Abstract
The aim of this study was to evaluate the replacement aspects of conventional methods (petroleum-based solvent and Folch assay) by alternative methods (bio-based and biodegradable solvent 2-methyltetrahydrofuran (MeTHF) and supercritical CO2 (SC-CO2)) for seed oil extraction from anise (Pimpinella anisum L.) and fennel (Foeniculum vulgare Mill.). Results showed that the highest oil yield of aniseeds was obtained by using Folch (24.07%) and MeTHF (23.65%) extraction methods whereas fennel seeds had 20.02% and 18.72%, respectively. Fatty acid composition of both seed oils obtained by the two green extraction methods was similar to the conventional ones with the predominance of petroselinic acid (54.22–61.25% in fennel and 42.39–48.97% in anise). Besides, SC-CO2 method allowed to obtain the maximum of sterol content in anise (3.85 mg/g of oil) and fennel (4.64 mg/g of oil) seed oils. Furthermore, anise and fennel seed oils extracted with MeTHF method significantly showed higher total phenolic content (2.43 and 1.32 mg GA/g oil, respectively), stronger antioxidant activity (9.23 and 5.04 μmol TEAC/g oil, respectively), and oxidative stability (8.23 and 10.15 h, respectively) than the other methods (p < 0.05). In conclusion, MeTHF appeared to be a good substitute to petroleum solvents for recovery of high oil quality from Pimpinella anisum and Foeniculum vulgare seeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil seeds are crucial for the nutritional security of the global population (Abert Vian et al. 2013). They are a source of nutritious human and animal food. Oil is recovered from plant either by mechanical expression or by chemical extraction processes (Akinoso and Adeyanju 2012). The first is often associated with low yields, and the latter uses solvents. Such solvents are dangerous to handle and are harmful to human health (Nyam et al. 2011). Frequently, hexane is widely used for oil extraction because of easy oil recovery, narrow boiling point (63–69 °C), and excellent solubilizing ability (Abert Vian et al. 2013). However, it is a noxious waste since it is released into the environment and reacts with pollutants to form ozone and photo. Moreover, several studies revealed that hexane is toxic both in short- and long-term expositions and affects the neural system when inhaled by humans (Misirli et al. 2008). In addition, the environmental contamination associated with its use has placed new demands on the food, cosmetic, and pharmaceutical industries to invest in clean technologies that could provide high-quality products for the highly competitive global market (Nyam et al. 2011). Hence, greener technologies are viable alternatives for oil extraction and are aimed to develop an environment friendly process with the elimination of the use of toxic solvents, the improvement of process efficiency and enhancement of extraction yields in a shorter time with less thermal degradation, and high quality of the oil (Virot et al. 2008). Greener solvents like ethanol, limonene, and 2-methyltetrahydrofuran (MeTHF) are mostly produced from agricultural sources and are greener processing fluids for bioactive compounds extraction and oil separation from oil seeds (Liu and Mamidipally 2005; Breil et al. 2016). In addition, supercritical carbon dioxide extraction is an eco-friendly technique that has been demonstrated to be useful in extracting edible oils from numerous sources (Kulkarni et al. 2017). Recent developments in supercritical carbon dioxide extraction technology have demonstrated that it can be a promising alternative to conventional extraction methods. Hence, supercritical CO2 has been shown to produce extracts with a natural aroma free from chemical alterations induced by heat and water, and without solvent residues and undesirable compounds such as organic and inorganic salt, sugars, amino acids, and tannins (Danh et al. 2013).
Fennel (Foeniculum vulgare Mill.) and anise (Pimpinella anisum L.) are important members of the Apiaceae family. Recently, much attention has been focused on these species due to the nutritional and health protective value of their seeds. They are a source of healthy promoting compounds as minerals, vitamins, phenolic compounds, and volatile oils (Bettaieb Rebey et al. 2018; Miguel et al. 2010). Moreover, they contain a noticeable yield of oils ranging from 11% in anise (Kozłowska et al. 2016; Bettaieb Rebey et al. 2018) to 13% in fennel, which are rich on monounsaturated fatty acids including oleic and petroselinic ones. Publications in the literature had reported oil extraction from anise and fennel seeds by organic solvent n-hexane (Bettaieb Rebey et al. 2016, 2018) and by SC-CO2 (Simándi et al. 1999; Moura et al. 2005; Shokri et al. 2011). However, there are no data about oil extraction from these two seeds using an agro-solvent as MeTHF.
Thus, the aim of the study was to obtain anise and fennel oil using MeTHF and supercritical carbon dioxide technique. The Soxhlet technique using n-hexane as the solvent and the Folch method were applied to obtain oils that were used for comparison purposes. In addition, the oil samples obtained were analyzed for their oil yield, fatty acid composition, sterol composition, antioxidant activity, and oxidative stability.
Materials and Methods
Plant Material
Fennel (Foeniculum vulgare Mill) and anise (Pimpinella anisum L.) seeds were harvested in June 2016, from Korba region in the northeastern part of Tunisia; latitude 36340 38.22″ (N); longitude 10510 29.63″ (E) and the altitude is 637 m. The precipitation average was 400–500 mm/year and the monthly average temperature was 17.7 ± 2 °C. Plants were identified by the botanist of the Biotechnology Center of Borj-Cedria (CBBC). A voucher specimen was deposited at the herbarium of the Laboratory of Bioactive Substances at CBBC under the “BC2011-2000” and “BC2011-2002” numbers, respectively, for fennel and anise seeds. Both seeds were air-dried at room temperature until constant weight. After drying, seeds were placed in dark glass bottles and stored in a refrigerator (Fisher Isotemp brand, USA) at 4 °C until use for further analysis.
Seed Oil Extraction
Soxhlet Extraction
Harvested seeds were finely grounded with a type A10 blade-carbide grinding (Ika-Werk, Staufen, Germany) and 30 g of a powdered sample were weighted in a 30 mm × 100 mm cellulose thimble (Schleicher and Schuele) and were placed in the extraction chamber of a 125-mL Soxhlet apparatus (type Gerhardt) fitted with a condenser, which was placed on a 500-mL distillation flask containing 250 mL of the solvent. Samples were extracted under reflux with n-hexane and MeTHF during 8 h at 85 °C. After extraction, solvents were evaporated under reduced pressure, using a rotary evaporator (Labobase/Laborota 4000 Heidolph WB/G4-intensive condenser) at 45 °C. The dried residues were weighed and oils were aliquoted in vials and stored at 4 °C until analysis. Oil content was determined as a percentage of the mass of lipids (g) obtained after extraction relative to the weight of dry sample (g) used for extraction.
Folch Method
Thirty grams of ground powdered plant seeds were extracted with 300 mL of a chloroform/methanol (2/1, (v/v)) solution at room temperature under shaking for 2 h (Folch et al. 1957). Then, the mixture was filtered through Whatman No. 1 paper filter into a separatory funnel and a 1 M KCl solution (70 mL) was added. After gentle manual shaking, the mixture was left overnight for separation into two phases. The lower phase was collected and solvents were evaporated under reduced pressure at 40 °C (Rotavapor R-215, Büchi Labortechnik, Switzerland). The extracted oil was weighed and flushed with nitrogen, and stored in a freezer (Thermo Fisher Scientific brand, USA) at − 20 °C until further analysis.
SC-CO2
Oil was extracted from fennel and anise seeds with pilot-scale equipment (Separex, France), using SC-CO2, as previously described by Koubaa et al. (2015) with slight modifications. The extracted oil was maintained at 200 bars pressure and 40 °C temperature. The extraction time was fixed to 180 min under a continuous flux of CO2 (14 mL/min), for all experiments. After finishing the extraction processes, total extraction yields (Y) were measured. Obtained extracts were transferred into the glass bottles, sealed and stored in a freezer (Thermo Fisher Scientific brand, USA) at − 20 °C to prevent any possible degradation of extract components until analysis.
Fatty Acid Analysis
Fatty acid composition was analyzed by gas chromatography (GC) after derivatization to fatty acid methyl esters (FAMEs) with a 2 M methanolic solution of potassium hydroxide (Cecchi et al. 1985). FAMEs were analyzed by gas chromatography using a Hewlett-Packard 6890 chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with a flame-ionization detector (FID) and an electronic pressure control (EPC) injector. They were separated on a RT-2560 capillary column (100 m length, 0.25 mm i.d., 0.20 mm film thickness). The oven temperature was kept at 170 °C for 2 min, followed by a 3 °C min−1 ramp to 240 °C and finally held there for an additional 15 min period. Nitrogen (U) was used as a carrier gas at a flow rate of 1.2 mL min−1. The injector and detector temperatures were maintained at 225 °C. Individual fatty acids were identified by comparing their retention times with a certified fatty acid methyl esters (FAME) mix and quantified as a percentage of the total fatty acids.
Sterol Analysis
The content and composition of the sterols were determined by GC following the procedure described by Anonymos (1997) Official Method. Each seed oil (50 mg) was saponified with 1 M KOH in methanol for 18 h at room temperature, then water was added and the unsaponifiables were extracted six times with n-hexane/methyl tert-butyl ether (1:1, v/v). The solvent was evaporated at ambient temperature under a stream of nitrogen. Dry residues were dissolved in 0.2 mL pyridine and silylated with 0.8 mL of Sylon BTZ (Supelco, Bellefonte, PA, USA). Sterol derivatives were separated on a Trace GC Ultra equipped with DB-35MS capillary column. A sample of 1.0 μL was injected in a splitless mode with an injection time of 5 min. The column temperature was held at 100 °C for 5 min, then increased to 250 °C at a rate of 25 °C/min, held for 1 min, then further increased to 290 °C at a rate of 3 °C/min and held for 20 min. The detector temperature was set at 300 °C. Hydrogen was used as a carrier gas at a flow rate of 1.5 mL/min. Sterols were identified by comparing their retention times with those of commercially available standards and results were expressed as milligram per gram (mg/g) of oil.
Total Phenolic Content Determination
Phenolic compounds of seeds were extracted by methanol-water solution and determined by Folin–Ciocalteu method described by Liu et al. (2012). In 5 mL hexane, 2.5 g of oil was dissolved and extraction was carried out by methanol–water solution (80:20% v/v). The aqueous phase was collected by centrifugation (Heraeus Labofuge 200 Centrifuge) at 3500 rpm for 5 min, followed by rotary vacuum drying (RE-2000 Model, China) at least than 40 °C and reduced pressure to dryness. Dried sample was dehydrated in 5 mL of methanol solution and was mixed with 2.5 mL of Folin reagent and 10 mL of sodium carbonate solution in 50 mL volumetric flask and was adjusted to volume with deionized water. The absorbance was evaluated at 765 nm after 30 min (Ultrospec 7000 Spectrophotometer UV-Vis). Gallic acid was used for calibration and the results were expressed as milligram (mg) gallic acid equivalent per 100 g of oil samples. Six replicate tests were performed for each sample.
Measurement of Antioxidant Activity (DPPH Assay)
The antioxidant activity of the methanolic extracts of seed oils and seed oil samples was determined using DPPH radicals as described by Kiralan et al. (2009), with some modifications. Of each methanolic extract of seed oils, 0.5 mL was diluted with 3.25 mL of methanol, and then 0.25 mL of 1 mM methanolic solution of DPPH was added. The mixture was vigorously mixed for 10 s in a vortex apparatus and left in darkness for 10 min. The absorbance was measured at 515 nm against pure methanol using a UV/Vis spectrophotometer. The radical scavenging activity was expressed as Trolox equivalent antioxidant capacity (TEAC) using a Trolox calibration curve (lmol TEAC/g of oil).
Oxidative Stability
Oxidative stability of anise and fennel seed oils was measured by Rancimat (Metrohm Rancimat; Metrohm, Riverview, FL, USA), based on the method of Tabee et al. (2008).
Statistical Analysis
All results were reported as means ± standard deviation (SD) of six replicates. Duncan’s test (p < 0.05) was performed to determine significant differences among means of six independent experiments.
Results and Discussion
Seed Oil Extraction
The seed oil yield mainly depends on which method and solvent were used to recover oil. As can be seen in Fig. 1, Soxhlet with n-hexane and Folch methods have been substituted by alternative solvent (MeTHF) and SC-CO2 method in order to compare their oil recovery performances. Indeed, aniseeds yielded the highest amount of oil using Folch (24.07%) and MeTHF (23.65%) extraction methods whereas fennel seeds had 20.02% and 18.72%, respectively. The result obtained for aniseed oil yield extracted by n-hexane (16.87%) was higher than that reported by Kozłowska et al. (2016) in the case of Poland aniseeds (9.03%) and Bettaieb Rebey et al. (2018) for Tunisian (11.60%) and Egyptian (9.82%) aniseeds. These dissimilarities may be mainly attributed to the geographic origin and the genera of seeds (Kozłowska et al. 2016). On the other hand, our results were in line with those obtained by Sayed Ahmad et al. (2018) who reported a vegetable oil content of 19.80% in fennel seeds after cyclohexane extraction.
Comparing the extraction methods, in our study, there were significant differences regarding their extraction yields (p < 0.05). Thus, conventional Folch and Soxhlet methods using MeTHF as a solvent deliver significantly higher yields than the other two methods (p < 0.05). Hence, Soxhlet method using MeTHF as solvent, proposed in this work as a preferable method, besides of being an environmentally friendly alternative, allowed to obtain one of the better oil yields in anise and fennel seeds. This was in good agreement with Breil et al. (2016) who stated that bio-based solvents could be an alternative to petrochemical solvents.
What’s more, the oil yield of these two seeds found by n-hexane was compared with that obtained by SC-CO2. Thus, even though obtaining almost the same oil yield, SC-CO2 extraction has offered many privileges compared with conventional hexane extraction. As a matter of fact, with hexane, a mixture of oil–hexane was achieved. Hexane should then be evaporated which presented a risk of alteration of oil quality by oxidation (Mhemdi et al. 2011). In spite of, SC-CO2 extraction permitted us to attain pure oil without residual organic solvent traces, and thus any chemical changes due to the processing technique, which gave extract of outstanding quality which is of large importance (Boutin and Badens 2009). In our study, the oil yield of anise and fennel seeds was procured when SC-CO2 was carried out at 40 °C temperature, 200 bars+ pressure, and 180 min extraction time. According to our study, high pressures (200–300 bar) and low temperatures (30–40 °C) were used for oil extraction with SC-CO2 from anise (Shokri et al. 2011) and fennel (Simándi et al. 1999; Moura et al. 2005) seeds. Similar findings were also observed in the case of SC-CO2 oil extraction from borage (Molero Gómez and Martı́nez de la Ossa 2002) and rosehip (Salgın et al. 2016) seeds. So, high pressures were generally recommended to increase the solubility of oil in CO2. However, the increase of the temperature generally resulted in the decrease of the extraction yield, due to the decrease of the solvents density, whose effect seemed to have dominated over the increase of the solute vapor pressure (Sovilj 2010; Shokri et al. 2011). Under these pressure and temperature conditions, extraction time was predicted to be 180 min as reported by Shokri et al. (2011).
Fatty Acid Composition
Vegetable oils are a mixture of mono-, di-, and triglycerides (97%) and other minor compounds with functional importance, such as vitamins, sterols, pigments, carotenoids, tocopherols, free fatty acids, hydrocarbons, and others (Pereira et al. 2010). The fatty acid composition of oil is its most useful chemical property. In this context, fatty acid composition of the seed oils obtained using conventional and alternative methods is summarized in Table 1. Anise and fennel seed oils were characterized by the highest contribution of unsaturated fatty acid, whereas the saturated fatty acid content was the lowest in the two studied oil samples.
Regardless of the extraction method, petroselinic acid (C18:1Δ6) was the most prevalent fatty acid, 54.22–61.25% and 42.39–48.97%, respectively for fennel and anise seed oils. Hence, our results prove also that anise and fennel vegetable oils were mainly a source of petroselinic acid followed by oleic and linoleic acids, whereas the levels of other components were present with lower concentrations as reported by previous studies (Kozłowska et al. 2016; Bettaieb Rebey et al. 2018; Sayed Ahmad et al. 2018). Typically, the major fatty acid component in Apiaceae plant seed oils is petroselinic acid.
Pimpinella anisum L. and Foeniculum vulgare Mill. seed oils, belonging to the Apiaceae family, are considered among the highest natural source of petroselinic acid by several authors (Bettaieb Rebey et al. 2018; Sayed Ahmad et al. 2018) and has awakened a great interest as a natural source of this fatty acid. Thus, one of the main goals of this study was to evaluate if fennel and anise seed oils produced with alternative extraction methods have the same petroselinic acid composition than traditionally extracted oils. As can be observed from Table 1, anise and fennel fatty acid profiles obtained from the different extraction methods were similar, nevertheless the statistical analysis showed few differences between them (p < 0.05). Comparable trends were also observed in the fatty acid composition of Corylus avellana (Bernardo-Gil et al. 2002) and Opuntia stricta (Koubaa et al. 2016) seed oils as extracted by organic solvent (hexane) and SC-CO2. Similarity in the fatty acid profile of SC-CO2 and Soxhlet-extracted oils had been observed in Sargassum hemiphyllum, although the fatty acid composition of SC-CO2-extracted oil had been reported to vary slightly with temperature and pressure (Cheung et al. 1998). However, Mariod et al. (2011) reported that the fatty acid profiles of Hibiscus cannabinus seed oil did not change with pressure and temperature of SC-CO2.
In our study, the proportion of the different fatty acids as well as the proportion of SFA, PUFA, or MUFA had not been changed by the new methods used in our experiment; in other words, the use of SC-CO2 and MeTHF as solvents did not introduce extraneous effects in the composition of the extracted oils. As reported by Chemat et al. (2017), green extraction methods can be considered interesting alternative technologies for conventional methods.
In the main, fatty acid profiles obtained from anise and fennel seeds are considered ideal for edible oils, because of its high percentage of UFA and low percentage of SFA, indicating the possible use of these oils in food industry (Pereira et al. 2017). Besides, oils containing high amount of PUFA are generally used in cosmetics and pharmaceutical industries (Stupp et al. 2008). Moreover, a recent trend is the use of vegetable oils for biodiesel production, especially those derived from agro-industrial wastes (Malacrida and Jorge 2012).
Sterol Content
Phytosterol content of anise and fennel oils was investigated due to their roles in the reduction of blood LDL cholesterol content and hence, their potential to decrease the risk of cardiovascular diseases (Dong et al. 2016). Moreover, they can be considered valuable tools for oil ranking and primary indexes for identification of fraud in edible oils (Ribas et al. 2017). As can be seen in Fig. 2, significant differences between the total sterol content in fennel and anise oils extracted from seeds with conventional and alternative methods (p < 0.05). Indeed, the contribution of total sterols reached, interestingly, the highest amount in both fennel (4.64 mg/g of oil) and anise oil (3.85 mg/g of oil) extracted with SC-CO2 method (Fig. 2). Besides, in fennel and anise oil extracted with the MeTHF, the total sterols content was about 1.5 times higher as compared with the Folch method. Eventually, sterol contents, of both seeds, obtained by MeTHF procedure were quite similar to those obtained by conventional extraction method using n-hexane as reference. Similarly, Sicaire et al. (2015) reported that rapeseed oil extracted with MeTHF was comparable with oil extracted with n-hexane in total sterol content. Mariod et al. (2011) showed that extraction of kenaf seed oil using SC-CO2 at high temperature (80 °C) gave higher sterol amount when compared with hexane extraction. So, the content of sterol in oils could be affected by several experimental factors, namely temperature, pressure, time, type of solvent, and type of oil extraction method. In brief, SC-CO2 method, proposed in this work, besides being an environmental friendly alternative, allowed to obtain the maximum sterol content in anise and fennel seed oils and there is a commercialization potential using this method.
The tested oil samples of our study were characterized by the presence of the following sterols: cholesterol, campesterol, stigmasterol, D7-campesterol, β-sitosterol, sitostanol, D5-avenasterol, and D7-avenasterol. The data is listed in Table 2. Cholesterol, an untypical sterol of plant lipids, was only detected in fennel oil at the level of 0.04–0.06 (mg/g oil). What’s more, stigmasterol was present in the highest amount in fennel, regardless of extraction method. Therefore, oil extracted from fennel seeds using SC-CO2 method contained 1.28 and 1.34 times higher amount of stigmasterol than that extracted with n-hexane and Folch method, respectively. Moreover, stigmasterol occurred in fennel seed oil obtained with MeTHF was 1.21 and 1.27 folds higher than that obtained by the two conventional methods used in our study. Besides, the content of β-sitosterol, the most prevalent sterol in aniseed oil, ranged from 1.45 mg/g in oil extracted with Folch method to 2.38 and 1.97 mg/g in aniseed oil obtained by SC-CO2 and MeTHF methods, respectively. The comparable amount of β-sitosterol in Polandian anise seeds was reported by Kozłowska et al. (2016). They also found significant content of stigmasterol in these seeds, which is in harmony with our research. Moreover, the highest content of campesterol was found in aniseed oil. Thus, Soxhlet with MeTHF gave an oil with the highest proportion of campesterol (1.34 mg/g oil) followed by SC-CO2 method (1.19 mg/g oil). Ben khedir et al. (2017) reported that β-sitosterol, campesterol, cholesterol, and stigmasterol have oxidative, anti-inflammatory, and antimutagenic activities.
Total Phenolic Content
Phenols constitute one of the major groups of compounds acting as antioxidants and having different therapeutic and protective effects on human health (Sayed Ahmad et al. 2018). The phenolic fraction of anise and fennel seed oils was isolated with methanol/water (80:20 v/v) as the case of black cumin, cumin (Ramadan et al. 2012), and grape (Konuskan et al. 2019) seed oils. In fact, the use of methanol/water (80:20 v/v) was reported as an efficient extraction solvent and it has been used in the official method of phenol determination from olive oil (Montedoro et al. 1992; Tasioula-Margari and Tsabolatidou 2015). In this study, the effect of oil extraction method on total phenolic contents (TPC) of anise and fennel seed oils was determined by Folin–Ciocaltieu assay.
As reported in Table 3, anise and fennel seed oils extracted using MeTHF showed significantly higher TPC (2.43 and 1.32 mg GA/g oil, respectively) than those extracted with hexane (1.94 and 0.93 mg GA/g oil, respectively), Folch (1.11 and 0.67 mg GA/g oil, respectively), and SC-CO2 (0.89 and 0.54 mg GA/g oil, respectively) methods (p < 0.05). Our results revealed that MeTHF solvent had better selectivity and gave oil with higher TPC than hexane and chloroform methanol (Folch method). Different results were obtained by Kozłowska et al. (2016) concerning TPC of Polandian aniseed oil extracted by chloroform/methanol and hexane methods having 2.52 and 0.42 mg GA/g oil, respectively. Such differences could be explained by the effect of origin, environmental conditions, and/or genetic factors on TPC of seed oils. Additionally, TPC of seed oils can be also affected by several experimental factors, namely temperature, pressure, time, type of solvent, and type of oil extraction method. To the best of our knowledge, this study is the first that reports the comparison of TPC of oils seeds and especially those of fennel and anise extracted using conventional and alternative methods.
Antioxidant Activity
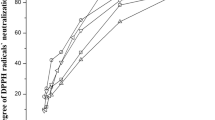
At present, most of the preservatives used by the food industry are artificial additives such as nitrates, sulfur dioxide, and benzoates. However, there is an increasing public concern over the use of artificial food additives and a growing demand for natural alternatives. Consequently, there is a constant demand in the food industry for natural food preservatives and antioxidant agents (Danh et al. 2013). In addition, consumption of foods rich in natural antioxidants has been reported to give protection against certain types of cancer and may also reduce the risk of cardiovascular and cerebrovascular events (Liu et al. 2009). Hence, the antioxidant ingredients and their health-related functionality and mechanism in the oil deserve further study. In this work, the fennel and anise seed oils extracted by conventional and alternative methods were investigated for their antioxidant activity through reduction of the DPPH free radicals. From Table 3, anise and fennel seed oils extracted with MeTHF exhibited the highest DPPH activity (5.04 and 9.23 μmol TEAC/g oil, respectively) followed by hexane (5.32 and 14.02 μmol TEAC/g oil, respectively), Folch (8.82 and 18.56 μmol TEAC/g oil, respectively), and SC-CO2 (5.04 and 19.58 μmol TEAC/g oil, respectively). Such differential scavenging activities can be explained through the strongest ability of MeTHF solvent to extract the adequate bioactive compounds responsible of this potent antiradical activity. In fact, solvents may influence the antioxidant activity of samples because they may affect the hydrogen-donating ability of antioxidants. Moreover, according to the “polar paradox” theory, polar antioxidants are more effective in the lipophilic media, while nonpolar antioxidants are more active in the polar media (Ramadan and Moersel 2006). Additionally, our findings pointed out a linear and positive correlation between phenol content and antioxidant activity of both seed oils, which supported the hypothesis that phenolics could be the major contributors to efficient DPPH radical scavenging capacity of both seed oils especially extracted by MeTHF solvent. In fact, anise and fennel seed oils, extracted by the green solvent MeTHF, were found to represent eventually a strong electron donor and could react with free radicals to convert them to more stable products and terminate the radical chain reaction (Koubaa et al. 2017).
Oxidative Stability
Oxidative stability (OS) is a very important parameter once it gives a good perception and estimation of the susceptibility to oxidation process (Malheiro et al. 2013). In our study, OS of fennel and aniseed oils was analyzed by Rancimat, where the ability of oil to resist peroxidation was measured as the induction period (Holser 2003). OS can be considered a valuable tool for oil ranking and a primary index for identification of fraud in edible oils. The values of induction period in aniseed oils were 10.15, 7.45, 5.26, and 3.02 h, respectively, for MeTHF, n-hexane, Folch, and SC-CO2 extracted oils. A similar trend was observed in fennel seed oil that was extracted by these four extraction processes. For both seed oils, the oxidative stability of MeTHF-extracted oil was higher than three other samples and indicated significant differences with them (p < 0.05). The higher oil stability may be attributed to the higher value of TPC and antioxidant activity of MeTHF-extracted oil. Enhancement of oil oxidative stability due to MeTHF extraction was reported for the first time in Apiaceae seeds.
Conclusion
In the course of time, green solvents and technologies are in great demand because of environmental, health, and energy issues. It is inevitable to develop a novel green technology for the oil extraction from various seed oils. As each seed oil comprises of different architecture, the process needs to look for suitability of technology in economic and technical ways. In this study, the production of anise and fennel seed oils was pronounced by using Folch (24.07% and 20.02%, respectively) and MeTHF (23.65% and 18.72%, respectively) extraction techniques. Fatty acid profiles of both seed oils obtained by the four extraction methods were comparable with the predominance of petroselinic acid (42.39–61.25%). SC-CO2 method allowed to obtain the maximum of sterol content in anise (3.85 mg/g of oil) and fennel (4.64 mg/g of oil) seed oils. Concerning MeTHF solvent, it recovered more bioactive compounds as phenolics (2.43 mg GA/g oil in anise and 1.32 mg GA/g oil in fennel) as well as enhanced the antioxidant activity (9.23 μmol TEAC/g oil in anise and 5.04 μmol TEAC/g oil in fennel) and the oxidative stability (8.23 h in anise and 10.15 h in fennel). Additionally, this bio-based MeTHF solvent derived from a renewable source has lower toxicity allowing it to be selected as a potential alternative of conventional solvents to practice in our further experimental studies and in pharmaceutical chemical processes.
References
Abert Vian, M., Dejoye Tanzi, C., & Chemat, F. (2013). Techniques conventionelles et innovantes, et solvants alternatifs pour l’extraction des lipides de micro-organismes. Oilseeds fats Crop. Lipids, 20, 1–6.
Akinoso, R., & Adeyanju, J. A. (2012). Optimization of edible oil extraction from ofada rice bran using response surface methodology. Food and Bioprocess Technology, 5(4), 1372–1378.
Anonymos (1997). Determination of the composition of the sterol fraction of animal and vegetable oils and fats by TLC and capillary GLC. AOCS, Official Method Ch 6–91.
Ben Khedir, S., Bardaa, S., Chabchoub, N., Moalla, D., Sahnoun, Z., & Rebai, T. (2017). The healing effect of Pistacia lentiscus fruit oil on laser burn. Pharmaceutical Biology, 55(1), 1407–1414.
Bernardo-Gil, M. G., Grenha, J., Santos, J., & Cardoso, P. (2002). Supercritical fluid extraction and characterization of oil from hazelnut. European Journal of Lipid Science and Technology, 104(7), 402–409.
Bettaieb Rebey, I., Rahali, F. Z., Saidani Tounsi, M., Marzouk, B., & Ksouri, R. (2016). Variation in fatty acid and essential oil composition of sweet fennel (Foeniculum vulgare Mill) seeds as affected by salinity. Journal of New Sciences, Agriculture and Biotechnology, IABC, 6, 1233–1240.
Bettaieb Rebey, I., Bourgou, S., Saidani Tounsi, M., Fauconnier, M. L., & Ksouri, R. (2018). Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosystems, 152(5), 971–978.
Boutin, O., & Badens, E. (2009). Extraction from oleaginous seeds using supercritical CO2: experimental design and products quality. Journal of Food Engineering, 92(4), 396–402.
Breil, C., Meullemiestre, A., Vian, M., & Chemat, F. (2016). Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules, 21, E196.
Cecchi, G., Biasini, S., & Castano, J. (1985). Méthanolyse rapide des huiles en solvant. Note de laboratoire. Revue Française des Corps Gras, 4, 163–164.
Chemat, F., Rombaut, N., Sicaire, A. G., Meullemiestre, A., Fabiano-Tixier, A. S., & Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540–560.
Cheung, P. C. K., Leung, A. Y. H., & Ang, P. O. (1998). Comparison of supercritical carbon dioxide and Soxhlet extraction of brown seaweed Sargassum hemiphyllum (turn.). Journal of Agricultural and Food Chemistry, 46(10), 4228–4232.
Danh, L. T., Han, L. N., Triet, N. D. A., Zhao, J., Mammucari, R., & Foster, N. (2013). Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food and Bioprocess Technology, 6(12), 3481–3489.
Dong, S., Zhang, R., Ji, Y. C., Hao, J. Y., Ma, W. W., Chen, X. D., & Yu, H. L. (2016). Soy milk powder supplemented with phytosterol esters reduced serum cholesterol level in hypercholesterolemia independently of lipoprotein E genotype: a random clinical placebo-controlled trial. Nutrition Research, 36(8), 879–884.
Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Holser, R. (2003). Seed conditioning and meadow foam press oil quality. Industrial Crops and Products, 17(1), 23–26.
Kiralan, M., Bayrak, A., & Mucahit, T. O. (2009). Oxidation stability of virgin olive oils from some important cultivars in East Mediterranean area in Turkey. Journal of the American Oil Chemists' Society, 86(3), 247–252.
Konuskan, D. B., Kamiloglu, O., & Demirkeser, Ö. (2019). Fatty acid composition, total phenolic content and antioxidant activity of grape seed oils obtained by cold- pressed and solvent extraction. Indian Journal of Pharmaceutical Education and Research, 53(1), 144–150.
Koubaa, M., Roselló-Soto, E., Šic Žlabur, J., Režek Jambrak, A., Brnčić, M., Grimi, N., Boussetta, N., & Barba, F. J. (2015). Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. Journal of Agricultural and Food Chemistry, 63(31), 6835–6846.
Koubaa, M., Mhemdi, H., Barba, F. J., Roohinejad, S., Greiner, R., & Vorobiev, E. (2016). Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: an overview. Food Research International, 85, 59–66.
Koubaa, M., Mhemdi, H., Barba, F. J., Angelotti, A., Bouaziz, F., Chaabouni, S. E., & Vorobiev, E. (2017). Seed oil extraction from red prickly pear using hexane and supercritical CO2: assessment of phenolic compound composition, antioxidant and antibacterial activities. Journal of the Sciences and Food Agricultural, 97(2), 613–620.
Kozłowska, M., Gruczyńska, E., Ścibisz, I., & Rudzińska, M. (2016). Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chemistry, 213, 450–456.
Kulkarni, N. G., Kar, J. R., & Singhal, R. S. (2017). Extraction of flaxseed oil: a comparative study of three-phase partitioning and supercritical carbon dioxide using response surface methodology. Food and Bioprocess Technology, 10(5), 1–9.
Liu, S. X., & Mamidipally, P. K. (2005). Quality comparison of rice bran oil extracted with limonene and hexane. Cereal Chemistry, 82(2), 209–215.
Liu, G., Xu, X., Hao, Q. F., & Gao, Y. X. (2009). Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT - Food Science and Technology, 42(9), 1491–1495.
Liu, C., Han, X., Cai, L., Lu, X., Han, X. X., & Ying, T. J. (2012). Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. Journal of Integrative Agriculture, 11(1), 159–165.
Malacrida, C. R., & Jorge, N. (2012). Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Brazilian Archives of Biology and Technology, 55(1), 127–134.
Malheiro, R., Casal, S., Teixeira, H., Bento, A., & Pereira, J. A. (2013). Effect of olive leaves addition during the extraction process of overmature fruits on olive oil quality. Food and Bioprocess Technology, 6(2), 509–521.
Mariod, A. A., Matthaȕs, B., & Ismail, M. (2011). Comparison of supercritical fluid and hexane extraction methods in extracting kenaf (Hibiscus cannabinus) seed oil lipids. Journal of the American Oil Chemists' Society, 88(7), 931–935.
Mhemdi, H., Rodier, E., Kechaou, N., & Fages, J. (2011). A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. Journal of Food Engineering, 105(4), 609–616.
Miguel, M. G., Nunes, S., Dandlen, S. A., Cavaco, A. M., & Antunes, M. D. (2010). Phenols and antioxidant activity of hydro-alcoholic extracts of própolis from Algarve, south of Portugal. Food and Chemical Toxicology, 48(12), 3418–3423.
Misirli, H., Domac, F. M., Somay, G., Araal, O., Ozer, B., & Adiguzel, T. (2008). N-hexane induced polyneuropathy: a clinical and electrophysiological follow up. Electroencephalography and Clinical Neurophysiology, 48, 103–108.
Molero Gómez, A., & Martı́nez de la Ossa, E. (2002). Quality of borage seed oil extracted by liquid and supercritical carbon dioxide. Chemical Engineering Journal, 88(1-3), 103–109.
Montedoro, G., Servili, M., Baldioli, M., & Miniati, E. (1992). Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. Journal of Agricultural and Food Chemistry, 40(9), 1571–1576.
Moura, L. S., Carvalho, R. N., Stefanini, M. B., Ming , L. C., & Meireles, M. A. A. (2005). Supercritical fluid extraction from fennel (Foeciculum vulgarae): global yield, composition and kinetic data. Journal of Supercritical Fluids, 35(3), 212–19.
Nyam, K. L., Tan, C. P., Lai, O. M., Long, K., & Man, Y. B. C. (2011). Optimization of supercritical CO2 extraction of phytosterol-enriched oil from Kalahari melon seeds. Food and Bioprocess Technology, 4(8), 1432–1441.
Pereira, C. G., Angela, M., & Meireles, A. (2010). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340–372.
Pereira, M. G., Hamerskia, F., Andradeb, E. F., Scheera, A. P., & Corazzaa, M. L. (2017). Assessment of subcritical propane, ultrasound-assisted and Soxhlet extraction of oil from sweet passion fruit (Passiflora alata Curtis) seeds. The Journal of Supercritical Fluids, 128, 338–348.
Ramadan, M. F., & Moersel, J. T. (2006). Screening of the radical action of vegetable oils. Journal of Food Composition and Analysis, 19(8), 838–842.
Ramadan, M. F., Asker, M. M. S., & Tadros, M. (2012). Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. European Food Research and Technology, 234(5), 833–844.
Ribas, S. A., Sichieri, R., Moreira, A. S. B., Souza, D. O., Cabral, C. T. F., Gianinni, D. T., & Cunha, D. B. (2017). Phytosterol-enriched milk lowers LDL-cholesterol levels in Brazilian children and adolescents: double-blind, cross-over trial. Nutrition, Metabolism, and Cardiovascular Diseases, 27, 971–977.
Salgın, U., Salgın, S., Din, D., Ekici, D. D., & Uludag, G. (2016). Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. Journal of Supercritical Fluids, 118, 194–202.
Sayed Ahmad, B., Talou, T., Saad, Z., Hijazi, A., Cerny, M., Kanaan, H., Chokr, A., & Merah, O. (2018). Fennel oil and by-products seed characterization and their potential applications. Industrial Crops and Products, 111, 92–98.
Shokri, A., Hatami, T., & Khamforoush, M. (2011). Near critical carbon dioxide extraction of anise (Pimpinella Anisum L.) seed: Mathematical and artificial neural network modeling. Journal of Supercritical Fluids, 58(1), 49–57.
Sicaire, A. G., Vian, M. A., Fine, F., Carré, P., Tostain, S., & Chemat, F. (2015). Experimental approach versus COSMO-RS assisted solvent screening for predicting the solubility of rapeseed oil. Oilseeds & fats Crops and Lipids, 22, 1–7.
Simándi, B., Deák, A., Rónyai, E., Yanxiang, G., Veress, T., Lemberkovics, E., Then, M., Sass-Kiss, A., & Vámos-Falusi, Z. (1999). Supercritical carbon dioxide extraction and fractionation of fennel oil. Journal of Agricultural and Food Chemistry, 47(4), 1635–1640.
Sovilj, M. N. (2010). Critical review of supercritical carbon dioxide extraction of selected oil seeds. Acta Periodica technologica, 41, 1–203.
Stupp, T., Freitas, R. A., Sierakowski, M. R., Deschamps, F. C., Wisniewski, A., & Biavatti, M. W. (2008). Characterization and potential uses of Copaifera langsdorfii seeds and seed oil. Bioresource Technology, 99(7), 2659–2663.
Tabee, E., Azadmard-Damirchi, S., Jägerstad, M., & Dutta, P. C. (2008). Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. Journal of Food Composition and Analysis, 21(2), 169–177.
Tasioula-Margari, M., & Tsabolatidou, E. (2015). Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants, 4(3), 548–562.
Virot, M. V., Tomao, C., Ginies, F., Visinoni, & Chemat, F. (2008). Microwave-integrated extraction of total fats and oils. Journal of Chromatography A, 1196, 147–152.
Funding
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR15CBBC06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bettaieb Rebey, I., Bourgou, S., Detry, P. et al. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol 12, 1798–1807 (2019). https://doi.org/10.1007/s11947-019-02341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02341-8