Abstract
The bacteriocin (pediocin) produced by Pediococcus acidilactici ITV26 is a bioconservative with antilisterial activity that can be inactivated when added in free form into foods. Proteolytic enzymes or interaction/binding with some food components are among the factors that can affect its antimicrobial activity. Hence, there is a need to protect these peptides by encapsulation into liposomes as well as to control their release. The aim of this research was to establish conditions to obtain stable liposomes with pediocin, to keep their activity and to control the release of the encapsulated bacteriocin. Pediocin was purified by adsorption-desorption and liposomes were prepared by using different phosphatidylcholine concentrations (1, 3, and 5% w/v) and different operating conditions in the microfluidizer (500–1000 bar and 1–2 cycles). The liposomes obtained were characterized by using dynamic light scattering, backscattering light, and cryo transmission electron microscopy. In addition, the effect of pH on the kinetics of pediocin release was studied, and its antimicrobial activity was tested by following the growth kinetics of Listeria innocua AST-062 under different treatments with pediocin-encapsulated liposomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriocins are antimicrobial peptides of ribosomal synthesis produced by lactic acid bacteria with the ability to inhibit the growth of pathogenic microorganisms (Cotter et al. 2005). Bacteriocins have a broad antimicrobial spectrum even with phylogenetically related strains (Papagianni 2003). Research interest has been centered on bacteriocin class II, mainly pediocin, due to its thermostability and strong activity against Listeria monocytogenes (Kemperman et al. 2003). Pediocin is produced by Pediococcus spp. good cell growth and pediocin production are obtained in complex media (MRS) which makes extraction and purification very expensive (De De Vuyst et al. 2003; Beaulieu et al. 2007).
The barrel-stave mechanism used to explain the antimicrobial action of pediocin (class II) begins with formation of pores between two or more amphipathic peptide species, resulting in ion output, loss of proton power, and finally on cell death (Jack et al. 1995; Mesa-Pereira et al. 2017). The sequence of events leading to loss of viability of Gram-positive bacteria after treatment with pediocin AcH includes (1) adsorption or binding of pediocin PA-1 molecules to specific receptors on the cell surface, (2) entry of pediocin molecules through the cell wall, (3) pediocin membrane contact, (4) destabilization of functional integrity by the output of small molecules such as ions and ATP, and (5) the loss of structural integrity of the membrane upon evidence of cellular lysis in some strains (Bhunia et al. 1991; Rodríguez et al. 2002; Drider et al. 2006).
In our research group, a bacteriocin identified as pediocin, produced by Pediococcus acidilactici ITV26 with a strong antilisterial activity was obtained. However, the activity of this bacteriocin is affected by several factors, such as inactivation by proteolytic enzymes or by additives, linking or interaction with food components, limited solubility, and high pH. Therefore, there is a need to protect these peptides by encapsulation and then to control their release.
Encapsulation improves antimicrobial activity and stability of active peptides in food systems. In this direction, nano and micro delivery systems using surfactants, lipids, carbohydrates, polymers, and protein have been fabricated to stabilize and enhance the biological activity of the bioactive compounds (Aditya et al. 2017). Liposomes can carry active peptides such as bacteriocins by trapping these peptides into the aqueous center or between bilayers of phospholipids due to their amphiphilic nature (Benech et al. 2002; Laridi et al. 2003). Liposomes are spherical vesicles made of one or more phospholipid bilamer (Taylor et al. 2005). Liposomal vesicles are a group of amphiphilic molecules, which structure may be described as a concentric series of molecules having a polar head, a non-polar tail, and where each molecule has two hydrophobic chains (Laridi et al. 2003; Were et al. 2004; Taylor et al. 2007; de Vos et al. 2010). Microfluidization allows encapsulating active agents without being exposed to sonication and organic solvents and forming stable liposomes without aggregation (Imran et al. 2012). However, factors such as the concentration of phospholipids, pH, and temperature can affect the release of bacteriocins, so it is important to characterize them physicochemically in order to ensure liposome stability and release of bacteriocin (da Silva Malheiros et al. 2010; Narsaiah et al. 2012). In this work, dynamic light scattering (DLS) and backscattered light (BSL) were used to determine liposome size and stability as well as cryo transmission electron microscopy (cryoTEM) for morphological characterization.
The antimicrobial activity of pediocin against L. monocytogenes has been proved in beef, sausages, meat extracts, and meat products, as well as in dairy products (Rodríguez et al. 2002). Therefore, liposomes loaded with pediocin produced by P. acidilactici ITV26 might increase the antibacterial effect against Listeria spp. in such food products and might be also used in bioactive packing films (Benech et al. 2002; Degnan et al. 1993; Imran et al. 2012).
Materials and Methods
Materials
Soy lecithin microbiological grade (L-α-phosphatidylcholine) was obtained from Sigma-Aldrich, Germany. All other chemicals used in the study were of analytical grade.
Bacterial Strains
A pediocin-producer P. acidilactici ITV26, isolated in our laboratory, and Listeria innocua AST-062 were both incubated at 37 °C for 18 h, without agitation in Man-Rogosa-Sharpe (MRS) and Lauria-Bertoni (LB) broths, respectively. Both microorganisms were kept frozen at − 70 °C (REVCO ULT1386-5-A30) in 40% (v/v) glycerol plus their corresponding broth.
Production and Purification of the Bacteriocin by Pediococcus acidilactici ITV26
The bacteriocin producer strain (P. acidilactici ITV26) and the sensitive strain (L. innocua AST-062) were activated in MRS and LB broth, respectively. Growth of P. acidilactici ITV26 was determined by plate count.
The bacteriocin obtained from a fresh culture of P. acidilactici ITV26 was purified by adsorption-desorption according to Yang et al. (1992) technique. One percent inoculum (1 × 107 CFU/mL) of P. acidilactici ITV26 was added to 400 mL of MRS medium and incubated at 37 °C for 18 h. Then, pH was adjusted to 5.5 and the culture submitted to a heat treatment (70 °C) for 20 min followed by a heat shock (T ≤ 25 °C) in order to inactivate enzymes and stop growth. The culture broth was centrifuged for 15 min (Eppendorf 5810R) at 12,000×g and 4 °C. The cell pellet obtained was washed three times with 5 mM PBS pH 6.8, then resuspended in 20 mL of 200 mM NaCl; the pH was adjusted to 2 with 5% phosphoric acid and kept under magnetic stirring for 4 h at 4 °C. Later, it was centrifuged at 20,000×g for 20 min. The pH of supernatant (bacteriocin desorbed) was adjusted to 6.8 with 1 M NaOH and sterilized by using a Millipore 0.22-μm membrane (Millex®GV). It was concentrated by ultrafiltration (3 and 10 kDa), lyophilized, and stored at − 20 °C.
The bacteriocin molecular weight was determined by sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) using a resolving gel (16.5%T, 3 %C) and a stacking gel (4%T, 3 %C). Where (%T) indicates the relative pore size of the resulting polyacrylamide gel and (%C) weight percentage of cross-linker. The ProteoSilver kit was used for staining the gel bands, and the antimicrobial activity of SDS-PAGE fractions was determined by bioassay in petri dish. The protein content of purified bacteriocin and bacteriocin in cell-free extract was determined by Bio-Rad protein assay using bovine serum albumin as the standard.
The activity units were determined by the agar diffusion technique and quantified as follows (Schillinger and Lucke 1989):
Preparation of Pediocin-Loaded Liposomes by Microfluidization
Pediocin-loaded liposomes with 1, 3, and 5 (%) of phosphatidylcholine from soybean were prepared using a rotor-stator homogenizer (Ultra-Turrax® IKA® T-25) at 13,500 rpm for 3 min; this pre-emulsion was passed through a microfluidizer (Microfluidics M-110) by using either 500 or 1000 bar of pressure and 2 cycles of microfluidization.
Pediocin Encapsulation Efficiency
Liposomes containing pediocin were recovered from emulsions by using size exclusion chromatography (Sephadex G-50 column).
The encapsulation efficiency (% EE) of the different formulations was calculated by the following equation:
where mc is the mass of bacteriocin-encapsulating liposomes and me is the mass of bacteriocin found in the extract (de Mello et al. 2013).
Physicochemical Characterization of Pediocin-Loaded Liposomes
The average particle size, zeta potential, and polydispersity index (PDI) were determined by dynamic light scattering (DLS) using a Zetasizer apparatus (Malvern Instruments, Inc) and analyzed by the Zetasizer Software 7.11. A Turbiscan was also used to evaluate if destabilization of liposomes occurred by backscattering light.
Cryogenic Transmission Electron Microscopy
Biological samples are extremely sensitive to the electron beam, and then a cryogenic transmission electron microscopy (cryoTEM) was necessary to characterize size and in morphology of the pediocin-loaded liposomes, using only 80 kV of acceleration voltage and a very special sample preparation procedure (Almgren et al. 2000) to protect them during TEM observations. First, commercial lacey carbon film on TEM cooper grids of 300 mesh were used, having holes but without polymer film. To make the C-Film hydrophilic, the Cu grids were subjected to a glow discharge in the air atmosphere. Then a drop of the well-diluted solution sample is deposited on the Cu grid with holey C-film, absorbing carefully the excess with a filter paper until a very thin (thickness less than 500 nm) aqueous film of the solution sample is reached. In order to keep the original liposome structure, the sample was vitrified plunging it into a liquid ethane medium. Separately, a special cryoTEM sample holder is evacuated and cooled to LN2 temperature (− 170 °C). The transference of the sample to the LN2 cooling TEM sample holder needs to be done in the Cryoplunge 3 system, where the sample was vitrified, i.e., into the liquid ethane medium; so the TEM holder was plunged also in it, and the sample was transferred to the TEM holder, which quickly should be introduced in the cryoTEM. The LN2 cooling TEM sample holder has an extra external recipient to refill as necessary with LN2.
The cryoTEM used at this work was a JEOL microscope, model JEM-2100, calibrated to a low electron beam intensity to avoid sample damage. Images with × 12,000–40,000 magnifications were taken to visualize different liposomes sizes.
In Vitro Pediocin Release Studies
Pediocin release studies were performed by suspending 50 mg of 2.0% (w/v) pediocin-loaded liposomes into 1 mL of 0.15 M NaCl/0.02 M phosphate buffer at pH 4.0, 5.0, 6.0, or 6.8. Suspensions were kept at 4 °C under shaking (150 rpm) for 5 min. One microliter sample from this suspension was loaded into a microtube and centrifuged at 3000×g for 10 min and filtered through a 0.22 μm Millipore filter. The pediocin content in the permeate was determined by Bradford. All experiments were performed in triplicate.
Growth Inhibition
The growth inhibition study was carried out following a modified method of Narsaiah et al. (2013). The biological activity of the pediocin-loaded liposomes was evaluated by determining the colony forming units (CFU) after incubation with 1% (v/v) suspension of L. innocua AST-062 in LB medium at 37 °C for 24 h.
To six flasks with 50 mL of LB broth, 100 μL of L. innocua AST-062 inoculum was added as follows: (a) control, no pediocin; (b) un-encapsulated pediocin (1 mL; 23,333 IU); (c) pediocin-encapsulated liposomes prepared with 3% (w/v) PC and by using 500 bar of pressure with 1 cycle of microfluidization (1 ml); (d) pediocin-encapsulated liposomes obtained with 3% (w/v) PC and by using 500 bar of pressure with 2 cycles of microfluidization (1 mL); (e) pediocin-encapsulated liposomes prepared with 5% (w/v) PC and by using 500 bar of pressure and 1 cycle of microfluidization; and (f) pediocin-encapsulated liposomes prepared with 5% (w/v) PC and by using 500 bar of pressure with 2 cycles of microfluidization. The broth was withdrawn from each flask, serially diluted, and plated on LB agar plate at 0, 4, 8, 12, 24, 36, 48, and 72 h and counted the colonies of L. innocua. In addition, control assays were set up in parallel with the equivalent amount of inoculum of L. innocua AST-062. All experiments were performed in triplicate.
Statistical Analysis
Statistical analysis was done by using Minitab software (2010), version 16.1.0 (Minitab Inc). The effect of different concentrations of phospholipids, pressure, and cycles of microfluidization were studied using one-way ANOVA, and means were compared by using the Tukey multiple range test (P < 0.05). To ensure the reproducibility of the results, each experiment was conducted at least two times in triplicate.
Results
Bacteriocin Production and Purification
The bacteriocin activity was detected after 6 h in the log growth phase (Fig. 1). The maximum activity of the bacteriocin (3500 activity units/mL) occurred between 18 and 21 h, when the strain was in the stationary phase (4 × 109 CFU/mL); pH also decreased with time until 4.14 due to lactic acid production.
After purification by adsorption-desorption, results from SDS-PAGE showed that bacteriocin from P. acidilactici ITV26 is a peptide of 4610 Da (Figs. 2 and 3). The activity of purified pediocin raised up to 20,333 units/mL compared with the initial 3500 activity units/mL from the cell-free extract (Table 1).
Effect of Encapsulation Conditions on Liposome Properties
Liposome average and size distribution
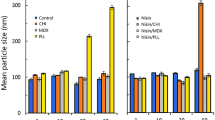
The study of liposome average size and its distribution is of interest because of its impact in the liposome stability and release of bacteriocin (Were et al. 2004). The effect of PC concentration and the pressure used during encapsulation on the liposome average size and size distribution are shown in Fig. 4. It can be seen that the average particle size of pediocin-encapsulating liposomes obtained by adding 3 and 5% PC, and by using 500 and 1000 bar of pressure with 1 cycle of microfluidization, was 144 nm. In addition, particle size distribution was unimodal in most cases when using 2 cycles of microfluidization, with a narrow polydispersity index (PDI) of 0.4. The particle size was reduced by 97.5% with respect to the pre-emulsion without treatment (cycle 0), and both the particle size and PDI did not show statistical difference between 1 and 2 cycles; therefore, no overprocessing of the sample was observed (Jafari et al. 2008). These results show that microfluidization operating conditions used were adequate for the encapsulation of the pediocin into liposomes.
In contrast to millimetric-sized carriers, nano-carriers provide more surface area and have the potential to increase solubility, enhance bioavailability, and to improve time-controlled release (Mozafari et al. 2008). Thus, nano-sizer results indicate that nanoliposomes containing pediocin formulated using soybean-lecithin are sufficiently smaller to carry out the abovementioned advantages (Imran et al. 2012). As the PC concentration decreases, the particle size decreases slightly, and two microfluidization cycles are required to obtain homogeneous liposomes according to the light-scattering values.
Zeta Potential
The zeta potential is usually used as indicator of accessible charges in liposome surface (de Mello et al. 2013). The zeta potentials of emulsions were below − 20 mV, which indicates that destabilization as coalescence, flocculation, sedimentation, or rupture of the phospholipid bilayer might occur. These will favor a faster release and a faster microorganism control.
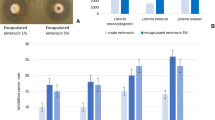
Even with the zeta potential values below − 20 mV (Fig. 5), the backscattering light analysis showed stability for most of the treatments.
Turbiscan Stability Index Kinetics
Turbiscan stability index (TSI) kinetics from the Turbiscan indicate sedimentation in the pre-emulsion. However, this result might not be representative due to the opacity of the emulsion, since the backscattered light was constant over the time of the analysis. Otherwise, results from the Turbiscan pointed out that liposomes obtained under one microfluidization cycle using either 500 or 1000 bar of pressure were the most stable, while slight destabilization phenomena such as flocculation or coalescence are suggested in liposomes obtained by using 1 and 2 cycles of microfluidization both under 500 and 1000 bar of pressure (Fig. 6).
Cryo-TEM
According to the definition of Mozafari et al. (2002), liposomes are “closed, continuous bilayer structures composed mainly of lipid and/or phospholipid molecules” (Colas et al. 2007). An important advantage of liposomes is to have both aqueous and lipid phases, which enable them to incorporate both hydrophilic and hydrophobic materials. CryoTEM can image both aqueous and lipid phases as it is shown in Fig. 7, where this technique attests the presence of liposomes predominantly with spherical and bilayer structures. As revealed by cryoTEM, liposome size diminishes as the number of microfluidization cycles increase (see Fig. 7), which is in good concordance with the previous DLS results (Fig. 4). Moreover, through cryoTEM, it was also possible to realize that the numerical density of liposome increases as the microfluidization cycles does (Fig. 7).
CryoTEM images of pediocin-encapsulated liposomes prepared with 5% phosphatidylcholine and obtained by using 0 (A, B), 1 (C, D), and 2 (E, F) cycles under 1000 bar of microfluidization pressure. Note that the liposomes size diminishes as the number of microfluidization cycle increases, but on the other side, its number density increases
Encapsulation and Inhibition Efficiency
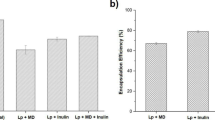
The encapsulation efficiency was related to the percentage of PC used; at higher PC concentration, higher encapsulation efficiency was observed, and average values of about 88% EE were obtained.
The bacteriocin was stable into liposomes and had a slow and controlled release during 120 h under all pH values tested (Fig. 8). However, at low pH value (i.e., 4.0), the ionic interactions involved in the stability of liposomes are modified, and a better release of the purified bacteriocin was observed, as suggested by Zimmermann and Mu (2001). Under this condition, the pediocin-encapsulated liposomes of about 143 nm were able to reduce 2.5 log units of L. innocua AST-062 (Fig. 9).
Results from this work demonstrated that nanoencapsulation of pediocin with PC allowed obtaining liposomes with inhibition activity higher or similar to those reported in literature.
Conclusions
Microfluidization allowed to significantly decrease liposome size regarding those obtained with rotor-stator. According to the TSI kinetics, just one cycle of microfluidization allowed the best stability of liposomes. The main reason for this may be because when using more than one microfluidization cycle, the energy used is higher; therefore, the liposomes are reduced in size, but the interaction forces between particles are also decreased, allowing higher instability such as flocculation and coalescence. These results, together with those from light scattering, zeta potential, and Turbiscan measurements, suggest that the vesicles are not sharp, and they can release fast enough for control of the sensitive microorganism. At acid pH, fast release of the bacteriocin from liposomes was observed, due to structural changes, mainly on both protonation and deprotonation on functional groups NH3/NH4 + and COOH/COO, respectively. Therefore, one microfluidization cycle would be the best encapsulation condition for the pediocin produced by P. acidilactici ITV26.
References
Aditya, N. P., Espinosa, Y. G., & Norton, I. T. (2017). Encapsulation systems for the delivery of hydrophilic nutraceuticals: food application. Biotechnology Advances, 35(4), 450–457. https://doi.org/10.1016/j.biotechadv.2017.03.012.
Almgren, M., Edwards, K., & Karlsson, G. (2000). Cryo transmission electron microscopy of liposomes and related structures. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 174(1–2), 3–21. https://doi.org/10.1016/S0927-7757(00)00516-1.
Beaulieu, J., Moles, A., Leitch, I., Bennett, M., Dickie, J., & Knight, C. (2007). Correlated evolution of genome size and seed mass. The New Phytologist, 173(2), 422–437. https://doi.org/10.1111/j.1469-8137.2006.01919.x.
Benech, R. O., Kheadr, E. E., Laridi, R., Lacroix, C., & Fliss, I. (2002). Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Applied and Environmental Microbiology, 68(8), 3683–3690. https://doi.org/10.1128/AEM.68.8.3683-3690.2002.
Bhunia, A. K., Johnson, M. C., Ray, B., & Kalchayanand, N. (1991). Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. Journal of Applied Microbiology, 70(1), 25–33. https://doi.org/10.1111/j.1365-2672.1991.tb03782.x.
Colas, J. C., Shi, W., Rao, V. S. N. M., Omri, A., Mozafari, M. R., & Singh, H. (2007). Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron, 38(8), 841–847.
Cotter, P. D., Hill, C., & Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nature Reviews. Microbiology, 3(10), 777–788.
da Silva Malheiros, P., Daroit, D. J., da Silveira, N. P., & Brandelli, A. (2010). Effect of nanovesicle-encapsulated nisin on growth of Listeria monocytogenes in milk. Food Microbiology, 27(1), 175–178. https://doi.org/10.1016/j.fm.2009.09.013.
de Mello, M. B., da Silva Malheiros, P., Brandelli, A., Pesce da Silveira, N., Jantzen, M. M., & de Souza da Motta, A. (2013). Characterization and antilisterial effect of phosphatidylcholine nanovesicles containing the antimicrobial peptide Pediocin. Probiotics and Antimicrobial Proteins, 5(1), 43–50. https://doi.org/10.1007/s12602-013-9125-3.
de Vos, P., Faas, M. M., Spasojevic, M., & Sikkema, J. (2010). Encapsulation for preservation of functionality and targeted delivery of bioactive food components. International Dairy Journal, 20(4), 292–302. https://doi.org/10.1016/j.idairyj.2009.11.008.
De Vuyst, L., Foulquié Moreno, M. R., & Revets, H. (2003). Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. International Journal of Food Microbiology, 84(3), 299–318. https://doi.org/10.1016/S0168-1605(02)00425-7.
Degnan, A. J., Buyong, N., & Luchansky, J. B. (1993). Antilisterial activity of pediocin AcH in model food systems in the presence of an emulsifier or encapsulated within liposomes. International Journal of Food Microbiology, 18(2), 127–138. https://doi.org/10.1016/0168-1605(93)90217-5.
Drider, D., Fimland, G., Héchard, Y., McMullen, L. M., & Prévost, H. (2006). The continuing story of class IIa bacteriocins. Microbiology and Molecular Biology Reviews: MMBR, 70(2), 564–582.
Imran, M., Revol-Junelles, A. M., René, N., Jamshidian, M., Akhtar, M. J., Arab-Tehrany, E., et al. (2012). Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocolloids, 29(2), 407–419. https://doi.org/10.1016/j.foodhyd.2012.04.010.
Jack, R. W., Tagg, J. R., & Ray, B. (1995). Bacteriocins of gram-positive bacteria. Microbiological Reviews, 59(2), 171–200.
Jafari, S. M., Assadpoor, E., He, Y., & Bhandari, B. (2008). Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocolloids, 22(7), 1191–1202. https://doi.org/10.1016/j.foodhyd.2007.09.006.
Kemperman, R., Kuipers, A., Karsens, H., Nauta, A., Kuipers, O., & Kok, J. (2003). Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Applied and Environmental Microbiology, 69(3), 1589–1597.
Laridi, R., Kheadr, E. E., Benech, R. O., Vuillemard, J. C., Lacroix, C., & Fliss, I. (2003). Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. International Dairy Journal, 13(4), 325–336.
Mesa-Pereira, B., O' Connor, P. M., Rea, M.C., P. D., Hill, C. & Ross, R. P. (2017). Controlled functional expression of the bacteriocins pediocin PA-1 and bactofecin A in Escherichia coli. Scientific Reports, 7(1), 1–11. https://doi.org/10.1038/s41598-017-0268-w.
Mozafari, M. R., Reed, C. J., Rostron, C., Kocum, C., & Piskin, E. (2002). Construction of stable anionic liposome-plasmid particles using the heating method: a preliminary investigation. Cellular and Molecular Biology Letters, 7(September), 923–927 Retrieved from http://www.cmbl.org.pl.
Mozafari, M. R., Johnson, C., Hatziantoniou, S., & Demetzos, C. (2008). Nanoliposomes and their applications in food nanotechnology. Journal of Liposome Research, 18(4), 309–327. https://doi.org/10.1080/08982100802465941.
Narsaiah, K., Jha, S. N., Wilson, R. A., Mandge, H. M., Manikantan, M. R., Malik, R. K., & Vij, S. (2012). Pediocin-loaded nanoliposomes and hybrid alginate–nanoliposome delivery systems for slow release of pediocin. BioNanoScience, 3(1), 37–42. https://doi.org/10.1007/s12668-012-0069-y.
Narsaiah, K., Jha, S. N., Wilson, R. A., Mandge, H. M., Manikantan, M. R., Malik, R. K., & Vij, S. (2013). Pediocin-loaded nanoliposomes and hybrid alginate-nanoliposome delivery systems for slow release of pediocin. BioNanoScience, 3(1), 37–42. https://doi.org/10.1007/s12668-012-0069-y.
Papagianni, M. (2003). Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnology Advances, 21(6), 465–499.
Rodríguez, J. M., Martínez, M. I., & Kok, J. (2002). Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Critical Reviews in Food Science and Nutrition, 42(2), 91–121. https://doi.org/10.1080/10408690290825475.
Schillinger, U., & Lucke, F. K. (1989). Antibacterial activity of lactobacillus-sake isolated from meat. Applied and Environmental Microbiology, 55(8), 1901–1906.
Taylor, T. M., Davidson, P. M., Bruce, B. D., & Weiss, J. (2005). Liposomal nanocapsules in food science and agriculture. Critical Reviews in Food Science and Nutrition, 45(7–8), 587–605. https://doi.org/10.1080/10408390591001135.
Taylor, T. M., Gaysinsky, S., Davidson, P. M., Bruce, B. D., & Weiss, J. (2007). Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophysics, 2(1), 1–9. https://doi.org/10.1007/s11483-007-9023-x.
Were, L. M., Bruce, B., Davidson, P. M., & Weiss, J. (2004). Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. Journal of Food Protection, 67(5), 922–927.
Yang, R., Johnson, M. C., & Ray, B. (1992). Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Applied and Environmental Microbiology, 58(10), 3355–3359.
Zimmermann, E., & Mu, R. H. (2001). Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLN™) dispersions in artificial gastrointestinal media. European Journal of Pharmaceutics and Biopharmaceutics, 52(2), 203–210.
Acknowledgements
The authors acknowledge the CNMN (Centro de Nanociencias y Micro y Nanotecnologías) of the National Polytechnic Institute for the samples analysis by cryoTEM.
Funding
This work was supported by the Tecnológico Nacional de México/I.T. Veracruz trough Proyect 6293.17-P and the CONACyT (Consejo Nacional de Ciencia y Tecnología).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Toledo, J.A., Torrestiana-Sánchez, B., Martínez-Sánchez, C.E. et al. Nanoencapsulation of a Bacteriocin from Pediococcus acidilactici ITV26 by Microfluidization. Food Bioprocess Technol 12, 88–97 (2019). https://doi.org/10.1007/s11947-018-2184-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2184-4