Abstract
Osmotic dehydration (OD) is a pre-treatment commonly used to incorporate solids and dehydrate fruits. In this study, the influence of mixed syrup (yacon and sucrose) concentration used in OD and drying air temperature on drying kinetics of banana osmotically dehydrated and characteristics of dried banana were evaluated. Yacon syrup (30°Brix) was produced and then sucrose was added, making mixed syrups of different concentrations (30–45°Brix). Banana slices (5 mm) were immersed in mixed syrups for five hours. Solid gain (SG) and water loss (WL) were calculated during OD. Afterward, the osmotically dried bananas were dried at different drying air temperatures (40–80 °C). Mathematical models were fitted to the moisture ratio data. The drying rate (DR) was calculated. The dried bananas were evaluated for moisture content, water activity, color and hardness. Optimal treatment was determined using the desirability function. Higher syrup concentration resulted in higher SG and WL during OD. Samples dehydrated osmotically using 32.2°Brix syrup and dried at 74.1 °C required shorter drying time (240 min), with a tendency of higher DR. High syrup concentrations and high temperatures resulted in dried bananas with low water activity and high hardness. The Midilli model was the one that best fitted. The optimal treatment was that using the mixed syrup of 45°Brix and drying temperature of 60 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Banana (Musa spp.) is one of the most produced fruits in the world, due to its high sensory acceptance and low cost. In its composition it presents carbohydrates (including dietary fibers), phenolic compounds, minerals and vitamins (B3, B6, B12, C and E) [1]. The consumption of this fruit occurs mostly in natura [2]. However, because it is a climacteric fruit and has high water activity, the banana has a short shelf life and undergoes large post-harvest losses [1,2,3]. Given this, the production of dried bananas is a good alternative to extend the shelf life of bananas. The dried banana is one of the products obtained from drying the fresh banana pulp, with moisture content between 20 and 25% (in wet basis) [4,5,6,7].
Osmotic dehydration (OD) is a drying method commonly used as a pre-treatment for the drying. It consists of immersing the product in an osmotic solution, in which water is removed from the food due to the difference in osmotic pressure between the food and the osmotic solution. At the same time as the water leaves, the solute is incorporated into the food. Sucrose, glucose, fructose, and sodium chloride are the most frequently used solutes in OD. However, several studies have been developed using other osmotic agents, such as corn syrup, honey, fructooligosaccharides (FOS), maltose, maltodextrin, ethanol and maple syrup. With that, OD has been a good way to incorporate solids in the food [8,9,10,11].
The incorporation of solids can be conducted to add functional, nutritional, technological or sensory properties to the product, to meet the growing consumer demand for foods with different characteristics. Furthermore, the enriched product has a higher added value by contributing to such properties [11,12,13].
Yacon (Smallanthus sonchifolius) is a tuberous root of Andean origin, where it is grown mainly for family consumption, due to its sweet flavor and crunchy texture. This crop has spread around the world since the 1960s, being consumed mainly raw, but also cooked, roasted, dehydrated (in the form of chips or flour) or processed (in the form of juice, jam, syrup). However, one of the highlights of the yacon is the high concentration of FOS. These carbohydrates are not hydrolyzed by enzymes in the upper part of the human gastrointestinal tract. As a result, FOS are fermented in the colon by a group of beneficial bacteria belonging to the intestinal microflora, improving gastrointestinal function. This characteristic makes yacon a functional food, in addition to providing low caloric value [14,15,16,17]. In this regard, the incorporation of FOS during OD is an efficient way to enrich the fruit, providing a functional compound to the diet, adding health benefits [18].
This work aimed to (i) evaluate the osmotic dehydration kinetics of banana using mixed syrup (yacon and sucrose), (ii) study the convective drying of the osmotically dehydrated banana (iii) examine the influence of the osmotic solution concentration and the drying temperature on water activity, colorimetric parameters and hardness of dried banana.
2 Material and methods
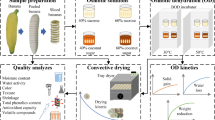
The experiment was conducted as shown in Fig. 1.
2.1 Material
Banana (Musa spp. cv Prata) (71.09% moisture content, in wet basis (w.b.)) and yacon (Smallanthus sonchifolius) were purchased at the market in the city of Alegre/ES, Brazil. The banana bunches were submerged in 200 ppm of a free residual chlorine solution for 10 min and rinsed. The bananas were removed from the bunches and were visually selected for integrity, uniformity and degree of ripeness (close to 4 on the 1 to 7 banana ripeness scale) [19]. The bananas were manually peeled and cut in the transverse direction with the aid of a stainless-steel food slicer, producing slices 5 mm thick.
The yacon roots were selected for integrity and uniformity, washed with running water to remove dirt, submerged in 200 ppm of a free residual chlorine solution for 10 min, rinsed and then peeled with the aid of a fixed blade peeler. The peeled roots were cut into slices 5 mm thick. Bleaching was performed by immersing the slices in boiling water for 8 min and then subjecting the slices to an ice bath for 10 min [16].
2.2 Preparation of mixed syrups
The blanched slices were inserted in a centrifuge (Mondial, model NCF-01) to extract the juice. The juice was disposed of in an evaporator with automatic stirring under heating for the elaboration of yacon syrup with 30°Brix. Mixed syrups were made by adding sucrose to the yacon syrup, to make syrups with a total soluble solids content of 30 (without sucrose added) at 45°Brix. The values of the concentration levels of the mixed syrups were calculated using a Central Composite Rotational Design (CCRD) (Table 1), with 5 repetitions at the central point.
2.3 Osmotic dehydration (OD)
Banana slices were immersed in mixed syrups, in a 1:11 ratio, to minimize the effect of dilution during OD [20]. The OD occurred without stirring, at atmospheric pressure (980 mbar) and 30 °C.
The samples were evaluated for solids gain (SG) (Eq. 1) and water loss (WL) (Eq. 2) at times 0, 15, 30, 45, 60, 90, 120, 180, 240, 300 min.
Where, Mo: initial mass (kg); Mt: mass at time t (kg); \( {\mathrm{x}}_{\mathrm{o}}^{\mathrm{S}} \): initial solids content (kg solids kg of sample−1); \( {\mathrm{x}}_{\mathrm{t}}^{\mathrm{S}} \): solids content at time t (kg solids kg of sample−1); \( {\mathrm{x}}_{\mathrm{o}}^{\mathrm{A}} \): initial water content (kg of water kg of sample−1); \( {\mathrm{x}}_{\mathrm{t}}^{\mathrm{A}} \): water content at time t (kg of water kg of sample−1).
The Peleg (1988) [21] (Eq. 3) and Azuara et al. (1992) [22] (Eq. 4) models were fitted to SG and WL data [23].
Where, t (min) is time; \( {\mathrm{K}}_1^{\mathrm{S}} \) and \( {\mathrm{K}}_1^{\mathrm{W}} \) (min g g−1) are Peleg’s rate parameters for solids and moisture, \( {\mathrm{K}}_2^{\mathrm{S}} \) and \( {\mathrm{K}}_2^{\mathrm{W}} \) (g g−1) are Peleg’s capacity parameters for solids and moisture, Ss and Sw (min−1) are Azuara’s parameter for solids and moisture, SGeq (g g−1) is estimated equilibrium solids gain, WLeq (g g−1) is estimated equilibrium water loss.
2.4 Convective drying
The drying of the osmotically dehydrated bananas was carried out in a tray dryer (50 × 40 cm), with forced air circulation (1.5 m s−1), at temperatures of 40 to 80 °C (Table 1). The trays were weighed at 15 min intervals during the first hour, 30 min during the second and third hour and, finally, at 60 min intervals. The samples remained in the dryer until the moisture content was 20% (w.b.). For this, the final mass, that is, the mass to interrupt the drying process in such a way that the product had the desired moisture content, was calculated by mass balance (Eq. 5).
Where, Wo, Wf, Mo e Mf are the initial and final masses and moisture content, respectively.
The moisture ratio (MR) during drying was calculated according to Eq. 6 [24].
Where X0, Xt and Xe are the water content (kg water 100 kg sample−1, in dry basis) at time zero, time t and equilibrium, respectively.
The Henderson & Pabis, Lewis, Midilli and Page models (Eqs. 7 to 10, respectively) were fitted to the data. The values of model constants were fitted by minimizing the sum of squares of deviations using nonlinear regression.
Where, MR: moisture ratio; t: time (min); k, a, n and b are parameters of the models.
The adjusted coefficient of determination (R2adj) (Eq. 11) and standard error of the regression (SE) (Eq. 12) were calculated to aid the comparison between models.
Where MRexp,i and MRpred,i are, respectively, experimental and predicted values of moisture ratio for the ith observation; \( \overline{\mathrm{MR}} \) is the mean value of the experimental moisture ratio; N is the number of observations; p is the number of constants in the model.
The drying rate (DR) was calculated according to Eq. 13 [24].
Where, DR is the drying rate, Xt + ∆t and Xt are the moisture content (kg of water kg of dry solid−1) at times t + ∆t and t, respectively, t is the time (min) and ∆t is the time difference (min).
DR was plotted as a function of moisture content on dry basis.
2.5 Characterization of dried bananas
The samples were characterized in terms of moisture content, water activity, color and hardness.
2.5.1 Moisture content
The moisture content was determined gravimetrically by drying the samples to constant weight at 105 °C [25].
2.5.2 Water activity (aw)
The aw of samples was determined by direct reading in a water activity meter (Aqualab, 3-TE model, Decagon Devices, Inc., Pullman, WA, USA), at 25 °C.
2.5.3 Color
The colorimetric parameters (L*, a* and b*) of samples were obtained using a colorimeter (Konica Minolta, Spectrophotometer CM-5, Tokyo, Japan). Chroma (C*) and hue (H°) were calculated, according to Eqs. 14 and 15, respectively.
2.5.4 Hardness
The hardness of the dried bananas was determined by a texture analyzer (Brookfield®, CT#3) by compression test, equipped with a load cell of 3.00 N and a knife-edge (TA7). The compression speed set before, during and after the test was 2, 1 and 10 mm s−1, respectively [7].
2.6 Statistical analysis
The experiment was conducted in three replications. The physical-chemical analyses were performed in triplicate.
The responses of the physical-chemical analyses were submitted to analysis of variance (ANOVA) of the regression, adopting a significance level of 5% and quadratic mathematical models (Eq. 16) were fitted to the data. Response surfaces were elaborated with the aid of Statistica software.
Where y is the estimated response, β0, β1, β11, β2, β22 and β12 are constants coefficients; T is the temperature of the drying air (°C) and S is the concentration of the syrup (°Brix).
The coefficient of determination (R2) of the fitted models was calculated according to Eq. 17.
Where yexp,i and ypred,i are, respectively, experimental and predicted values of the response for the ith observation; \( \overline{\mathrm{y}} \) is the mean value of experimental response; N is the number of observations.
2.7 Optimization
The determination of the optimal treatment was performed with the aid of the desirability function. For this, initially, the individual desirability (di) of each response was calculated within the range 0 ≤ di ≤ 1 [26]. Eqs. 18 and 19 were used for the responses that were desired to minimize and maximize, respectively.
Where, y is the response; Ti is the target value desired; Ui is the maximum value for responses that desired to minimize; Li is the minimum value for responses that desired to maximize.
Overall desirability (D) was calculated from individual desirability [26], according to Eq. 20.
Where N is the number of individual desirabilities.
The optimal condition corresponds to the highest value of the overall desirability.
3 Results and discussion
3.1 Osmotic dehydration
The kinetics of solids gain (SG) and water loss (WL) are shown in Fig. 2a and b, respectively. Throughout the OD, SG increased, especially in the first minutes. According to Torreggiani (1993) [27], the SG occurs, mostly, during the first 30 min. Thereafter, there is usually a very significant reduction in the SG rate. The flow of solids and water occurs due to the concentration gradient between the food and the osmotic solution [10].
The increase in the concentration of the osmotic solution (mixed syrup) increased SG (Fig. 2a). Higher concentrations of the osmotic solution increase the pressure gradient between the solution and the food, promoting the driving force for SG [10]. Besides the higher concentration, the 45°Brix syrup had a higher amount of added sucrose, which is a lower molecular weight sugar compared to FOS. Lower molecular weight solutes penetrate the food matrix more easily [10, 20]. Matusek et al. (2008) [18] studied the OD of apple comparing the penetration of sucrose and FOS and it was observed that the GS was higher when the first sugar was used.
According to Genta et al. (2009) [15], the yacon root is the plant with the highest concentration of fructooligosaccharides (FOS). Yacon syrup is a product obtained by concentrating the juice of the yacon root and can have 40 to 50% FOS [15]. FOS are carbohydrates capable of resisting enzymatic hydrolysis in the upper part of the human gastrointestinal tract and are fermented in the colon, promoting the development of fermentative bacteria, which perform several beneficial functions in the organism [15, 17]. Through the gain of solids during OD, including using 30°Brix syrup (without sucrose added), FOS may have been incorporated into the banana, which leads to an increase in the nutritional and functional value of the banana, consequently increasing the added value of fruit [18]. Furthermore, the OD process increases the sensory acceptance of dried bananas [2].
WL presented a behavior similar to that of SG, presenting higher values during the OD and with the increase of the syrup concentration (Fig. 2b). Verma et al. (2014) [3] also observed in the banana OD that the WL was higher according to the higher concentrations of the osmotic solution. Furthermore, WL occurred mainly at the beginning of OD, with a minimum increase from the sixth hour of OD [3].
The behavior of SG and WL responses during OD corroborates those found by Ayetigbo et al. (2019) [28], Fernandes et al. (2019) [29], Barragán-Iglesias et al. (2019) [9] and Chaguri et al. (2017) [30] when performing the OD of white-flesh cassava, mangoes, papaya and green banana, respectively.
The values found for the coefficient of determination (Table 2) indicated that the Peleg and Azuara models fitted satisfactorily to the SG and WL data, thus being able to represent the behavior of these responses during OD. Ayetigbo et al. (2019) [28] also found that these models fit well with the cassava SG and WL data.
For both SG and WL, there was a tendency to reduce the value of the K1 parameter in the Peleg model, as well as an increase in the K2 parameter (Table 2), by increasing the concentration of the mixed syrup. Likewise, there was also a tendency to increase the S parameter of the Azuara model as the syrup concentration increased. These same trends were observed by Ayetigbo et al. (2019) [28], in cassava OD.
3.2 Convective drying
The moisture contents of the osmotically pre-dehydrated bananas using the 30.0, 32.2, 37.5, 42.8 and 45.0°Brix syrups were 67.09, 66.52, 65.71, 64.63 and 63.17% (in wet basis (w.b.)), respectively. The drying kinetics of osmotically pre-dehydrated bananas is shown in Fig. 3. The time to reduce the moisture content of the samples to 20% (w.b.) ranged from 240 to 1260 min. It can be observed that the concentration of the mixed syrup used in the OD and the temperature of the drying air influenced the dispersion behavior of the MR data (Fig. 3).
Lower syrup concentrations favored a reduction in drying time (Fig. 4). In the OD higher concentrations of the syrup resulted in greater incorporation of solids, increasing the number of chemical bonds of water present in the food, making it difficult to eliminate water during convective drying, leading to an increase in drying time.
Although the concentration of the syrup also affected the drying time, it can be observed that temperature was the factor with the greatest influence. As expected, higher drying air temperatures resulted in shorter drying times (Eq. 21). Similar results have been reported by Song et al. (2019) [24].
Where, DT is drying time (min), T is air drying temperature (°C) and S is syrup concentration (°Brix).
The minimum drying time estimated by the mathematical model (Eq. 21) was 239.69 min, corresponding to the mixed syrup concentration of 34.99°Brix and the drying air temperature of 74.86 °C.
Henderson & Pabis, Lewis, Midilli and Page models fitted satisfactorily to the moisture ratio data, since they presented high values of determination coefficient (R2), between 0.894 and 0.999 (Table 3).
The parameter k of the models indicates the effect of external drying conditions [31, 32]. The values of parameter k showed a tendency to increase with increasing temperature and with a reduction in syrup concentration (Table 3).
The parameter n relates to the effect of internal drying conditions, where, n > 1 indicates super-diffusion and n < 1 indicates sub-diffusion [31, 32]. It was observed that, with the increase of the syrup concentration, there was a reduction of a and n. On the other hand, as the drying air temperature increased, there was an increase in the value of a and n (Table 3).
Comparing the models to each other, the Midilli model was that which best fitted the MR data, due to the higher value of the adjusted coefficient of determination (R2adj) and the lower standard error of the regression (SE) in all treatments (Table 3). The comparison between the experimental values and the values predicted by the Midilli model are shown in Fig. 5. It was observed that both values were close since the data were grouped around a 45° straight line [33], presenting itself as another way of observing the good fit of the Midilli model.
Low syrup concentrations and high drying air temperatures resulted in lower DR (Fig. 6). This behavior is because, during OD, the lower concentrations of the osmotic solution resulted in lower SG and WL. The low SG provides a lower proportion of solid materials to interact and make chemical bonds with water, making water more conducive to removal. Moreover, the low WL during OD provides a higher moisture content to be removed during convective drying. Regarding the drying temperature, the DR increased with increasing temperature. Similar results have been reported by Song et al. (2019) [24]. This is due to higher temperatures providing a higher rate of heat and mass transfer, in addition to increasing the water vapor pressure in the samples, increasing water outflow from the food to the drying air [34]. The same fact was observed by Kayacan et al. (2018) [34], drying bee pollen.
The period of constant rate in the drying at 74.1 and 80 °C was short and non-existent in the drying at 40, 45.9 and 60 °C. The falling rate domain indicates that the diffusion of moisture is the main drying mechanism in the present study [24].
3.3 Characterization of dried bananas
3.3.1 Moisture content
The convective drying of the samples was interrupted when they had a moisture content of 20%. Thus, after drying, the dried bananas had a moisture content of 20.75 ± 1.77% (w.b.), with no significant difference between treatments (p > 0.05). According to the standard adopted worldwide, dried bananas have a moisture content between 20 and 25% (w.b.) [3,4,5,6, 35].
3.3.2 Water activity (aw)
The aw of dried bananas was influenced (p < 0.05) by the syrup concentration during OD and the temperature of the drying air (Fig. 7a), showing values between 0.591 and 0.738. The mathematical model fitted satisfactorily to the data of aw, with R2 of 0.90 (Table 4). Values close to aw were found by Cano-Chauca et al. (2004) [4], Verma et al. (2014) [3] and Batista et al. (2014) [35].
It was observed that the use of syrups of higher concentration and higher drying temperatures resulted in samples with less aw. However, the concentration had a greater effect, resulting in a more significant reduction with higher levels of this factor. The incorporation of solids during OD can increase the water trapping in the product, by increasing the number of interactions and chemical bonds of water with the incorporated solids, resulting in a reduction in aw. As a result, the use of OD using an osmotic solution with a high concentration can contribute to the increase in the shelf life of dried bananas, since lower aw disadvantages the growth of microorganisms and reduces the rate of food spoilage reactions [8, 10].
3.3.3 Color
The L* parameter represents the brightness of the sample. The concentration of the syrup and the temperature of the drying air significantly influenced (p < 0.05) the L* of the dried bananas (Fig. 7b and Table 4). The temperature was the main influencing factor in this response and that higher drying temperatures resulted in samples with lower L* values. Similar results have been reported by Song et al. (2019) [24]. Furthermore, a small reduction in L* was observed with the increase in the concentration of the mixed syrup. As they have high sugar content, especially after OD, the samples are susceptible to non-enzymatic browning reactions. These reactions are enhanced when the product is exposed to high temperatures, leading to the formation of dark pigments, especially near the end of the drying [36].
The parameters a* and b* of dried bananas were not significantly influenced (p > 0.05) by the concentration of the syrup and the drying air temperature, with average values of 6.76 ± 0.91 and 45.49 ± 3.02, respectively. The parameter a* indicates the color variation from green (−a) to red (+a). The parameter b* indicates the color variation from blue (−b) to yellow (+ b). Thus, dried bananas showed a slight tendency towards red and a strong tendency towards yellow.
Chroma (C*) and hue (H°) were also not significantly influenced (p > 0.05) by the factors. The C* represents the purity/saturation of the color and presented an average value of 45.65 ± 0.23. The H° showed an average value of 81.64 ± 0.25, being close to the fully yellow tint (90°).
3.3.4 Hardness
Hardness has physical and sensory definitions. Physically, hardness is a force necessary to attain a given deformation. Sensorially, hardness is the force required to compress a substance between molar teeth, in the case of solids food [37, 38].
Instrumental texture analysis can detect and quantify only a few parameters, however, it is unable to replace human beings, as they are the only means able to perceive and describe the sensory properties of food [37]. However, instrumental hardness analysis has excellent correlation with the same analysis performed by sensory analysis [37, 38], with the advantages of eliminating the recruitment of a large number of people, obtaining results quickly, at lower cost and with greater repeatability [39].
The hardness of dried bananas was significantly influenced (p < 0.05) by the concentration of the syrup during OD and the drying air temperature (Fig. 7c and Table 4), whereas higher levels of these factors resulted in greater hardness. Higher concentrations of the osmotic solution used in OD resulted in higher WL, being responsible for higher stress generated in the cellular structure of food, resulting in several physical changes, including increased hardness [9]. Macedo et al. (2019) [6] produced dried bananas with and without osmotic pre-treatment and found that OD was responsible for increasing the hardness of the samples. Besides the concentration of the osmotic solution, it was also observed in the present study that higher drying air temperatures also resulted in greater physical changes, increasing the sample hardness. Vilela et al. (2016) [11] studied OD using osmotic solutions of sorbitol, maltitol, FOS, galactooligosaccharides and fructose, and it was found that FOS was the agent that resulted in the increased hardness of orange peel and citron fruit. Barragán-Iglesias et al. (2019) [9] stated that the hardness of the dry product is greater than that of the fresh product, applying OD or not, as the reduction in the moisture content of the product elevates this texture parameter.
3.4 Optimization
To determine the optimal treatment, the desire was to maximize the responses of solids gain, water loss and luminosity. The responses of solids gain and water loss obtained in the final OD time were used. For the responses of drying time, water activity and hardness, the desire was to minimize them.
Treatment 7 (mixed syrup of 45°Brix and drying temperature of 60 °C) was that with the highest overall desirability, with D = 0.74.
4 Conclusion
Higher syrup concentrations resulted in higher solids gain and water loss during osmotic dehydration (OD). The Peleg and Azuara models fitted satisfactorily to the OD data.
The reduction in drying time occurred according to the use of syrups of lower concentrations and the use of higher drying air temperatures. The Henderson & Pabis, Lewis, Midilli and Page models fitted well to the drying kinetics data, with emphasis on the Midilli model, which was the most suitable for all the treatments studied.
Higher concentrations of the mixed syrup resulted in dried bananas with less water activity and greater hardness. High drying air temperatures were responsible for less water activity, less luminosity and greater hardness of dried bananas.
The optimal treatment was that using the highest concentration of the mixed syrup (45°Brix) and the intermediate drying temperature (60 °C).
Banana OD using yacon syrup can promote the incorporation of yacon solids, among them fructooligosaccharides, therefore, it is a promising technique to be used as pre-treatment for drying by convection of fruits.
Data availability
There are no data available.
References
Amini Khoozani A, Birch J, Bekhit AE-DA (2019) Production, application and health effects of Banana pulp and Peel flour in the food industry. J Food Sci Technol 56(2):548–559. https://doi.org/10.1007/s13197-018-03562-z
Thippanna KS, Tiwari RB (2015) Quality Changes in Osmotically Dehydrated Banana Var. ‘Robusta’ and ‘Ney Poovan’ as Affected by Syrup Concentration and Immersion Time. J Food Sci Technol 52(1):399–406. https://doi.org/10.1007/s13197-013-1008-9
Verma D, Kaushik N, Rao PS (2014) Application of high hydrostatic pressure as a pretreatment for osmotic dehydration of Banana slices (Musa Cavendishii) finish-dried by dehumidified air drying. Food Bioprocess Technol 7(5):1281–1297. https://doi.org/10.1007/s11947-013-1124-6
Cano-Chauca M, Ramos AM, Stringheta PC, Marques JA, Silva PI (2004) Curvas de Secagem e Avaliação Da Atividade de Água Da Banana Passa. Bol do Cent Pesqui Process Aliment 22(1):121–132. https://doi.org/10.5380/cep.v22i1.1184
Borges SV, Mancini MC, Corrêa JLG, Leite J (2010) Secagem de Bananas Prata e d’água Por Convecção Forçada. Ciência e Tecnol Aliment 30(3):605–612. https://doi.org/10.1590/S0101-20612010000300006
Macedo LL, Araújo CS, Vimercati WC, Saraiva SH, Teixeira LJQ (2019) Influência Da Desidratação Osmótica Na Umidade e Textura de Banana-Passa. Rev. Agrotecnologia 10(1):81–87. https://doi.org/10.12971/2179-5959/agrotecnologia.v10n1p81-87
Macedo LL, Vimercati WC, Araújo C, Saraiva SH, Teixeira LJQ (2020) Effect of drying air temperature on drying kinetics and physicochemical characteristics of dried Banana. J Food Process Eng 43(April):1–10. https://doi.org/10.1111/jfpe.13451
Ahmed I, Qazi IM, Jamal S (2016) Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov Food Sci Emerg Technol 34:29–43. https://doi.org/10.1016/j.ifset.2016.01.003
Barragán-Iglesias J, Rodríguez-Ramírez J, Sablani SS, Méndez-Lagunas LL (2019) Texture Analysis of Dried Papaya (Carica papaya L., Cv. Maradol) pretreated with calcium and osmotic dehydration. Dry Technol 37(7):906–919. https://doi.org/10.1080/07373937.2018.1473420
Muñiz-Becerá S, Méndez-Lagunas LL, Rodríguez-Ramírez J (2017) Solute transfer in osmotic dehydration of vegetable foods: a review. J Food Sci 82(10):2251–2259. https://doi.org/10.1111/1750-3841.13857
Vilela A, Sobreira C, Abraão AS, Lemos AM, Nunes FM (2016) Texture quality of candied fruits as influenced by osmotic dehydration agents. J Texture Stud 47(3):239–252. https://doi.org/10.1111/jtxs.12177
Piasecka E, Klewicki R (2009) Odwadnianie Osmotyczne Owoców w Roztworach Zawierających Fruktooligosacharydy. Zywn Technol Jakosc 2(63):138–153
Rascón MP, Huerta-Vera K, Pascual-Pineda LA, Contreras-Oliva A, Flores-Andrade E, Castillo-Morales M, Bonilla E, González-Morales I (2018) Osmotic dehydration assisted impregnation of lactobacillus Rhamnosus in Banana and effect of water activity on the storage stability of probiotic in the freeze-dried product. Lwt 92(November 2017):490–496. https://doi.org/10.1016/j.lwt.2018.02.074
de Almeida Paula HA; Abranches MV; de Luces Fortes Ferreira, C. L. Yacon ( Smallanthus sonchifolius ) (2015). A Food with Multiple Functions. Crit. Rev. Food Sci. Nutr., 55 (1), 32–40. https://doi.org/10.1080/10408398.2011.645259
Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, Sánchez S (2009) Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr 28(2):182–187
Macedo LL, Silva Araújo C, Vimercati WC, Saraiva SH, Teixeira LJQ (2019) Evaluation of different bleaching methods applied to Yacon. J Food Process Eng:1–7. https://doi.org/10.1111/jfpe.13276
Caetano BFR; de Moura NA; Almeida APS; Dias MC; Sivieri K; Barbisan LF (2016). Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients, 8 (7). https://doi.org/10.3390/nu8070436
Matusek A, Czukor B, Merész P (2008) Comparison of sucrose and Fructo-oligosaccharides as osmotic agents in apple. Innov Food Sci Emerg Technol 9(3):365–373. https://doi.org/10.1016/j.ifset.2007.10.003
PBMH; PIF. Programa Brasileiro Para a Modernização Da Horticultura & Produção Integrada de Frutas. Normas de Classificação de Banana. São Paulo: CEAGESP 2006
Ramya V, Jain NK (2017) A review on osmotic dehydration of fruits and vegetables: an integrated approach. J Food Process Eng 40(3):1–22. https://doi.org/10.1111/jfpe.12440
Peleg M (1988) An empirical model for the description of moisture sorption curves. J Food Sci 53(4):1216–1217. https://doi.org/10.1111/j.1365-2621.1988.tb13565.x
Azuara E, Beristain C, Garcia H (1992) Development of a mathematical model to predict kinetics of osmotic dehydration. J Food Sci Technol 29:239–242
Barbosa Júnior JL, Cordeiro Mancini M, Hubinger MD (2013) Mass transfer kinetics and mathematical Modelling of the osmotic dehydration of Orange-fleshed honeydew melon in corn syrup and sucrose solutions. Int J Food Sci Technol 48(12):2463–2473. https://doi.org/10.1111/ijfs.12237
Song XD, Mujumdar AS, Law CL, Fang XM, Peng WJ, Deng LZ, Wang J, Xiao HW (2019) Effect of drying air temperature on drying kinetics, color, carotenoid content, antioxidant capacity and oxidation of fat for Lotus pollen. Dry Technol:1–14. https://doi.org/10.1080/07373937.2019.1616752
AOAC - Association Of Official Analytical Chemists (1995) Official methods of analysis, 16th edn. Association of Official Analytical, Arlington
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12(4):214–219. https://doi.org/10.1080/00224065.1980.11980968
Torreggiani D (1993) Osmotic dehydration in fruit and vegetable processing. Food Res Int 26(1):59–68. https://doi.org/10.1016/0963-9969(93)90106-S
Ayetigbo O, Latif S, Abass A, Müller J (2019) Osmotic dehydration kinetics of biofortified yellow-flesh cassava in contrast to white-flesh cassava (Manihot Esculenta). J Food Sci Technol 56(9):4251–4265. https://doi.org/10.1007/s13197-019-03895-3
Fernandes FAN, Braga TR, Silva EO, Rodrigues S (2019) Use of Ultrasound for Dehydration of Mangoes (Mangifera indica L.): Kinetic Modeling of Ultrasound-Assisted Osmotic Dehydration and Convective Air-Drying. J Food Sci Technol 56(4):1793–1800. https://doi.org/10.1007/s13197-019-03622-y
Chaguri L, Sanchez MS, Flammia VP, Tadini CC (2017) Green Banana (Musa Cavendishii) osmotic dehydration by non-caloric solutions: modeling, physical-chemical properties, color, and texture. Food Bioprocess Technol 10(4):615–629. https://doi.org/10.1007/s11947-016-1839-2
Simpson R, Ramírez C, Nuñez H, Jaques A, Almonacid S (2017) Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices. Trends Food Sci Technol 62:194–201. https://doi.org/10.1016/j.tifs.2017.01.003
dos Santos FS, de Figueirêdo RMF, Queiroz AJ d M, Santos D d C (2017) Drying kinetics and physical and chemical characterization of white-fleshed ‘Pitaya’ peels. Rev Bras Eng Agrícola e Ambient 21(12):872–877. https://doi.org/10.1590/1807-1929/agriambi.v21n12p872-877
Junqueira JR d J, Corrêa JLG, de Oliveira HM, Ivo Soares Avelar R, Salles Pio LA (2017) Convective Drying of Cape Gooseberry Fruits: Effect of Pretreatments on Kinetics and Quality Parameters. LWT - Food Sci Technol 82:404–410. https://doi.org/10.1016/j.lwt.2017.04.072
Kayacan S, Sagdic O, Doymaz I (2018) Effects of hot-air and vacuum drying on drying kinetics, bioactive compounds and color of bee pollen. J Food Meas Charact 12(2):1274–1283. https://doi.org/10.1007/s11694-018-9741-4
Batista D d VS, Cardoso RL, de Godoy RCB, Evangelista-Barreto NS (2014) Estabilidade Físico-Química e Microbiológica de Banana Passa Orgânica. Ciência Rural 44(10):1886–1892. https://doi.org/10.1590/0103-8478cr20130442
Sagar VR, Suresh Kumar P (2010) Recent advances in drying and dehydration of fruits and vegetables: a review. J Food Sci Technol 47(1):15–26. https://doi.org/10.1007/s13197-010-0010-8
Szczesniak AS (2002) Texture is a sensory property. Food Qual Prefer 13(4):215–225. https://doi.org/10.1016/S0950-3293(01)00039-8
Nishinari K, Fang Y, Rosenthal A (2019) Human Oral processing and texture profile analysis parameters: bridging the gap between the sensory evaluation and the instrumental measurements. J Texture Stud 50(5):369–380. https://doi.org/10.1111/jtxs.12404
Li P; Wu G; Yang D; Zhang H; Qi X; Jin Q; Wang X (2020). Applying Sensory and Instrumental Techniques to Evaluate the Texture of French Fries from Fast Food Restaurant. J Texture Stud, No. January, 1–11. https://doi.org/10.1111/jtxs.12506
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Espírito Santo (FAPES).
Author information
Authors and Affiliations
Contributions
Macedo designed the study, collected test data, interpreted the results and drafted the manuscript.
Araújo and Vimercati collected test data and interpreted the results.
Saraiva and Teixeira designed the study and review the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing for financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Macedo, L.L., da Silva Araújo, C., Vimercati, W.C. et al. Influence of yacon syrup concentration and drying air temperature on properties of osmotically pre-dehydrated dried banana. Heat Mass Transfer 57, 441–451 (2021). https://doi.org/10.1007/s00231-020-02966-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-020-02966-y