Abstract
Encapsulation may provide increased stability and antimicrobial efficiency to bacteriocins. In this work, the antilisterial peptide pediocin was encapsulated in nanovesicles prepared from partially purified soybean phosphatidylcholine. The maintenance of antimicrobial activity and properties of free and encapsulated pediocin was observed during 13 days at 4 °C, and after this period, the encapsulated pediocin retained 50 % its initial activity. The maintenance of the bioactive properties of free and encapsulated pediocin was observed against different species of Listeria, inhibiting Listeria monocytogenes, Listeria innocua and Listeria ivanovii. The size of vesicles containing pediocin was determined by dynamic light scattering as an average of 190 nm, with little change throughout the observation period. Polydispersity index values were around 0.201 and are considered satisfactory, indicating an adequate size distribution of liposomes. The efficiency of encapsulation was 80 %. Considering these results, the protocol used was appropriate for the encapsulation of this bacteriocin. Results demonstrate the production of stable nanoparticulate material. The maintenance of the properties of pediocin encapsulated in liposomes is fundamental to prospect the stability in different conditions of the food matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriocins are ribosomally synthesized, heat-stable, antimicrobial peptides produced by bacteria, which are immune to their own bacteriocin(s) and are active against other bacteria, either in the same species (narrow spectrum of activity) or showing a broad spectrum of activity [6]. However, the bacteriocins of lactic acid bacteria (LAB) have received much attention in terms of food safety due to their generally recognized as safe (GRAS) status microorganisms [29]. Nisin is the most studied bacteriocin of LAB, showing a broad antimicrobial spectrum, stability in some products, and allowed for use in food protection.
Another bacteriocin that attracts research interest is pediocin produced by Pediococcus acidilactici and is commercially exploited as a bacteriocin-containing fermentate powder [30]. Although this antimicrobial compound is mainly used in meat products, the extension of its application to dairy products is being evaluated due to its antilisterial activity and stability [26]. Pediocin is part of a group of bacteriocins belonging to the class IIa, characterized as “antilisterial” bacteriocins [27]. Pediocin AcH (same of PA-1) is a 44 amino acid bacteriocin (molecular mass of 4,629 Da; pI of 9.6) produced primarily by strains of the genus Pediococcus [10]. These compounds present a great interest to the food-processing industry as natural preservatives and potential substitutes for chemical preservatives [1, 12].
However, certain conditions can reduce the availability and effectiveness of bacteriocins in food. Under experimental conditions, when free nisin is added to milk, the majority of the nisin adheres to fat and protein surfaces resulting in a lower accessibility to bacterial cells. The antimicrobial efficacy of bacteriocins may be also reduced because of (a) chemical and physical changes during food process operations and (b) undesirable interactions with other food components, requiring the addition of large peptide quantities [37]. To overcome these limitations, the encapsulation of bacteriocins may offer the following potential advantages: reduce or prevent bacteriocin affinity to food components, avoid interference with starter cultures during fermentation, act as long-term preservative in food stored for long periods of time, and protect the antimicrobial from inhibitors or unfavorable conditions that naturally occur in the food matrix [14, 28, 34].
Liposomes or lipid vesicles are aggregates formed from aqueous dispersions of amphiphilic molecules such as polar lipids that tend to produce bilayer structures [15]. Additional processing methods, such as sonication or extrusion, may produce small unilamellar liposomes with sizes in the nanometer range that can be utilized to encapsulate active compounds [25]. Nanoencapsulation of antimicrobial peptides may represent an efficient approach to increasing the physical stability of these substances, protecting them from the undesirable interactions with the medium components and, because of the subcellular size, increasing their bioactivity [3]. Encapsulation can increase the concentration of the antimicrobial compound in food parts where microorganisms are preferably located, for example water-rich phases or liquid–solid interfaces [36]. Most studies on bacteriocin encapsulation are related to nisin, and the maintenance of the antimicrobial activity after incorporation in nanoparticles is often observed [13, 16, 33, 35]. The antimicrobial activity of lysozyme and peptide P34 was also retained after encapsulation in nanoliposomes [19, 38]. Despite the use of nanoencapsulated bacteriocins in food matrices is poorly reported, encapsulated nisin can reduce Listeria monocytogenes counts in whole and skimmed milk [17], and in soft cheese [20].
Relatively few studies on the encapsulation of bacteriocins in nanostructures have been reported, and promising bacteriocins like pediocin have not been investigated [8]. The objective of the present study was to evaluate the antimicrobial activity, stability, size and encapsulation efficiency of commercial pediocin in soybean phosphatidylcholine (PC) liposomes. Thereafter, the effect of free and nanovesicles-encapsulated pediocin was evaluated against Listeria species.
Materials and Methods
Bacterial Strains, Pediocin, and Media
Listeria monocytogenes ATCC 7644 was used as the indicator organism for the bacteriocin activity assay, since this bacterium is related to food-borne outbreaks involving dairy and meat products. The strain was maintained on tryptic soy agar plates with yeast extract (TSA-YE 0.6 %, Himedia, India) at 4 °C and subcultured periodically. Before each experiment, this strain was grown in TSA-YE medium at 37 °C for 18–24 h. Pediocin (ALTA™ 2345) was provided by Kerry Ingredients & Flavours, USA. According to the manufacturer’s guidelines, pediocin ALTA™ 2345 should be applied at approximately 0.05–1.0 % (Kerry Inc.). Before each experiment, pediocin was diluted with 10 mM sodium phosphate buffer (pH 7.0) to reach a concentration of 1.0 %.
Purification of Crude Soybean Lecithin
The purification of PC was carried out as described by Malheiros et al. [16]. Samples of crude soybean lecithin were provided by Solae S.A. (Esteio, Brazil). The crude soybean lecithin (10 g) was dissolved in 50 ml of ethyl acetate (Merck, Germany). Then, slowly and under agitation, 2 ml of distilled water was added, resulting formation of two phases. The upper phase was separated from the lower phase and discarded. The lower phase, having a gel aspect, was dispersed in 30 ml of acetone, forming clusters that were crushed using a glass stick. Then, the acetone was separated by decanting and a new aliquot of 30 ml acetone was added, repeating the shredding process. The precipitate was vacuum filtered and dried in a desiccator, providing a mass of 8.75 g, which was maintained under freezing temperature (−18 °C). The sample was designated as PC-1, composed of 75 % distearoylphosphatidylcoline, 12 % dioleoylphosphatidylcholine, and 8 % dipalmitoylphosphatidylcoline [22].
Pediocin Encapsulation
Encapsulation of pediocin in PC-1 liposomes was carried out by the thin-film hydration method with bath-type sonicator [16]. Briefly, 0.076 g of PC-1 was dissolved with 10 ml of chloroform in a round-bottom flask, and the organic solvent was removed by a rotary evaporator until a thin film was formed on the flask walls. Traces of organic solvents were removed by storage for 18 h in vacuum desiccator. The resulting dried lipid film was dispersed by the addition of 10 mM sodium phosphate buffer pH (7.0) containing pediocin. Pediocin-free liposomes were prepared by addition of 10 mM phosphate buffer only. The protocol follows five repeated cycles consisting of 2 min heating the sample to 60 °C and 1 min with agitation in vortex device. Sonication of the preparation was carried out in a bath-type ultrasound (40 kHz, Unique USC 700/Unique Group, São Paulo/Brazil) for 30 min at 45 °C [16]. The mean particles size and polydispersity (PDI) were evaluated by light scattering performed on a Brookhaven Instruments standard setup (BI-200 M goniometer, BI-9000AT digital correlator) with a He–Ne laser (λ = 632.8 nm) as light source [34].
Antimicrobial Activity Assay
Antimicrobial activity was determined essentially as described previously [23]. An aliquot of 20 μl of the free pediocin, liposome-encapsulated pediocin, pediocin-free liposomes and 10 mM phosphate buffer (pH 7.0) were applied to cellulose discs (6 mm) onto TSB agar plates previously inoculated with a swab submerged in indicator strain L. monocytogenes ATCC 7644 suspension, which corresponded to a 0.5 McFarland turbidity standard solution (approximately 107 CFU ml−1). Plates were incubated at 37 °C for 24 h. The antimicrobial activity titer was determined by the serial twofold dilution method described by Mayr-Harting et al. [21]. Activity was defined as the reciprocal of the last dilution giving an inhibition zone and expressed as activity unit (AU) per milliliter. The AU ml−1 was determined against L. monocytogenes ATCC 7644 as indicator strain.
The number of L. monocytogenes cells killed by different pediocin concentrations (AU ml−1) was determined in BHI broth containing 100, 200, and 400 AU ml−1 pediocin. Tubes were inoculated with L. monocytogenes ATCC 7466 (104 CFU ml−1), and viable cell counts were determined after 24 h incubation at 37 °C.
Effect of Free and Encapsulated Pediocin in Liquid Medium
The effect of free and encapsulated pediocin was evaluated by determination of colony forming units (CFU) of L. monocytogenes ATCC 7466, in liquid medium. For this experiment, 1 ml of each treatment (free pediocin, encapsulated pediocin, pediocin-free liposomes, and 10 mM phosphate buffer pH 7.0) was added to tubes containing 10 ml of BHI (Brain Heart Infusion) broth. The tubes were inoculated with a culture of L. monocytogenes ATCC 7644 giving an initial concentration of 104 CFU ml−1 and incubation was performed at 37 °C. The sampling points for evaluation of CFU ml−1 were made at days 0, 2, 6, 9, and 13. The experiment was conducted in triplicate for each treatment.
Stability and pH of Free and Encapsulated Pediocin
The encapsulated pediocin had its stability assessed over an incubation period of 13 days under refrigeration at 4 °C. This assessment was compared to the same concentrations of free pediocin. The evaluation was done by determining the antimicrobial activity with culture indicator L. monocytogenes ATCC 7644. The evaluation of the pH of the preparations of free and encapsulated pediocin were made with pH strip (Merck, Germany).
Zeta-potential Measurements
The zeta-potential analyses of filtered and unfiltered liposomes were carried out after dilution of the formulations in 1 mM NaCl and equilibrated at 25 °C in the Peltier-controlled cuvette using the Zetasizer®nano-ZS ZEN 3600 equipment (Malvern Instruments, Herrenberg, Germany). The zeta potential was determined by measuring the direction and velocity of the liposomes in the applied electric field. The electrophoretic mobility data were converted into zeta-potential values by the software through the Smoluchowski equation [31]. The measurements were made in triplicate.
Entrapment Efficiency
The entrapment efficiency (EE) of liposomes produced by film hydration method was determined using agar diffusion method. Encapsulated pediocin was separated from unencapsulated pediocin by ultrafiltration (Ultracel YM-10 Membrane, Millipore). Samples of pediocin-free liposomes and free pediocin solution were also submitted to ultrafiltration as controls. The antimicrobial activity of the filtrates and retained was measured. EE was calculated using the following equation [14]:
Transmission Electron Microscopy
The liposome-encapsulated pediocin was prepared in 10 mM phosphate sodium pH (7.0) and evaluated by transmission electron microscopy (TEM) with negative staining. Liposome-encapsulated pediocin suspension was diluted ten-fold in phosphate buffer, and the sample was deposited on a sample grid with and negatively stained with uranyl acetate solution 2 % (w/v). All preparations were observed with JEOL JEM 1200ExII transmission electron microscope (JEOL, Tokyo) operating at 120 kV.
Results and Discussion
Antimicrobial Activity of Free and Encapsulated Pediocin
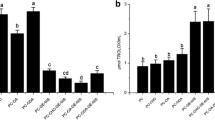
Liposomes prepared with partially purified soybean phosphatidylcholine were used for encapsulation of pediocin. The antimicrobial activity of the pediocin solution used for encapsulation was 800 AU ml−1. In this work, pediocin was encapsulated by the methodology of the hydration of lipid film using bath-type ultrasound. This method has been used with more satisfactory results considering the maintenance of biological activity and production of smaller and more homogeneous liposomes [16]. The antimicrobial activity of the pediocin was preserved by encapsulation, yielding an encapsulation efficiency of 80 %. The inhibition of L. monocytogenes was evidenced by the agar diffusion method, and the halos for free and encapsulated pediocin were 21 and 19 mm, respectively. Absence of antimicrobial activity was observed for liposomes prepared without pediocin and for 10 mM sodium phosphate buffer, corroborating the antimicrobial effect was due to pediocin activity (Fig. 1).
Inhibition of Listeria monocytogenes by encapsulated pediocin. TSB agar plates were previously inoculated with L. monocytogenes and aliquots (20 μl) of free pediocin (P), liposome-encapsulated pediocin (LP), empty liposomes (L), and 10 mM phosphate buffer pH 7.0 (T) were applied to 6 mm cellulose discs. Plates were incubated at 37 °C for 24 h, and the diameters of inhibition zones were measured
Free and encapsulated pediocin were evaluated for 13 days to verify the maintenance of the antimicrobial activity. L. monocytogenes ATCC 7644 was sensitive to both free and encapsulated pediocin during the evaluation period (Table 1). The evaluation of antimicrobial activity is needed to understand the behavior of the encapsulated compound during the time, considering the potential application of pediocin in foods. It was observed that free pediocin showed greater antimicrobial activity compared to encapsulated pediocin at the same incubation conditions. However, at the end of 13 days of incubation, both free and encapsulated pediocin retained 50 % of their initial activities. In addition, other strains of Listeria were tested and showed sensitivity to free and encapsulated pediocin (Table 2). The pH of the solution of free and encapsulated pediocin was 6.0 and remained constant over time (data not shown).
The effect of free and encapsulated pediocin on L. monocytogenes was monitored during incubation in BHI broth. In 48 h, the treatment with encapsulated pediocin resulted in viable counts 2.5 log and 1.0 log lower than that observed for empty liposomes and free pediocin, respectively (Fig. 2). After this time, free pediocin showed an improved inhibitory effect than encapsulated pediocin. These results were similar to that presented by Malheiros et al. [17], which indicate that nisin-loaded liposomes were less efficient in controlling L. monocytogenes growth when compared to free nisin, in both BHI medium and skim milk at 30 °C. Other studies showed a similar effect of both free and encapsulated bacteriocin. The addition of nanovesicles containing bacteriocin P40 to a cell suspension of L. monocytogenes resulted in a decrease in viable counts of 2 log cycles within 5 min of incubation. A complete cell death was observed after 12 min of incubation. This kinetics was almost identical to that observed with free bacteriocin P40 [34].
Encapsulation of bacteriocins into liposomes may enhance their efficacy while protecting against detrimental interactions with food components [18, 36]. The maintenance of antimicrobial activity of nisin after encapsulation in PC liposomes has been described [16, 32, 37]. Similarly, the antimicrobial peptide P34 was encapsulated in nanovesicles, and no significant differences in the biological activity of free and encapsulated P34 were observed through 24 days storage at 4 °C [19]. In agreement with those previous reports, the results of this work indicate that encapsulated pediocin also retain its bioactive properties.
Although nisin has been intensively investigated, including its incorporation in nanovesicles [5, 13, 17, 35], there are difficulties in using nisin in raw-meat applications. Thus, other bacteriocins have been examined, and the most promising results in meats have been obtained with pediocin PA-1/AcH from Pediococcus acidilactici [4, 12]. Several bacteriocins from Pediococcus species have been biochemically and genetically characterized, and their mode of action has also been studied [10, 12]. However, studies on the encapsulation of this peptide are scarce [8, 9]. In this work, pediocin was encapsulated in nanovesicles, retaining its antimicrobial activity against L. monocytogenes during the period studied. The inhibition of this microorganism is relevant because this pathogen is associated with food-borne disease and outbreaks in dairy and meat products [11]. Similar sensitivity was also observed for other strains of Listeria, indicating that isolates from different sources are also sensitive to free and encapsulated pediocin.
Although experimental data in food matrices are scarce, encapsulated nisin was capable to inhibit L. monocytogenes in both whole and skimmed milk [17]. The effectiveness of both free and encapsulated nisin was greater in skimmed milk, in agreement with the previously described negative effect of fat on the antimicrobial activity of bacteriocins, caused by the adsorption of nisin onto fat globules [2]. Also, PC nanovesicles containing nisin or peptide P34 resulted in better inhibitory effect after 10 days of storage of cheese in comparison with other treatments, suggesting that the bacteriocins are being released from the PC liposomes after this time [20].
No detailed investigation on the interaction of encapsulated bacteriocins with microbes in food matrices is available. However, some studies in defined culture media suggest that the bacteriocin would be continuously released from the liposomes, then acting on the target microbe. Electron microscopy studies on encapsulated peptide P34 showed that L. monocytogenes cells are surrounded by the nanoliposomes after 1 h at 30 °C. Liposomes appeared to be adhered but not fused with the bacteria. Free peptide P34 acts through vesiculization of the protoplasm, pore formation, and disintegration of L. monocytogenes cells [24]. In this sense, it is suggested that the peptide needs to be released from the liposomes to act in the cell membrane of target bacteria. Colas et al. [5] demonstrated that empty PC nanoliposomes surrounded B. subtilis cells, and a few of them fused with the bacterial membrane after 2 h at 37 °C.
Properties of Encapsulated Pediocin
Other properties of interest for nanoparticles are the particle size, polydispersity index, elasticity, structure, and encapsulation efficiency [18, 25].
Analyses using dynamic light scattering give information on the size and polydispersity of the nanovesicles. The liposomes containing pediocin had its size measured over a period of 13 days with a mean of 190 nm (Table 3). The values for the polydispersity did not change during the period, with an average value of 0.201.
The pediocin encapsulated in liposomes of soybean phosphatidylcholine was analyzed by transmission electron microscopy, and a homogeneous size of liposomes was observed. The liposomes appeared as clusters with spherical morphology (Fig. 3). The methodology of the thin-film hydration with the use of ultrasound bath often provides liposomes with sizes in the nanometer range, as previously described for nisin [16].
The encapsulation efficiency is an important parameter to be evaluated. Degnan and Luchansky [8] and Degnan et al. [9] observed that pediocin was encapsulated in conventional phosphatidylcholine liposomes by the film hydration method with an efficiency of about 18 %. This value is low considering the application in a food matrix. In this study, an encapsulation efficiency of 80 % was reached for pediocin, suggesting a better method to be used for pediocin incorporation in liposomes. The encapsulation efficiency of nisin has been also evaluated for neutral phospholipids such as PC, and diverse values for this parameter have been reported [18]. The encapsulation of pediocin AcH within PC liposomes resulted in an increased recovery of pediocin activity in heated muscle (27.5 %) and heated tallow (28.9 %) when compared to similar slurries containing free pediocin [8]. Significant loss of pediocin AcH activity was observed within 1.5 min (24–60 % recovery of original activity) following the addition of 30,000 AU of pediocin per ml. This shows the aptitude of these peptides to interact with food components, becoming unavailable to act against pathogenic and spoilage microorganisms in the product.
Laridi et al. [14] found an encapsulation efficiency of 34 % for nisin by the use of pro-liposome H containing a smaller amount of negatively charged phosphatidylinositol, when compared with the use of other liposomes (9.5–26 %). The encapsulation efficiency of nisin on partially purified PC was 94 % with a pH of 4.5 [16]. Those authors also performed the encapsulation of antimicrobial peptide P34 by the same method and under the same conditions. The value for the encapsulation efficiency was 100 % [19]. This parameter is a function of lipid composition and can be attributed to the electrostatic and hydrophobic interactions between the antimicrobial and lipid [37].
The size of vesicles containing the bacteriocin pediocin was about 190 nm with little change throughout the observation period. PDI values were around 0.201 and are considered satisfactory, indicating a low polydispersity of liposomes (Table 3). Using similar methodology, Malheiros et al. [19] showed that nanovesicles containing the antimicrobial peptide P34 were physically stable, and the encapsulated nisin showed values of constant size and PDI (132–149 nm) for a period of 24 days [16].
The zeta potential was approximately −44 mV and remained constant for 13 days (Table 3), indicating stability of the preparation. The zeta potential is usually utilized as an indicator for accessible charges in liposome surface. For instance, empty phosphatidylcholine liposomes present a zeta potential of 8.3 mV at 25 °C, whereas PC:PG (8:2) and PC:PG (6:4) showed zeta potentials of −52.28 and −72.60 mV, respectively [32]. To statistical analysis on the data in Table 3, the Tukey test was used. During the period of 13 days the data size, polydispersity and zeta potential of encapsulated did not vary, suggesting maintaining their properties over the period of study. The assessment made by transmission electron microscopy showed that encapsulated pediocin formed clumpy structures with spherical morphology and homogeneous form and size (Fig. 3). The methodology of the film hydration using the bath-type ultrasound has been reported as responsible for the formation of vesicles with more homogeneous appearance, contributing to the maintenance of stability of these structures. The technique of negative staining is important to visualize these structures, assisting in the investigation and study of nanovesicles. To evaluate the ability of Mozafari method, this technique was used to observed nanoliposomes and was observed the presence of predominantly spherical and bilayered structure [5]. Similar images were obtained with the encapsulated pediocin in this work.
Regarding the safety aspects, toxicity studies on the use of nanoparticles in food are scarce. A recent review on this subject describes the toxic effects of single-walled carbon nanotubes to eukaryotic cells and some concern on incorporation of metallic nanoparticles to packaging, while incorporation of nanoclay appears to be safe [7]. In this work, the nanoliposomes were prepared with food grade materials (soybean PC and commercial pediocin) and the resulting nanocomposites would expect to be safe. Nanoparticulate materials have unique interactions with biological systems, and therefore, future investigation on cytotoxicity of nanoencapsulated bacteriocins must be warranted.
Conclusion
Encapsulation of pediocin by the film hydration method in lipid nanovesicles was successfully achieved using liposomes prepared from soybean lecithin with a high EE of 80 %. The stability of liposome vesicles was evaluated and showed potential for application in foods. Pediocin-loaded nanovesicles may provide an important tool for controlling spoilage and pathogenic organisms in food and may improve pediocin stability and efficacy in food matrices.
References
Arauz LJ, Jozala AF, Mazzola PG, Penna TC (2009) Nisin biotechnological production and application: a review. Trends Food Sci Technol 20:146–154
Bhatti M, Veeramachaneni A, Shelef LA (2004) Factors affecting the antilisterial effect of nisin in milk. Int J Food Microbiol 97:215–219
Brandelli A (2012) Nanostructures as promising tools for delivery of antimicrobial peptides. Mini Rev Med Chem 12:731–741
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Colas JC, Shi WS, Rao VSNM, Omri A, Mozafari MR, Singh H (2007) Microscopical investigations of nisin-loaded nanolipossomes prepared by Mozafari method and their bacterial targeting. Micron 38:841–847
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E (2012) Nanotechnologies in the food industry: recent developments, risks and regulation. Trends Food Sci Technol 24:30–46
Degnan AJ, Luchansky JB (1992) Influence of beef tallow and muscle on the antilisterial activity of pediocin ACH and liposome-encapsulated pediocin ACH. J Food Prot 55:552–554
Degnan AJ, Buyong N, Luchansky JB (1993) Antilisterial activity of pediocin AcH in model food systems on the presence of an emulsifier or encapsulated within liposomes. Int J Food Microbiol 18:127–138
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Classe IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106
Gandhi M, Chikindas ML (2007) Liseria; a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15
Jamuna M, Jeevaratnam K (2004) Isolation and partial characterization of bacteriocins from Pediococcus species. Appl Microbiol Biotechnol 65:433–439
Kopermsub P, Mayen V, Warin C (2011) Potential use of liposomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res Int 44:605–612
Laridi R, Kheadr EE, Benech RO, Vuillemard JC, Lacroix C, Fliss I (2003) Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. Int Dairy J 13:325–336
Lasch J, Weissig V, Brandl M (2003) Preparation of liposomes. In: Torchilin VP, Weissig V (eds) Liposomes: a practical approach. Oxford University Press, New York, pp 3–30
Malheiros PS, Micheletto YMS, Silveira NP, Brandelli A (2010) Development and characterization of phosphatidylcholine nanovesicles containing the antimicrobial peptide nisin. Food Res Int 43:1198–1203
Malheiros PS, Daroit DJ, Silveira NP, Brandelli A (2010) Effect of nanovesicle-encapsulated nisin on growth of Listeria monocytogenes in milk. Food Microbiol 27:175–178
Malheiros PS, Daroit DJ, Brandelli A (2010) Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci Technol 21:284–292
Malheiros PS, Sant’Anna V, Micheletto YMS, Silveira NP, Brandelli A (2011) Nanovesicle encapsulation of antimicrobial peptide P34: physicochemical characterization and mode of action on Listeria monocytogenes. J Nanoparticle Res 13:3545–3552
Malheiros PS, Sant’Anna V, Barbosa MS, Brandelli A, Franco BDGM (2012) Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int J Food Microbiol 156:272–277
Mayr-Harting A, Hedjes AJ, Berkeley CW (1972) Methods for studying bacteriocins. In: Norris JB, Ribbons D (eds) Methods in microbiology, vol 7. Academic Press, New York, pp 315–412
Mertins O, Sebben M, Schneider PH, Pohlmann AR, Silveira NP (2008) Characterization of soybean phosphatidylcholine purity by 1H and 31P NMR. Quim Nova 3:1856–1859
Motta AS, Brandelli A (2002) Characterization of an antimicrobial peptide produced by Brevibacterium linens. J Appl Microbiol 92:63–70
Motta AS, Flores FS, Souto AA, Brandelli A (2008) Antibacterial activity of a bacteriocin-like substance produced by Bacilus sp. P34 that targets the bacterial cell envelope. Antonie Van Leeuwenhoek 93:275–284
Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C (2008) Nanoliposomes and their applications in food nanotechnology. J Lipos Res 18:309–327
Nes IF, Diep DB, Havarstein LS, Brurberg MB, Eijsink V, Holo H (1996) Biosynthesis of bacteriocins in lactic acid bacteria. Anton van Leeuw 70:113–128
Papagianni M (2003) Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv 21:465–499
Sant’Anna V, Malheiros PS, Brandelli A (2011) Liposome-encapsulation protects bacteriocin-like substance P34 against inhibition by Maillard reaction products. Food Res Int 44:326–330
Settanni L, Corsetti A (2008) Application of bacteriocins in vegetable food preservation. Int J Food Microbio 121:123–138
Sobrino-Lópes A, Martín-Belloso O (2008) Use of nisin e other bacteriocins for preservation of dairy products. Int Dairy J 18:329–343
Sze A, Erickson D, Ren L, Li D (2003) Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J Colloid Interface Sci 261:402–410
Taylor TM, Gaysinski S, Davidson PM, Bruce BD, Weiss J (2007) Characterization of antimicrobial-bearing liposomes by zeta-potential, vesicle-size and encapsulation efficiency. Food Biophys 2:1–9
Taylor TM, Bruce BD, Weiss J (2008) Listeria monocytogenes and Escherichia coli O157:H7 inhibition in vitro by liposomes-encapsulated nisin and ethylene diaminetetraacetic acid. J Food Saf 28:183–197
Teixeira ML, Santos J, Silveira NP, Brandelli A (2008) Phospholipid nanovesicles containing a bacteriocin-like substance for control of Listeria monocytogenes. Inn Food Sci Emerg Technol 9:49–53
Xiao D, Davidson PM, Zhong O (2011) Spray-dried zein capsules with coencapsulated nisin and thymol as antimicrobial delivery system for enhanced antilisterial properties. J Agric Food Chem 59(13):7393–7404
Weiss J, Takhistov P, McClements L (2006) Functional materials in food nanotechnology. J Food Sci 71:R107–R116
Were LM, Bruce BD, Davidson PM, Weiss J (2003) Size, stability, and entrapment efficiency of phospholipids nanocapsules containing polypeptide antimicrobials. J Agric Food Chem 51:8073–8079
Were LM, Bruce B, Davidson PM, Weiss J (2004) Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J Food Prot 67:922–927
Acknowledgments
Authors thank to Dr. Sylvia Stanisçuaski Guterres from UFRGS, for the use of the Zetasizer equipment and to Center of Electronic Microscopy (UFRGS) for technical support. This research was financially supported by CNPq, Brazil. We declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Mello, M.B., da Silva Malheiros, P., Brandelli, A. et al. Characterization and Antilisterial Effect of Phosphatidylcholine Nanovesicles Containing the Antimicrobial Peptide Pediocin. Probiotics & Antimicro. Prot. 5, 43–50 (2013). https://doi.org/10.1007/s12602-013-9125-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-013-9125-3