Abstract
The harvest period is one of the most important factors influencing olive oil quality. This period is extended for several months and the late-extracted olive oils are characterized by quality loss and reduced resistance to oxidation. The aim of this work was to verify the effect of olive leaves addition during the oil extraction process in the olive oils quality and composition. In two consecutive years (2009 and 2010), different olive leaves amounts (1%, 2.5%, 5% and 10% w/w) were added during the extraction process of cv. Cobrançosa olive fruits, collected in the late season. Standard quality parameters, oxidative stability, fatty acids profile, tocopherols, chlorophylls, and carotenoids contents were evaluated. Olive leaves addition induces a slight increase in acidity, peroxide value, K232, and K270 without compromising olive oils classification, but the resistance to oxidation was significantly improved. Vitamin E increased nearly 30% with 10% of leaves added mainly due to the considerable increase in α-tocopherol. A similar effect was observed in the contents of chlorophylls (chlorophyll a and pheophytin a) and carotenoids (lutein and β-carotene), that attributed a more intense greener pigmentation and enhanced nutritional attributes. Significant correlations were observed for several parameters with the amounts of leaves added. Moreover, leaves addition modified the characteristics and composition of the olive oils in a way that was possible to discriminate and to classify each group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the last statistics published by the International Olive Council, the olive oil extraction predicted worldwide for the 2010/2011 crop season achieved almost 3 million tons (IOC 2010). The main production areas are concentrated in the Mediterranean basin countries which compress nearly 95% of the olive oil world production (IOC 2010).
Harvest time is a critical aspect for obtaining high-quality olive oils. Along the olive fruit maturation process, the chemical characteristics of the oil undergo important modifications (Matos et al. 2007). In the majority of the olive-producing regions, the harvest period is extended for several months usually beginning in the end of October and being extended in some cases up to February. Several factors contribute for the delay of olives harvest. In some regions, social aspects are crucial (like workers shortage, aged farmers, and their lack of knowledge). However, in the majority of the producing regions, the extraction capacity of the olive mills is the core problem, being not enough to process all the fruits at the optimum harvest time (García et al. 1996a).
Olive oils processed in the beginning of the season, with olives in the physiological ripening stage, are usually of superior quality, with low free acidity, peroxide value and specific extinction coefficients at 232 and 270 nm (K232 and K270). Those oils have also very good sensorial attributes that allow their classification as extra virgin olive oils (EVOO; Rotondi et al. 2004). Olive oils extracted from fruits with high maturation index, usually from the mid to the end of the crop season, despite being classified also as EVOO in some cases, present lower resistance to oxidation and loss of sensorial attributes with evident chemical composition changes (García et al. 1996b; Rotondi et al. 2004). Olive oils extracted later in the season and proceeding from olives with overmaturation index are normally characterized by reduced conservation ability and lower quality comparatively with those obtained at the proper harvest time (García et al. 1996b).
Olive leaves are one of the olive oil extraction by-products and can reach 10% of the total weight of processed olives (Bouaziz et al. 2008). In most cases, those leaves represent a problem for the industrials, which have a great quantity of low-economic value raw material without adequate destination. Recent studies however, have highlighted olive leaves high-added value, being an excellent source of compounds with biologic properties (Briante et al. 2002; Ferreira et al. 2007; Pereira et al. 2007; Korukluoglu et al. 2010; Sudjana et al. 2009), particularly phenolic compounds (Briante et al. 2002; Meirinhos et al. 2005), exhibiting a strong protective effect against oils oxidation (Gutiérrez et al. 2001; Paiva-Martins et al. 2007).
In this context, we intend to study if the addition of olive leaves during the extraction process of olive oil introduces a benefic effect in the chemical composition and quality of olive oils. Therefore, during two consecutive crop seasons, 2009/2010 and 2010/2011, and by using one widespread olive cultivar (cv. Cobrançosa), different lots with overmature olives and with increasing quantities of olive leaves (0%, 1%, 2.5%, 5%, and 10% w/w) of the same cultivar were processed. The extracted olive oils were characterized for several quality parameters, oxidative stability (through Rancimat method), fatty acids composition (gas chromatography/flame ionization detector (FID), tocopherols and tocotrienols composition (high-performance liquid chromatography (HPLC) with fluorescence detection), and chlorophylls and carotenoids content (HPLC/diode-array detection (DAD)).
Material and Methods
Olive Leaves and Fruits Sampling
The study was conducted in two consecutive seasons (2009/2010 and 2010/2011). Fruits and leaves from cv. Cobrançosa were collected in the first week of January of 2010 (crop season of 2009/10) and in the last week of December 2010 (crop season of 2010/2011) in an olive grove located in Vila Flor (Trás-os-Montes region, northeast of Portugal). Fruits with the index of maturation 6 or 7 were handpicked from around the whole perimeter of each tree at the operator height and put in plastic containers. The index of maturation was evaluated according to Hermoso et al. (1991). A sample of 100 fruits were recovered and separated based on epidermis and pulp color that varies between 0 and 7. Therefore, the fruit is classified as 0 if the epidermis is green, 1 if the epidermis is yellowish green, 2 if the epidermis shows red spots in less than half of the fruit, 3 if the epidermis is red or purple in more than half of the fruit, 4 if the epidermis is black and pulp is white, 5 if the epidermis is black and less than half od the pulp is purple, 6 if the epidermis is black and more than half of the pulp is purple (without reaching the stone), and 7 if the epidermis is black and the total pulp is purple (reaching the stone). The index of maturation was calculated as follows: \( {\text{index}}\,{\text{of}}\,{\text{maturation}} = \left( {a \times 0 + b \times {1} + c \times {2} + d \times {3} + e \times {4} + f \times {5} + g \times {6} + h \times {7}} \right)/{1}00 \), where, the letters are the number of fruits in each class. Leaves were also picked from the same trees. Six independent lots of 10 kg each were collected. In each lot, subsamples of 2 kg were separated and different quantities of leaves were added (w/w) before extraction, namely 0% (control samples), and 1%, 2.5%, 5%, and 10%.
Olive Oil Extraction and Olive Oil Samples Preparation
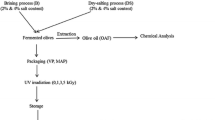
The extraction of the olive oils was conducted within the first 24 h after harvest. An Abencor analyzer (Comercial Abengoa S.A., Seville, Spain) was used to process the olives in a pilot extraction plant. The unit consists of three essential elements: the mill, the thermobeater, and the pulp centrifuge. The oil was separated by decanting, transferred into dark glass bottles and stored in the dark at 4 °C. Before the analytical procedures, the samples were dehydrated with anhydrous sodium sulfate and subsequently filtered through Whatman no. 4 paper.
Physical and Quality Parameters Evaluated
The free acidity, peroxide value (PV), specific extinction coefficients at 232 and 270 nm (K232 and K270), and fatty acid composition were determined according to official methods described in EEC Reg. 2568/91. The other parameters were either based on international standards or literature reports as briefly described below.
Free Acidity
The free acidity, expressed as free oleic acid percentage, was determined by titration of an accurate sample solution, dissolved in ethanol/ether (1:1, v/v), with 0.1 M sodium hydroxide solution, using phenolphthalein as indicator.
Peroxide Value
The PV, expressed in miliequivalents of active oxygen per kilogram (mEq O2/kg), was determined as follows: a mixture of oil and chloroform/acetic acid 2:3 (v/v) was left to react in the dark with saturated potassium iodine solution and the free iodine titrated with a sodium thiosulfate standard solution.
K232 and K270 Extinction Coefficients
The extinction coefficients K232 and K270 (absorption of 1% solution (m/v) in isooctane at 232 and 270 nm, respectively, with 1 cm of path length) were measured using a UV spectrophotometer (Genesys 10UV).
Oxidative Stability (Rancimat)
The oxidative stability was estimated by measuring the oxidation induction time on a Rancimat 743 apparatus (Metrohm CH, Switzerland). Air (20 L/h) was bubbled through the oil (3.0 g) heated at 120 ± 1.6 °C, with the volatile compounds being collected in water, and the increasing water conductivity continuously measured. The time taken to reach the conductivity inflection was recorded.
Fatty Acids Composition
Fatty acids were evaluated as their methyl esters after cold alkaline transesterification with methanolic potassium hydroxide solution (EEC Reg. 2568/91) and extraction with n-heptane. The fatty acid profile was determined with a Chrompack CP 9001 chromatograph equipped with a split-splitless injector, an FID detector, an autosampler Chrompack CP-9050 and a 50 m × 0.25 mm i.d. fused silica capillary column coated with a 0.19 μ film of CP-Sil 88 (Varian). Helium was used as carrier gas at an internal pressure of 110 kPa. The temperatures of the detector and injector were 250 °C and 230 °C, respectively. The split ratio was 1:50 and the injected volume was of 1 μL. The results are expressed in relative percentage of each fatty acid calculated by internal normalization of the chromatographic peak area eluting between myristic and lignoceric methyl esters. A control sample (olive oil 47118, Supelco) and a fatty acids methyl esters standard mixture (Supelco 37 FAME Mix) was used for identification and calibration purposes (Sigma, Spain).
Tocopherols and Tocotrienols Composition
Tocols were evaluated following the international standard ISO 9936 (2006), with some modifications as described by Casal et al. (2010). Tocopherols and tocotrienols standards (α, β, γ, and δ) were purchase from Calbiochem (La Jolla, San Diego, CA, USA) and Sigma (Spain), while the internal standard 2-methyl-2-(4,8,12-trimethyltridecyl)chroman-6-ol (tocol) was from Matreya Inc. (Pleasant Gap, PA, USA). A 50-mg amount of filtered olive oil was blended with an appropriate amount of internal standard solution (tocol) in a 1.5-mL volume of n-hexane and homogenized by stirring. Sample preparation was conducted in dark and tubes containing the samples were always wrapped in aluminum foil. The mixture was centrifuged for 5 min at 13,000×g and the supernatant analyzed by HPLC. The liquid chromatograph consisted of a Jasco integrated system (Japan) equipped with a Jasco LC–NetII/ADC data unit, a PU-1580 Intelligent Pump, a LG-1580-04 Quaternary Gradient Unit, a DG-1580-54 Four Line Degasser, and an FP-920 fluorescence detector (λexc = 290 nm and λem = 330 nm). The chromatographic separation was achieved on a Supelcosil ™ LC-SI column (3 μm) 75 × 3.0 mm (Supelco, Bellefonte, PA, USA), operating at constant room temperature (23 °C). A mixture of n-hexane and 1,4-dioxane (97.5:2.5) was used as eluent at a flow rate of 0.7 mL/min. Data were analyzed with the ChromNAV Control Center, JASCO Chromatography Data Station (Japan). The compounds were identified by chromatographic comparisons with authentic standards, by coelution and by their UV spectra. Quantification was based on the internal standard method using the fluorescence signal response.
Chlorophylls and Carotenoids Composition
The extracts were prepared in accordance with Achir et al. (2010). Briefly, olive oil samples from the 2010 sampling only (200 mg) were added with an appropriate amount of internal standard β-apo-carotenal (Sigma-Aldrich), mixed with 2 mL acetone, vortexed for 10 s, and left overnight at −20 °C for triacylglycerol crystallization. Triacylglycerols were separated by rapid sampling followed by centrifugation at 13,000 rpm. The extract was directly injected into the HPLC column, a Phenomenex Luna C18 (250 × 3.5 mm internal diameter) at 23 °C and eluted with a 30-min linear gradient from 80% aqueous methanol (v/v) containing 0.05% triethylamine and 20% ethyl acetate (containing 0.05% triethylamine) at 1 mL/min. The analysis was performed on the same HPLC equipment described for the tocols, except for the use of a DAD detector (JASCO MD-2015-Plus, Japan). Lutein, β-carotene, and chlorophyll a were obtained from Sigma. Pheophytin a was prepared from acidified chlorophyll a solution and calibrated spectrophotometrically at 406 nm using an E1% 1 cm of 1,290 in acetone. Calibration curves were constructed at 440 nm for lutein and β-carotene and at 412 nm for chlorophyll a and pheophytin a.
Statistical Analysis
Principal Component Analysis
Principal components analysis (PCA) was applied for reducing the number of variables in the 2009 samples (six variables corresponding to the quality parameters—free acidity, peroxide value, K232, K270, ΔK, and Rancimat oxidative stability; five variables corresponding to tocopherols and tocotrienols content; and 24 variables corresponding to the fatty acids profile; with a total 35 variables) to a smaller number of new derived variables (principal component or factors) that adequately summarize the original information, i.e., the effect of olive leaves added during the extraction process of cv. Cobrançosa olive oil in 2009. Moreover, it allowed recognizing patterns in the data by plotting them in a multidimensional space, using the new derived variables as dimensions (factor scores). PCA was performed by using SPSS software, version 19.0 (IBM Corporation, New York, USA).
Linear Discriminant Analysis
A linear discriminant analysis (LDA) was used as a supervised learning technique to classify the cv. Cobrançosa olive oils extracted in 2009 with different percentages of olive leaves according to their quality parameters, tocols content, and fatty acids profile. A stepwise technique, using the Wilk’s lambda method with the usual probabilities of F (3.84 to enter and 2.71 to remove), was applied for variable selection. This procedure uses a combination of forward selection and backward elimination procedures, where before selecting a new variable to be included, it is verified whether all variables previously selected remain significant (Rencher 1995; Maroco 2003; López et al. 2008). With this approach, it is possible to identify the significant variables among all variables in study. To verify which canonical discriminant functions were significant, the Wilks’ lambda test was applied. To avoid overoptimistic data modulation, a leaving-one-out cross-validation procedure was carried out to assess the model performance. Moreover, the sensibility and specificity of the discriminant model were computed from the number of individuals correctly predicted as belonging to an assigned group (Rencher 1995; López et al. 2008). Sensibility was calculated by dividing the number of samples of a specific group correctly classified by the total number of samples belonging to that specific group. Specificity was calculated by dividing the number of samples of a specific group classified as belonging to that group by the total number of samples of any group classified as belonging to that specific group. The LDA was performed by using the SPSS software, version 19.0 (IBM Corporation, New York, USA).
Analysis of Variance
An analysis of variance (ANOVA) with type III sums of squares was performed using the general linear model procedure of the SPSS software, version 19.0 (IBM Corporation, New York, USA). The fulfillment of the ANOVA requirements, namely the normal distribution of the residuals and the homogeneity of variance were evaluated by means of the Kolmogorov–Smirnov with Lilliefors correction (if n > 50) or the Shapiro–Wilk’s test (if n < 50) and the Levene’s tests, respectively. All dependent variables were analyzed using a one-way ANOVA with or without Welch correction, depending if the requirement of the homogeneity of variances was fulfilled or not. The main factor studied was the effect of the percentage of olive leaves added during the extraction of cv. Cobrançosa olive oils during two consecutive years on the quality parameters (free acidity, peroxide value, K232, K270, ΔK and Rancimat oxidative stability), fatty acids profile, tocols, and chlorophylls and carotenoids contents. If a statistical significant effect was found, means were compared using Tukey’s honestly significant difference multiple comparison test or Dunnett’s T3 test also depending if equal variances could be assumed or not. All statistical tests were performed at a 5% significance level.
Regression Analysis
A regression analysis, using Excel from Microsoft Corporation, was established between the percentage of olive leaves added and the quality parameters (free acidity, peroxide value, K232, K270, ΔK, and Rancimat oxidative stability) fatty acids profile, tocopherols and tocotrienols, and chlorophylls and carotenoids content of the olive oils obtained in 2009 and 2010.
Results and Discussions
Quality Parameters
Free Acidity
The results obtained in the free acidity of cv. Cobrançosa olive oils, from 2009 and 2010, extracted with different percentages of olive leaves are presented in Table 1.
In the olive oils from 2009, a smooth increase in the acidity value along with the increment of leaves was observed varying the values between 0.28% and 0.45%. Concerning the olive oils extracted in 2010, the variation was even smaller (0.37% and 0.47%) and the addition of olive leaves during the extraction of olive oil did not change significantly the free acidity in these samples (P = 0.851). Comparative to the results obtained by Casal et al. (2010) and Pereira et al. (2002) for the same cultivar, our free acidity values are higher. This observation should be related with the use of olives with an advanced stage of maturation which consequently implies higher acidity values by an increase in the lipolytic enzymatic activity (Martinéz-Suárez 1973). The addition of olive leaves may also increase the presence of those lipolytic enzymes which consequently entails an increase in the free acidity, explaining the observed increments in this parameter. Furthermore, olives standing longer in the olive tree become more sensitive to the olive pests attack and mechanical damages, suffering wounds from where microorganisms may enter causing infections and consequently also higher enzymatic activity (Salvador et al. 2001). Despite the discussed aspects, all the samples reported a free acidity lower than 0.8%, which means that regarding this parameter, and accordingly to the EC Reg. 1989/2003, the olive oils could still be classified as EVOO.
Peroxide Value
The PV determination is of major importance once that it constitutes an indicator of the extension of primary oxidation of lipids, allowing us to measure the oxidative rancidity. Together with free acidity and the specific coefficients of extinction (K232 and K270), PV is one of the most frequently evaluated quality parameters during olive oil production, storage, and marketing being considered a guide of the olive oil quality (Nouros et al. 1999).
For both years, the PV of cv. Cobrançosa olive oils extracted with different percentages of olive leaves are reported in Table 1. Comparing the results from the olive oils from 2009 to 2010, it is noticeable that the samples without leaves (control) were the ones that reported lower peroxide values (7 ± 1 and 6 ± 0 mEq O2/kg, respectively, in 2009 and 2010). In the olive oils from 2009, an up and down in the results was observed with higher peroxide values (12 mEq O2/kg) in the olive oils with 1% and 5% of added leaves. In the olive oils from 2010, an increasing tendency was observed with leaves addition (Table 1). Higher values were detected in the olive oils with 10% of leaves, nearly 33% above the control. In both years, olive leaves addition influenced significantly the peroxide values (P < 0.001).
As observed in all olive oils, leaves addition increases PV. Such fact could be related with the presence of leaves which favor gas changes related with the respiration process that could increase the availability of oxygen or other related species that favor the peroxidation process. Nevertheless, and despite being prepared with over-ripe olives, our PV are lower than those obtained with olive oils from the same cultivar (Pereira et al. 2004; Casal et al. 2010) attesting the quality of the olive oil samples in the present study. Also, and as observed for free acidity, the PV remain far below the legal maximum limits established (EC 1989/2003) for extra virgin olive oils (20 mEq O2/kg of oil).
Specific Extinction Coefficients K232 and K270
UV spectrophotometrical analysis can provide a series of information about oil quality, its conservation status and possible changes occurred during the technological process. Over 90% of hydroperoxides formed by lipoperoxidation have a conjugated dienic system resulting from stabilization of the radical state by double-bound rearrangement. These compounds absorb in the UV range (235 nm) forming a shoulder on the main absorption peak of nonconjugated double bounds (200–210 nm) (Laguerre et al. 2007). Several secondary oxidation products, as well as triene-conjugated double bounds, absorb in the 270 nm region, and their presence is usually indicative of extensive oxidation. For olive oil characterization, the absorption at 232 nm and in the 270 nm region are of special importance, giving information on the oxidative status and enabling to distinguish virgin olive oils categories (EEC 1991; EC 2003).
In the 2009 crop season, lower K232 and K270 values were observed in control oils than in oils extracted with leaves (Table 1). A slight increase was observed for oils with 1%, 2.5%, and 5% of leaves added. However, a significant increase was observed in the olive oils with 10% of leaves for both parameters (P < 0.001). In 2010, a similar tendency was observed for K270 (Table 1) but the K232, despite being higher, was quite invariable to the leaves addition.
The changes observed in the coefficients of extinction values could indicate a slight increase in the oxidation with leaves addition in accordance with the observations made for the PV. Respective to the ΔK parameter, the values are below the maximum admissible value (≤0.01) and in all samples from both years, the same behavior was observed with the values decreasing with the amount of leaves added.
The increase of the K232 values in both years, mainly in samples with high content of olive leaves denote a slight deterioration of olive oils due to the formation of primary oxidation products as already witnessed by the peroxide value determination. Special attention to this point should be accounted once that the use of high quantity of leaves can declassify olive oil category (EC 1989/2003). However, the formation of secondary products of oxidation was even in less extent than the former ones as observed in the results obtained in the specific coefficient of extinction K270.
Oxidative Stability
Oxidative stability is a very important parameter once it gives a good perception and estimation of the susceptibility to oxidation process. In the 2009 olive oils, the oxidative stability increased with the amount of olive leaves added during the extraction process (Table 1). A significant increase (P < 0.001) was observed comparatively to control oils (between 44% and 74%). However, those values must be seen only as a tendency, once that the determination of oxidative stability of the oils from the crop season of 2009 were performed only after 1 year of storage at room temperature in the dark, simultaneously with the 2010 samples. Nevertheless, this increased long-term stability with added leaves can be an interesting result that reinforces the importance of such strategy.
In 2010, all values were higher than in 2009, as expected from the previous discussion. Despite being lower in the control samples, the increased resistance with the leaves added was not so evident, highlighting that this effect could be of interests for longer storage periods (Table 1). These results are clearly associated with changes induced in the composition of olive oils due to leaves addition, namely by extraction of anti-oxidative components into the olive oils. According to Gutiérrez et al. (2001), the phenolic compounds are responsible for approximately 50% of the stability reported by olive oils. In our case, the olive leaves of cv. Cobrançosa added are rich sources of phenolic compounds as demonstrated by Pereira et al. (2007) in previous studies, contributing to the overall oxidative stability obtained (Briante et al. 2002; Pereira et al. 2007; Laguerre et al. 2009; Lee et al. 2009; Kiritsakis et al. 2010). Several authors also observed substantial improvements in the oxidative stability of several edible vegetable oils by enriching or adding different extracts of olive leaves (Farag et al. 2003; Salta et al. 2007; Bouaziz et al. 2008) proving that the natural antioxidant display an important role in their stability. As far as we know, this is the first time that the improvement on oxidative stability of olive oils extracted with olive leaves and not from olive oils with olive leaves extracts added is reported.
Fatty Acid Profile
The fatty acids composition of the olive oils extracted with different percentages of olive leaves was analyzed and the respective profiles are given in Table 2. The fatty acids profiles obtained in all samples from both years are in accordance with those regulated for olive oil (EC 1989/2003). As expected, the most abundant fatty acid in all samples was the oleic acid (C18:1), followed by palmitic acid (C16:0) and linoleic acid (C18:2) independently of the percentage of olive leaves added. In 2009, the content of the three main fatty acids did not follow any tendency with the increasing quantity of olive leaves added. However in 2010, a considerable decrease in the oleic acid amounts was observed with 10% of leaves (from 75.0% to 71.7%; P < 0.001). Palmitic acid increased nearly 1% on its content from 0% to 10% of leaves (P < 0.001). The major increase was noticed in the linoleic acid that rises from 7.0% in the olive oil without leaves to 10.0% with 10% of leaves (P < 0.001).
The results observed influenced the content of the major fractions that compose the fat of the olive oil. SFA varied from 15.3% to 16.0% (samples with 1% and 5% of leaves, respectively) in 2009 and from 16.6% to 17.0% (samples with 1% and 10% of leaves, respectively) in 2010 mainly due to the contributions of palmitic and stearic fatty acids. Mono-unsaturated fatty acid (MUFA) showed the same tendency as oleic acid in both years once that this fatty acid is the major responsible for MUFA content. In 2010, the MUFA content decreased considerably comparatively to 2009 olive oils (P < 0.001, Table 2). Contrarily to the MUFA, PUFA showed a considerable increase in the olive oils extracted in 2010 (P < 0.001) after being observed with a smooth increase in 2009 mainly due to the content of linoleic acid.
The cv. Cobrançosa olive oils present a high oleic acid content, high oleic/palmitic acid (from 6.8 to 7.2), and high MUFA/SFA (between 4.5 and 4.8) ratios, altogether important factors indicating that moderate consumption of this olive oils associated to the Mediterranean diet can reduce the risk for cardiovascular diseases when part of a healthy lifestyle (Hooper et al. 2002). However, the MUFA/PUFA ratio in 2010 varied from 10.6 in the samples without leaves to 7.2 in the samples with 10% of leaves, reflecting by one hand the linolenic acid increase, but decreasing the oxidative stability by the other, once that the reaction of oxygen with polyunsaturated fatty acids is the major cause of deterioration of lipids or lipid-containing foods leading to losses in quality and nutritional value and to the development of off flavors and hazard compounds (Malheiro et al. 2011a). This situation could be, in part, responsible for the observed results in the oxidative stability of the olive oils extracted in 2010, once that the samples with 5% and 10% were those with higher PUFA content, which may lead to a decrease in the oxidative stability comparatively with the samples with 1% and 2.5% of leaves (Table 1).
Tocopherols and Tocotrienols Content
Tocols are naturally present in oils and play an important part in their resistance to oxidation processes. Table 3 described the changes on the tocopherols and tocotrienols contents in olive oils extracted with olive leaves from the two consecutive years. Three tocopherols (α-, β-, and γ-tocopherol) and one tocotrienol (α-tocotrienol) were identified and quantified, α-tocopherol being the most abundant in all olive oils studied. In 2009, the content of α-tocopherol was not significantly different between the olive oils without leaves and those up to 5% of leaves added (Table 3). However, statistical differences (P < 0.001) were observed when olive oils were extracted with 10% of leaves, raising the content of α-tocopherol by about 13% (30 mg/kg) in comparison with the control. In the 2010 samples, significant increased in α-tocopherol contents (P < 0.001) were also observed in the samples with 5% and 10% of leaves (275.7 and 313.3 mg/kg, respectively). Overall, the 2010 samples were also characterized by increased α-tocopherol contents than the 2009 ones (in the order of 27% between controls), in agreement with the reduced PV and increased ROS.
α-Tocotrienol and β-tocopherol amounts were reduced but significantly higher in the 2010 samples (P < 0.001). No consistent variations were observed within each year with leaves addition (Table 3). γ-Tocopherol apparently increased with leave addition, with this observation being of particular significance in the 2009 samples (P < 0.001), although no differences were observed between the different amounts of leaves added. As regards to the 2010 samples, reduced differences were observed between the control and the 1% and 2.5% samples. The olive oils extracted with 5% and 10% of leaves reported significant differences (P < 0.001) from the remaining samples and from each other (4.0 and 5.1 mg/kg, respectively).
Total vitamin E content, taking all tocols together, raised in the samples with leaves on both years with a major increase being observed in the samples extracted in 2010 (15% in 2009 against 30% in 2010). This result is related mainly with the α-tocopherol content. In fact, some authors (Lucas et al. 2002) considered olive leaves as an alternative source of α-tocopherol. In the present work, during the extraction process of the olive oil in samples with olive leaves, α-tocopherol extraction could occur. Besides being an important aspect due to its natural antioxidant activity, from a nutritional point of view this increase allows a greater availability of vitamin E, increasing olive oil health benefits and prevention of deficiency symptoms (Morrissey and Sheehy 1999).
Chlorophylls and Carotenoids Content
Chlorophyll and carotenoid compounds play important roles in olive oils. They interfere in the oxidative stability acting as antioxidants in the dark and acting as prooxidants when exposed to light (Gutierrez et al. 1992). Besides that, these compounds are responsible for the yellow green pigmentation of olive oils, increasing consumer’s acceptability.
Chlorophylls and carotenoids determination was only possible to perform in the olive oils extracted in 2010, and their contents are reported in Table 4. Four pigments were identified and quantified, namely lutein, β-carotene, chlorophyll a, and pheophytin a. Chlorophyll content of the olive oils extracted did not differ significantly (P = 0.317) with the addition of leaves, while significant differences were observed in the remaining pigments (P < 0.001), mainly pheophytin a. This pigment nearly doubles its content when 1% of leaves was added (from 5.8 to 10.4 mg/kg) and almost triplicate with the addition of 10% of leaves (15.7 mg/kg). This major increase is probably due to the increased extracted chlorophyll a from the leaves that naturally leads to the formation of derivate products, particularly pheophytin a (Choe and Min 2006). The increase in the addition of olive leaves also turned the olive oils greener, this visual observation being clearer in the olive oils with 5% and 10% of leaves, and probably associated to the increased pheophytin a content. Aware that these compounds act as prooxidants in the light, particularly pheophythin, these olive oils should be preserved in the dark and in adequate bottles. The increased lutein and β-carotene contents are also interesting from a nutritional point of view due to their pro-vitamin A activity.
Correlations between the Parameters Studied and the Leaves Addition
A regression analysis was performed between the percentage of olive leaves added and the quality parameters (free acidity, peroxide value, K232, K270, ΔK, and Rancimat oxidative stability), fatty acids profile, tocopherols and tocotrienols, chlorophylls, and carotenoids contents in the olive oils obtained in both years (Table 5). Concerning the quality parameters, it was observed that the olive leaves addition during the extraction of olive oil was extremely correlated with the results obtained in all the parameters evaluated in 2009, except with the peroxide value (R 2 = 0.055, P = 0.079). In 2010, the leaves addition affected the peroxide value and K270, but the free acidity and the oxidative stability values were not correlated with the amount of leaves (R 2 = 0.036 and P = 0.497; R 2 = 0.015 and P = 0.611, respectively).
Concerning the fatty acids profile (Table 2), in both years linoleic acid (C18:2) (R 2 = 0.208 and P ≤ 0.001; R 2 = 0.906 and P ≤ 0.001, respectively in 2009 and 2010) and PUFA (R 2 = 0.330 and P ≤ 0.001; R 2 = 0.889 and P ≤ 0.001) contents were extremely positively correlated with the percentage of leaves added. Oleic acid (C18:1) content showed to be correlated with the amount of leaves added during the extraction in 2009 (R 2 = 0.121 and P = 0.013) and extremely correlated in 2010 (R 2 = 0.887 and P ≤ 0.001). Palmitic (C16:0) and stearic (C18:0) fatty acids contents were not correlated with the amount of leaves added in 2009 but were strongly correlated in the following year.
The vitamin E content and of its vitamers were extremely correlated in both years, except for β-tocopherol that was only significantly correlated with the amount of olive leaves added during the extraction of olive oils in 2009 (Table 5). The addition of leaves also showed an extreme significant correlation with the values of the pigments identified (lutein, β-carotene, and pheophytin a) in the olive oils from 2010 except for chlorophyll with only significant correlation (R 2 = 0.395 and P = 0.012). These data shows that the addition of leaves during the extraction of olive oil is correlated with the majority of the parameters tested and influences the composition of the olive oils in a concentration dependent manner.
Discrimination and Classification of cv. Cobrançosa Olive Oils
With the data obtained from the olive oils extracted in 2009, two different statistical tools were applied in order to tentatively discriminate and classify the different olive oils. First, a PCA was applied to discriminate followed by a LDA to create a discriminant model capable to classify the olive oils according to their group.
The unsupervised PCA method has already demonstrated that it is capable to discriminate olive products in several cases and with a wide range of compounds and parameters studied: adulteration (Gurdeniz and Ozen 2009; Lizhi et al. 2010), geographical origin, and olive variety (Mannina et al. 2003; Oliveros et al. 2005; Di Bella et al. 2007; Malheiro et al. 2011b; c; Lin et al. 2009), olives maturation index (Matos et al. 2007). The performed PCA was applied to the quality parameters (free acidity, peroxide value, K232, K270, ΔK, and Rancimat oxidative stability), fatty acids profile, and tocopherols and tocotrienols content. Principal component analysis allowed explaining 50.8% of the total variance of the data by using two principal components. In Fig. 1, the two-dimensional representation of the two principal components factor scores obtained from the data of the olive oils extracted with olive leaves is shown. In the figure, it is observed that the five olive oils types are separated into five groups. It can also be observed that the olive oils with 10% of leaves are completely isolated from the remaining samples, indicating that its composition is clearly different from the other olive oils (Fig. 1). The first principal component factor, which comprises 33.7% of the total variance, separates mainly the samples of 0% and 1% of leaves (located in the negative region) from the remaining olive oils (placed in the positive region). This happens because the samples with 0% of leaves reported the lowest values in some quality parameters, like free acidity, oxidative stability, and K232 and K270 values. On the other hand, the samples with 1% of leaves reported higher oleic acid content and consequently also higher MUFA content. The second factor, which explains 17.1% of the total variance observed, was able to separate mainly the samples with 1% and 10% of leaves (located in the positive region) from the remaining samples. Both principal component factors allowed to isolate the samples with 10% of leaves (located in both positive regions of both principal component factors). These samples were isolated because they reported higher values in the quality parameters. Furthermore, the samples with 10% of leaves were dominant in α-tocopherol and vitamin E total content, as well as in the content of linolenic acid (C18:3).
Principal components analysis using quality parameters (free acidity, peroxide value, K232, K270, ΔK, and Rancimat oxidative stability), fatty acids profile, tocopherols, and tocotrienols contents of cv. Cobrançosa olive oils extracted with different percentages of olive leaves in 2009. The PCA factors explain 50.8% of the total variance
The use of a stepwise LDA resulted in a discriminate model with four significant discriminant functions that explained 100% of the variance, although only the first two were used, since they explain 96.4% of the variance of the experimental data (the first explaining 86.5% and the second 9.9%, Fig. 2). From the initial 35 variables, only nine were used to build the discriminate model (peroxide value, K270, ΔK, stearic and lignoceric (C18:0 and C24:0, respectively), saturated fatty acids, α-, β-, and γ-tocopherols). The built model showed a very satisfactory classification performance allowing correct classification of all the olive oils for the original groups as well as for the cross-validation procedure (sensitivities and sensibilities of 100%). The results obtained showed that the application of LDA could be a used to identify olive oils with different percentages of olive leaves added during the extraction process, also due to the changes caused by the leaves in the composition and quality parameters of the olive oils.
Conclusions
The addition of different olive leaves percentages during extraction of olive oils from overmature cv. Cobrançosa olives clearly brought changes in the chemical composition and quality of the olive oils, overall with increased oxidative resistance, nutritional qualities, and enhanced appearance. Leaves addition induces at a very low extent the occurrence of oxidation and hydrolysis with slight increase of peroxide values, K232, K270, and free acidity without compromising their classification. Preliminary sensorial evaluation indicated that the overall sensorial quality increases, and the positive attributes, like green fruity and bitter taste, increased. However, such results are merely indicative and further studies are needed to clarify this topic.
Meanwhile, the inclusion of several antioxidant compounds extracted from the leaves in the olive oil lead to a considerable increase in the oxidative stability and nutritional quality, mainly in the samples with smaller amounts of leaves added. PUFA and pheophytin increased content could be related with potential loss of oxidative stability, requiring adequate measures for their adequate preservation from light and oxygen. Overall, the olive oils with olive leaves were enriched by several compounds and constituents that improved their appearance, resistance, and composition. Despite being applied to overmature olives, as a way to improve olive oil quality, this technology could be also applied to other olive oils, with similar results expected, particularly regarding increased long-term oxidative stability and enhanced nutritional quality.
References
Achir, N., Randrianatoandro, V. A., Bohuon, P., Laffargue, A., & Avallone, S. (2010). Kinetic study of β-carotene and lutein degradation in oils during heat treatment. European Journal of Lipid Science and Technology., 112, 349–361.
Bouaziz, M., Fki, I., Jemai, H., Ayadi, M., & Sayadi, S. (2008). Effect of storage on refined and husk olive oils composition; stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chemistry, 108, 253–262.
Briante, R., Patumi, M., Terenziani, S., Bismuto, E., Febbraio, F., & Nucci, R. (2002). Olea europaea L. leaf extract and derivatives: antioxidant properties. Journal of Agricultural and Food Chemistry, 50, 4934–4940.
Casal, S., Malheiro, R., Sendas, A., Oliveira, B. P. P., & Pereira, J. A. (2010). Olive oil stability under deep-frying conditions. Food and Chemical Toxicology, 48, 2972–2979.
Choe, E., & Min, D. B. (2006). Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety, 5, 169–186.
Commission Regulation (EC) No 1989/2003 of 6 November 2003, amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis.
Commission Regulation (EEC) No 2568/91 of 11 July 1991, on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis.
Di Bella, G., Maisano, R., La Pera, L., Lo Turco, V., Salvo, F., & Dugo, G. (2007). Statistical characterization of Sicilian olive oils from the Peloritana and Maghrebian zones according to the fatty acids profile. Journal of Agricultural and Food Chemistry, 55, 6568–6574.
Farag, R. S., El-Baroty, G. S., & Basuny, A. M. (2003). The influence of phenolic extracts obtained from the olive plant (cvs. Picual and Kronakii), on the stability of sunflower oil. International Journal of Food Science and Technology, 38, 81–87.
Ferreira, I. C. F. R., Barros, L., Soares, M. E., Bastos, M. L., & Pereira, J. A. (2007). Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chemistry, 103, 188–195.
García, J. M., Gutiérrez, F., Barrera, M. J., & Albi, M. A. (1996a). Storage of mil olives on an industrial scale. Journal of Agricultural and Food Chemistry, 44, 590–593.
García, J. M., Seller, S., & Pérez-Camino, M. C. (1996b). Influence of fruit ripening on olive oil quality. Journal of Agricultural and Food Chemistry, 44, 3516–3520.
Gurdeniz, G., & Ozen, B. (2009). Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chemistry, 116, 519–525.
Gutierrez, F., Garrido, J., Gallardo, L., Gandul, B., & Minguez, M. I. (1992). Action of chlorophylls on the stability of virgin olive oil. Journal of the American Oil Chemists’ Society, 69, 866–871.
Gutiérrez, F., Arnaud, T., & Garrido, A. (2001). Contribution of polyphenols to the oxidative stability of virgin olive oil. Journal of the Science of Food and Agriculture, 81, 1463–1470.
Hermoso, M., Uceda, M., García, A., Morales, B., Frias, M.L. & Fernández, A. (1991). Elaboración de Aceite de Calidad; Consejeria de Agricultura y Pesca, Serie Apuntes 5/92; Sevilla, Spain.
Hooper, L., Bartlett, C., Smith, G. D., & Ebrahim, S. (2002). Systematic review of long-term effects of advice to reduce dietary salt in adults. British Medical Journal, 325, 628–632.
International Olive Council (2010). Available at: http://www.internationaloliveoil.org. Accessed 8 July 2011.
ISO 9936 (2006). Animal and vegetable fats and oils—determination of tocopherol and tocotrienol contents by high-performance liquid chromatography.
Kiritsakis, K., Kontominas, M. G., Kontogiorgis, C., Hadjipavlou-Litina, D., Moustakas, A., & Kiritsakis, A. (2010). Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. Journal of the American Oil Chemists’ Society, 87, 369–376.
Korukluoglu, M., Sahan, Y., Yigit, A., Ozer, E. T., & Gucer, S. (2010). Antibacterial activity and chemical constitutions of Olea europaea L. leaf extracts. Journal of Food Processing and Preservation, 34, 383–396.
Laguerre, E. P., Lecomte, J., & Villeneuve, P. (2007). Evaluation of the ability of antioxidants to counteract lipid oxidation; existing methods, new trends and challenges. Progress in Lipid Research, 46, 244–282.
Laguerre, M., Giraldo, L. J. L., Piombo, G., Figueroa-Espinoza, M. C., Pina, M., Benaissa, M., et al. (2009). Characterization of olive-leaf phenolics by ESI-MS and evaluation of their antioxidant capacities by the CAT assay. Journal of the American Oil Chemists’ Society, 86, 1215–1225.
Lee, O.-H., Lee, B.-Y., Lee, J., Lee, H.-B., Son, J.-Y., Park, C.-S., et al. (2009). Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresource Technology, 100, 6107–6113.
Lin, P., Chen, Y., & He, Y. (2009). Identification of geographical origin of olive oil using visible and near-infrared spectroscopy technique combined with chemometrics. Food and Bioprocess Technology. doi:10.1007/s11947-009-0302-z.
Lizhi, H., Toyoda, K., & Ihara, I. (2010). Discrimination of olive oil adulterated with vegetable oils using dielectric spectroscopy. Journal of Food Engineering, 96, 167–171.
López, A., García, P., & Garrido, A. (2008). Multivariate characterization of table olives according to their mineral nutrient composition. Food Chemistry, 106, 369–378.
Lucas, A., Martinez de la Ossa, E., Rincón, J., Blanco, M. A., & Garcia, I. (2002). Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. Journal of Supercritical Fluids, 22, 221–228.
Malheiro, R., Casal, S., Ramalhosa, E. & Pereira, J.A. (2011a). Microwave heating: a time saving technology or a way to induce vegetable oils oxidation? In: Grundas S (Ed.) Advances in induction and microwave heating of mineral and organic materials (pp 597–614). InTech, Rijeka, Croatia.
Malheiro, R., Sousa, A., Casal, S., Bento, A., & Pereira, J. A. (2011b). Cultivar effect on the phenolic composition and antioxidant potential of stoned green table olives. Food and Chemical Toxicology, 49, 450–457.
Malheiro, R., Casal, S., Sousa, A., Guedes de Pinho, P., Peres, A. M., Dias, L. G., et al. (2011c). Effect of cultivar on sensory characteristics, chemical composition, and nutritional value of stoned green table olives. Food and Bioprocess Technology. doi:10.1007/s11947-011-0567-x.
Mannina, L., Dugo, G., Salvo, F., Cicero, L., Ansanelli, G., Calcagni, C., et al. (2003). Study of the cultivar–composition relationship in Sicilian olive oils by GC, NMR and statistical methods. Journal of Agricultural and Food Chemistry, 51, 120–127.
Maroco, J. (2003). Análise Estatística, com utilização do SPSS. Lisboa: Edições Sílabo.
Martinéz-Suárez, J. M. (1973). Recientes estúdios de la almazara experimental del instituto de la grasa. Rivista Italiana delle Sostanze Grasse, 50, 325–330.
Matos, L. C., Cunha, S. C., Amaral, J. S., Pereira, J. A., Andrade, P. B., Seabra, R. M., et al. (2007). Chemometric characterization of three varietal olive oils (Cvs. Cobrançosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chemistry, 102, 406–414.
Meirinhos, J., Silva, B. M., Valentão, P., Seabra, R. M., Pereira, J. A., Dias, A., et al. (2005). Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Natural Product Research, 19, 189–195.
Morrissey, P. A., & Sheehy, P. J. A. (1999). Optimal nutrition: vitamin E. Proceedings of the Nutrition Society, 58, 459–468.
Nouros, P. G., Georgiou, C. A., & Polissiou, M. G. (1999). Direct parallel flow injection multichannel spectrophotometric determination of olive oil peroxide value. Analytica Chimica Acta, 389, 239–245.
Oliveros, C. C., Boggia, R., Casale, M., Armanino, C., & Forina, M. (2005). Optimisation of a new headspace mass spectrometry instrument: discrimination of different geographical origin olive oils. Journal of Chromatography. A, 1076, 7–15.
Paiva-Martins, F., Correia, R., Felix, S., Ferreira, P., & Gordon, M. (2007). Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. Journal of Agricultural and Food Chemistry, 55, 4139–4143.
Pereira, J. A., Casal, S., Bento, A., & Oliveira, M. B. P. P. (2002). Influence of olive storage period on oil quality of three Portuguese cultivars of Olea europaea, Cobrançosa, Madural and Verdeal Transmontana. Journal of Agricultural and Food Chemistry, 50, 6335–6340.
Pereira, J. A., Alves, M. R., Casal, S., & Oliveira, M. B. P. P. (2004). Effect of olive fruit fly infestation on the quality of olive oil from cultivars Cobrançosa, Madural and Verdeal Transmontana. Italian Journal of Food Science, 16, 355–365.
Pereira, A. P., Ferreira, I. C. F. R., Marcelino, F., Valentão, P., Andrade, P. B., Seabra, R., et al. (2007). Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules, 12, 1153–1162.
Rencher, A. C. (1995). Methods of multivariate analysis. New York: Wiley.
Rotondi, A., Bendini, A., Cerretani, L., Mari, M., Lercker, G., & Toschi, T. G. (2004). Effect of olive ripening degree on the oxidative stability and organoleptic properties of Cv. Nostrana di Brisighella extra virgin olive oil. Journal of Agricultural and Food Chemistry, 52, 3649–3654.
Salta, F. N., Mylona, A., Chiou, A., Boskou, G., & Andrikopoulos, N. K. (2007). Oxidative stability of edible vegetable oils enriched in polyphenols with olive leaf extract. Food Science and Technology International, 13, 413–421.
Salvador, M. D., Aranda, F., & Fregapane, G. (2001). Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality. A study of four successive crop seasons. Food Chemistry, 73, 45–53.
Sudjana, A. N., D’Orazio, C., Ryan, V., Rasool, N., Ng, J., Islam, N., et al. (2009). Antimicrobial activity of commercial Olea europaea (olive) leaf extract. International Journal of Antimicrobial Agents, 33, 461–463.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Malheiro, R., Casal, S., Teixeira, H. et al. Effect of Olive Leaves Addition during the Extraction Process of Overmature Fruits on Olive Oil Quality. Food Bioprocess Technol 6, 509–521 (2013). https://doi.org/10.1007/s11947-011-0719-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0719-z