A. (2009). Volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Drying Technology, 27(2), 248–257. https://doi.org/10.1080/07373930802606097
Braga, Alice M. P., Silva, M. A., Pedroso, M. P., Augusto, F., & Barata, L. E. S. (2010). Volatile composition changes of pineapple during drying in modified and controlled atmosphere. International Journal of Food Engineering, 6(1). https://doi.org/10.2202/1556-3758.1835
Carvalho, G. R., Rojas, M. L., Silveira, I., & Augusto, P. E. D. (2020). Drying accelerators to enhance processing and properties: Ethanol, isopropanol, acetone and acetic acid as pre-treatments to convective drying of pumpkin. Food and Bioprocess Technology, 13(11), 1984–1996. https://doi.org/10.1007/s11947-020-02542-6
Corrêa, J. L. G., Braga, A. M. P., Hochheim, M., & Silva, M. A. (2012). The Influence of Ethanol on the Convective Drying of Unripe, Ripe, and Overripe Bananas. Drying Technology, 30(8), 817–826. https://doi.org/10.1080/07373937.2012.667469
Corrêa, J. L. G., Ernesto, D. B., & de Mendonça, K. S. (2016). Pulsed vacuum osmotic dehydration of tomatoes: Sodium incorporation reduction and kinetics modeling. LWT - Food Science and Technology, 71, 17–24. https://doi.org/10.1016/j.lwt.2016.01.046
Cruz, L., Clemente, G., Mulet, A., Ahmad-Qasem, M. H., Barrajón-Catalán, E., & García-Pérez, J. V. (2016). Air-borne ultrasonic application in the drying of grape skin: Kinetic and quality considerations. Journal of Food Engineering, 168, 251–258. https://doi.org/10.1016/j.jfoodeng.2015.08.001
da Cunha, R. M. C., Brandão, S. C. R., de Medeiros, R. A. B., da Silva Júnior, E. V., Fernandes da Silva, J. H., & Azoubel, P. M. (2020). Effect of ethanol pretreatment on melon convective drying. Food Chemistry, 333, 127502. https://doi.org/10.1016/j.foodchem.2020.127502
Damodaran, S. (2017). Water and Ice. In S. Damodaran & K. L. Parkin (Eds.), Fennema’s food chemistry (fifth, pp. 19–88). CRC Press.
Dehghannya, J., Bozorghi, S., & Heshmati, M. K. (2018). Low temperature hot air drying of potato cubes subjected to osmotic dehydration and intermittent microwave: Drying kinetics, energy consumption and product quality indexes. Heat and Mass Transfer, 54(4), 929–954. https://doi.org/10.1007/s00231-017-2202-5
Deng, Y., & Zhao, Y. (2008). Effect of pulsed vacuum and ultrasound osmopretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT - Food Science and Technology, 41(9), 1575–1585. https://doi.org/10.1016/j.lwt.2007.10.018
Dermesonlouoglou, E. K., Giannakourou, M., & Taoukis, P. S. (2016). Kinetic study of the effect of the osmotic dehydration pre-treatment with alternative osmotic solutes to the shelf life of frozen strawberry. Food and Bioproducts Processing, 99(2004), 212–221. https://doi.org/10.1016/j.fbp.2016.05.006
Gamboa-Santos, J., Montilla, A., Cárcel, J. A., Villamiel, M., & Garcia-Perez, J. V. (2014). Air-borne ultrasound application in the convective drying of strawberry. Journal of Food Engineering, 128, 132–139. https://doi.org/10.1016/j.jfoodeng.2013.12.021
Giusti, M. M., & Wrolstad, R. E. (2001). Characterization and measurement of anthocyanins by UV-visible spectroscopy. Current Protocols in Food Analytical Chemistry, 00(1), F1.2.1-F1.2.13. https://doi.org/10.1002/0471142913.faf0102s00
González-Pérez, J. E., Ramírez-Corona, N., & López-Malo, A. (2021). Mass transfer during osmotic dehydration of fruits and vegetables: Process factors and non-thermal methods. Food Engineering Reviews, 13(2), 344–374. https://doi.org/10.1007/s12393-020-09276-3
Jay, J. M., Loessner, M. J., & Golden, D. A. (2005). Protection of foods by drying. In Modern Food Microbiology (pp. 443–456). Springer US. https://doi.org/10.1007/0-387-23413-6_18
Karam, M. C., Petit, J., Zimmer, D., Baudelaire Djantou, E., & Scher, J. (2016). Effects of drying and grinding in production of fruit and vegetable powders: A review. Journal of Food Engineering, 188, 32–49. https://doi.org/10.1016/j.jfoodeng.2016.05.001
Khubber, S., Chaturvedi, K., Taghi Gharibzahedi, S. M., Cruz, R. M. S., Lorenzo, J. M., Gehlot, R., & Barba, F. J. (2020). Non-conventional osmotic solutes (honey and glycerol) improve mass transfer and extend shelf life of hot-air dried red carrots: Kinetics, quality, bioactivity, microstructure, and storage stability. Lwt, 131(March), 109764. https://doi.org/10.1016/j.lwt.2020.109764
Kroehnke, J., Szadzińska, J., Radziejewska-Kubzdela, E., Biegańska-Marecik, R., Musielak, G., & Mierzwa, D. (2021). Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa) - The influence of ultrasound on process kinetics and product quality. Ultrasonics Sonochemistry, 71. https://doi.org/10.1016/j.ultsonch.2020.105377
Lazou, A., Dermesonlouoglou, E. K., & Giannakourou, M. C. (2020). Modeling and evaluation of the osmotic pretreatment of tomatoes (S. lycopersicum) with alternative sweeteners for the production of candied products. Food and Bioprocess Technology, 13(6), 948–961. https://doi.org/10.1007/s11947-020-02456-3
Li, X., Bi, J., Chen, Q., Jin, X., Wu, X., & Zhou, M. (2019). Texture improvement and deformation inhibition of hot air-dried apple cubes via osmotic pretreatment coupled with instant control pressure drop (DIC). Lwt, 101, 351–359. https://doi.org/10.1016/j.lwt.2018.11.035
Liu, Z. L., Staniszewska, I., Zielinska, D., Zhou, Y. H., Nowak, K. W., Xiao, H. W., Pan, Z., & Zielinska, M. (2020). Combined hot air and microwave-vacuum drying of cranberries: Effects of pretreatments and pulsed vacuum osmotic dehydration on drying kinetics and physicochemical properties. Food and Bioprocess Technology, 13(10), 1848–1856. https://doi.org/10.1007/s11947-020-02507-9
López-Ortiz, A., Méndez-Lagunas, L. L., Delesma, C., Longoria, A., Escobar, J., & Muñiz, J. (2020). Understanding the drying kinetics of phenolic compounds in strawberries: An experimental and density functional theory study. Innovative Food Science and Emerging Technologies, 60, 102283. https://doi.org/10.1016/j.ifset.2019.102283
Lyu, J., Yi, J., Bi, J., Chen, Q., Zhou, L., & Liu, X. (2017). Effect of sucrose concentration of osmotic dehydration pretreatment on drying characteristics and texture of peach chips dried by infrared drying coupled with explosion puffing drying. Drying Technology, 35(15), 1887–1896. https://doi.org/10.1080/07373937.2017.1286670
Macedo, L. L., Corrêa, J. L. G., da Araújo, C., Vimercati, W. C., & Pio, L. A. S. (2021). Process optimization and ethanol use for obtaining white and red dragon fruit powder by foam mat drying. Journal of Food Science, 86(2), 426–433. https://doi.org/10.1111/1750-3841.15585.
Macedo, L. L., da Silva Araújo, C., Vimercati, W. C., Saraiva, S. H., & Teixeira, L. J. Q. (2021b). Influence of yacon syrup concentration and drying air temperature on properties of osmotically pre-dehydrated dried banana. Heat and Mass Transfer, 57(3), 441–451. https://doi.org/10.1007/s00231-020-02966-y
Macedo, L. L., Silva Araújo, C., Vimercati, W. C., Saraiva, S. H., & Teixeira, L. J. Q. (2019). Evaluation of different bleaching methods applied to yacon. Journal of Food Process Engineering, 42(7). https://doi.org/10.1111/jfpe.13276
Macedo, L. L., Vimercati, W. C., Araújo, C., Saraiva, S. H., & Teixeira, L. J. Q. (2020). Effect of drying air temperature on drying kinetics and physicochemical characteristics of dried banana. Journal of Food Process Engineering, 43(April), 1–10. https://doi.org/10.1111/jfpe.13451
Méndez-Lagunas, L., Rodríguez-Ramírez, J., Cruz-Gracida, M., Sandoval-Torres, S., & Barriada-Bernal, G. (2017). Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chemistry, 230, 174–181. https://doi.org/10.1016/j.foodchem.2017.03.010
Mendonça, K. S., Corrêa, J. L. G., de Junqueira, J. R., & J., Cirillo, M. A., Figueira, F. V., & Carvalho, E. E. N. . (2017). Influences of convective and vacuum drying on the quality attributes of osmo-dried pequi (Caryocar brasiliense Camb.) slices. Food Chemistry, 224, 212–218. https://doi.org/10.1016/j.foodchem.2016.12.051
Mierzwa, D., & Kowalski, S. J. (2016). Ultrasound-assisted osmotic dehydration and convective drying of apples: Process kinetics and quality issues. Chemical and Process Engineering - Inzynieria Chemiczna i Procesowa, 37(3), 383–391. https://doi.org/10.1515/cpe-2016-0031
Nahimana, H., Zhang, M., Mujumdar, A. S., & Ding, Z. (2011). Mass transfer modeling and shrinkage consideration during osmotic dehydration of fruits and vegetables. Food Reviews International, 27(4), 331–356. https://doi.org/10.1080/87559129.2010.518298
Omolola, A. O., Jideani, A. I. O., & Kapila, P. F. (2017). Quality properties of fruits as affected by drying operation. Critical Reviews in Food Science and Nutrition, 57(1), 95–108. https://doi.org/10.1080/10408398.2013.859563
Page, G. E. (1949). Factors influencing the maximum rates of air drying shelled corn in thin layers. Purdue University.
Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36–60. https://doi.org/10.1007/s11947-012-0867-9
Peleg, M. (1988). An empirical model for the description of moisture sorption curves. Journal of Food Science, 53(4), 1216–1217. https://doi.org/10.1111/j.1365-2621.1988.tb13565.x
Ramya, V., & Jain, N. K. (2017). A review on osmotic dehydration of fruits and vegetables: An integrated approach. Journal of Food Process Engineering, 40(3), 1–22. https://doi.org/10.1111/jfpe.12440
Rojas, M. L., Augusto, P. E. D., & Cárcel, J. A. (2020a). Ethanol pre-treatment to ultrasound-assisted convective drying of apple. Innovative Food Science & Emerging Technologies, 61, 102328. https://doi.org/10.1016/j.ifset.2020.102328
Rojas, M. L., Silveira, I., & Augusto, P. E. D. (2020b). Ultrasound and ethanol pre-treatments to improve convective drying: Drying, rehydration and carotenoid content of pumpkin. Food and Bioproducts Processing, 119, 20–30. https://doi.org/10.1016/j.fbp.2019.10.008
Rojas, Meliza L., Silveira, I., & Augusto, P. E. D. (2019). Improving the infrared drying and rehydration of potato slices using simple approaches: Perforations and ethanol. Journal of Food Process Engineering, 42(5). https://doi.org/10.1111/jfpe.13089
Rojas, M. L., & Augusto, P. E. D. (2018a). Ethanol and ultrasound pre-treatments to improve infrared drying of potato slices. Innovative Food Science and Emerging Technologies, 49(April), 65–75. https://doi.org/10.1016/j.ifset.2018.08.005
Rojas, M. L., & Augusto, P. E. D. (2018b). Microstructure elements affect the mass transfer in foods: The case of convective drying and rehydration of pumpkin. Lwt, 93(March), 102–108. https://doi.org/10.1016/j.lwt.2018.03.031
Rojas, M. L., & Augusto, P. E. D. (2018c). Ethanol pre-treatment improves vegetable drying and rehydration: Kinetics, mechanisms and impact on viscoelastic properties. Journal of Food Engineering, 233, 17–27. https://doi.org/10.1016/j.jfoodeng.2018.03.028
Rufino, M., & do S. M., Alves, R. E., de Brito, E. S., Pérez-Jiménez, J., Saura-Calixto, F., & Mancini-Filho, J. . (2010). Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chemistry, 121(4), 996–1002. https://doi.org/10.1016/j.foodchem.2010.01.037
Santos, P. H. S., & Silva, M. A. (2009). Kinetics of L-ascorbic acid degradation in pineapple drying under ethanolic atmosphere. Drying Technology, 27(9), 947–954. https://doi.org/10.1080/07373930902901950
Sawale, P. D., Shendurse, A. M., Mohan, M. S., & Patil, G. R. (2017). Isomaltulose (Palatinose) - An emerging carbohydrate. Food Bioscience, 18, 46–52. https://doi.org/10.1016/j.fbio.2017.04.003
Schwieterman, M. L., Colquhoun, T. A., Jaworski, E. A., Bartoshuk, L. M., Gilbert, J. L., Tieman, D. M., Odabasi, A. Z., Moskowitz, H. R., Folta, K. M., Klee, H. J., Sims, C. A., Whitaker, V. M., & Clark, D. G. (2014). Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE, 9(2). https://doi.org/10.1371/journal.pone.0088446
Sette, P., Salvatori, D., & Schebor, C. (2016). Physical and mechanical properties of raspberries subjected to osmotic dehydration and further dehydration by air- and freeze-drying. Food and Bioproducts Processing, 100, 156–171. https://doi.org/10.1016/j.fbp.2016.06.018
Silva, M. G., Celeghini, R. M. S., & Silva, M. A. (2018). Effect of ethanol on the drying characteristics and on the coumarin yield of dried guaco leaves (Mikania laevigata schultz BIP. Ex Baker). Brazilian Journal of Chemical Engineering, 35(3), 1095–1104. https://doi.org/10.1590/0104-6632.20180353s20160481
Simpson, R., Ramírez, C., Nuñez, H., Jaques, A., & Almonacid, S. (2017). Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices. Trends in Food Science and Technology, 62, 194–201. https://doi.org/10.1016/j.tifs.2017.01.003
Szczesniak, A. S. (2002). Texture is a sensory property. Food Quality and Preference, 13(4), 215–225. https://doi.org/10.1016/S0950-3293(01)00039-8
Tatemoto, Y., Mizukoshi, R., Ehara, W., & Ishikawa, E. (2015). Drying characteristics of food materials injected with organic solvents in a fluidized bed of inert particles under reduced pressure. Journal of Food Engineering, 158, 80–85. https://doi.org/10.1016/j.jfoodeng.2015.03.006
Turkiewicz, I. P., Wojdyło, A., Tkacz, K., Lech, K., & Nowicka, P. (2020). Osmotic dehydration as a pretreatment modulating the physicochemical and biological properties of the Japanese quince fruit dried by the convective and vacuum-microwave method. Food and Bioprocess Technology, 13(10), 1801–1816. https://doi.org/10.1007/s11947-020-02522-w
Tylewicz, U., Mannozzi, C., Romani, S., Castagnini, J. M., Samborska, K., Rocculi, P., & Dalla Rosa, M. (2019). Chemical and physicochemical properties of semi-dried organic strawberries enriched with bilberry juice-based solution. Lwt, 114(February), 108377. https://doi.org/10.1016/j.lwt.2019.108377
Tylewicz, U., Oliveira, G., Alminger, M., Nohynek, L., Dalla Rosa, M., & Romani, S. (2020). Antioxidant and antimicrobial properties of organic fruits subjected to PEF-assisted osmotic dehydration. Innovative Food Science and Emerging Technologies, 62(March), 102341. https://doi.org/10.1016/j.ifset.2020.102341
Wang, X., Feng, Y., Zhou, C., Sun, Y., Wu, B., Yagoub, A. E. A., & Aboagarib, E. A. A. (2019). Effect of vacuum and ethanol pretreatment on infrared-hot air drying of scallion (Allium fistulosum). Food Chemistry, 295(May), 432–440. https://doi.org/10.1016/j.foodchem.2019.05.145
Waterhouse, A. L. (2003). Determination of total phenolics. In Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc. https://doi.org/10.1002/0471142913.fai0101s06
Acknowledgements
The authors gratefully acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
Leandro Macedo: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft. Jefferson Corrêa: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, writing—review and editing. Cintia Araújo: formal analysis, investigation, methodology, writing—review and editing. Wallaf Vimercati: formal analysis, investigation, methodology, writing—review and editing. Irineu Petri Júnior: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Macedo, L.L., Corrêa, J.L.G., da Silva Araújo, C. et al. Convective Drying with Ethanol Pre-treatment of Strawberry Enriched with Isomaltulose. Food Bioprocess Technol 14, 2046–2061 (2021). https://doi.org/10.1007/s11947-021-02710-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02710-2