Abstract
Pomegranate juice was concentrated by conventional heating and microwave heating at different operational pressures (12, 38.5, and 100 kPa), and their effects on evaporation rate and quality attributes of concentrated juice were investigated. The final juice concentration of 40° Brix was achieved in 140, 127, and 109 min at 100, 38.5, and 12 kPa, respectively, by using conventional heating. Applying microwave energy decreased required times to 118, 95, and 75 min. The changes in color, anthocyanin content, and antioxidant capacity during concentration processes were investigated. L*, a*, and b* parameters were measured to estimate the intensity of color loss. All Hunter color parameters decreased with time. Results showed that the degradation of color, anthocyanins, and antioxidant activity were more important in conventional heating compared to microwave heating method. Degradation rates increases by increasing process pressure. A first-order kinetics model was applied to modeling changes in total solid content, anthocyanin content, and antioxidant capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pomegranate fruit (Punica granatum L.) is a popular edible fruit native to Iran. The total production of pomegranate in Iran was about 800,000 t in 2006, the majority of which is converted to juice and juice concentrate (Anonymous 2006). Epidemiological studies show that the consumption of fruits and vegetables with high phenolic content is correlated with reduced cardio diseases and cancer mortality (Hertog et al. 1997; Sumner et al. 2005). Thus, red fruit juices such as those of grapes and different berries have received attention due to their high anthocyanin content and antioxidant activity. The juice of the pomegranate has been found to be effective in reducing heart disease risk factors, including LDL oxidation (Sumner, et al. 2005).
Anthocyanins are the water-soluble pigments in plants that contribute to the brilliant blue, red, and purple colors of leaves, flowers, and fruits. Anthocyanins represent a class of important antioxidants, and they are common in human foods. Strawberry and pomegranate juices contain 55.7 mg/L (Torregiani et al. 1999) and 250.87 mg/L of anthocyanins, respectively (Perez-Vicente et al. 2004); thus, the anthocyanin content of pomegranate is relatively high (Mousavinejad et al. 2009).
Pomegranate is a tropical and seasonal fruit, and its production occurs during August and September. Therefore, many processes such as cold storage, concentration, reducing to paste, or drying are used to conserve pomegranates or their juice. Fruits and related juices contain high amounts of water (75–90%), which makes them susceptible to enzymatic and microbial deterioration reactions due to the high enzymatic and microbial activities in water. Thus, extracted juice is concentrated for long-term storage and easier transportation. Concentration is a common step in fruit juice processing. During this process, the majority of water or volatile solvent could be separated by boiling the juice, obtaining a concentrated solution. Due to the significant effects of color, aroma, and flavor on the quality of the final products, the concentration of fruit juices is an important operations unit in fruit processing. The commercial pressing of pomegranate into juice and its concentration facilitate its handling and storage, enabling its application in the food industry (Maskan 2006).
Color is the most important quality attribute of food as well fruit juices. Complex mixtures of water-soluble anthocyanins, including 3,5-diglucoside of cyanidin, pelargonidin, delphinidin, and maldivian, are the main determining factors in pomegranate juice color (Mousavinejad et al. 2009). On the other hand, the main quality judgment made by the consumer at the point of sale of food is optical properties (Mujumdar 2000). Color deterioration reactions such as nonenzymatic browning reactions, pigment destruction, and polymerization could change the visual properties of concentrated fruit juices during thermal processing (Maskan 2006). Color is an important sensory property that plays a role in determining product quality; therefore, minimizing pigment losses during processing is of primary concern to the processor (Markakis 1982; Bridle and Timberlake 1997).
Other techniques used as alternative methods for concentrating fruit juices are membrane concentration, ultrafiltration, nanofiltration, and reverse osmosis. Water can be removed without heating during these processes, but they are restricted from use as standard concentration methods of fruit juice products due to inherent problems including higher cost and other operational limitations in obtaining high solid contents.
Compared with conventional heating, microwave heating has the following advantage: the heating rate can be greatly increased while largely maintaining the nutritional value, color, and original flavor. There are many examples in the literature of microwave application in the food industry, especially for dehydration, blanching, etc. (Alvarez and Dorantes 2005; Vadivambal and Jayas 2010). However, microwave heating has been used by several researchers for thermal concentration due to its benefits, including rapid and volumetric heating (Erle 2005; Krokida and Maroulis 1999). Maskan (2006) showed that this method could be used to heat juices more rapidly and uniformly, which is necessary for reducing damage to color and other quality attributes. However, the effect of heating on the nutritional value of pomegranate juice such as its antioxidant activity and polyphenolic compounds was not investigated. The main objective of this study is to investigate the effect of different heating methods, including conventional heating and microwave heating at different operational pressures, on the evaporation rate and quality attributes of pomegranate juice during concentration.
Materials and Methods
Sample Preparation
Pomegranates (cv. Malas) at the same level of maturation were purchased from a local market in Saveh, Iran. Then, they were washed in cold tap water to remove dirt and foreign materials. Only sweet, mature fruits were selected for these experiments (16° Brix and acidity 1.36 g acid citric/100 mL). The juice was extracted manually and stored at 4 °C overnight to settle the suspended particles. Finally, fresh juice was clarified using a spiral ultrafiltration system with a molecular weight cutoff equal to 40 kDa (Polyethersulphone, Osmonic, USA). The membrane area was 2.5 m2, and transmembrane pressure was kept at 150 psi during ultrafiltration. The cold sterile single-strength clarified juice with 14.2% of total soluble solid (TSS) was frozen at −25 °C and used for further experiments.
Juice Concentration
Clarified juice was concentrated using two different thermal methods from an initial concentration of 14.2º Brix to a final concentration of 40º Brix as follows:
-
1.
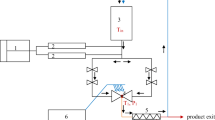
Microwave heating: a programmable domestic microwave oven (Butane MR-1, Iran, with a maximum output of 900 W at 2,450 MHz) was modified for microwave evaporation and used in its intermittent mode. A hermetic jar (V = 1,000 mL) containing 600 mL of juice sample was placed at the center of the microwave, which was connected to a vacuum pump (Robinair, USA), and microwave energy was applied. Some undesirable results such as foaming and sample charring were observed with power above 300 W, so the study was carried out at 300 W (Fig. 1).
As shown in Fig. 1, a digital thermometer with a very thin probe (thermometer: EBRO TFI500 −60 °C to 760 °C, with a probe: TESTO 0602–0593 NiCrNi, d = 1.5 mm, L = 300 mm) was placed in the heating system. The probe of thermometer was immerging in the solution at the center of a jar which was completely sealed. While sampling, pump 5 is on, valve 9 open, and 10 were closed. When the pump is on, sample flows to the sampling point. To return the sample to the jar, the vacuum causes the sample suction to the evaporation jar. So, the pump was off and valves (9 and 10) opened carefully. The pressure applied by pump was kept constant using a vacuum controller device (Vacuu brand, CVC 2111, England).
Operational parameters such as microwave power and pressure were controlled using a PC, and the temperature was recorded periodically. Samples (3 mL) were taken periodically for necessary measurements. Microwave heating was conducted at three different operational pressures: 100 (atmospheric), 38.5, and 12 kPa, and their effects were investigated.
-
2.
Conventional heating: A rotary vacuum evaporator (Heidolph, Heizbad HB Contr, Germany) was used for the 38.5- and 12-kPa pressures, and atmospheric evaporation. Soy bean oil was used as operative liquid (because of its high boiling temperature, 120 °C). Sampling was carried out by the same method as for microwave heating, and data were recorded during concentration without any interruption in the process.
Analytical Methods
Total Soluble Solid Content Measurement
The total soluble solid (TSS) content of concentrated juice was determined through evaporation processes using an Abbe refractometer (Atago Rx-7000a, Tokyo, Japan) at 20 °C and expressed in degrees Brix.
Color Measurement
Sample color was measured before concentrating and at pre-specified time intervals during this period by a spectrophotometer (HACH, DR 4000U, USA). The difference between the color parameters of concentrated and fresh pomegranate juice was calculated. At least five measurements were performed for each sample, and the measured values (mean values) were calculated. The L, a, and b values of the measurements are reported, where L represents the light–dark spectrum with a range from 0 (black) to 100 (white), a represents the green–red spectrum with a range from −60 (green) to +60 (red), and b represents the blue–yellow spectrum with a range from −60 (blue) to +60 (yellow).
The three measured color parameters were converted to browning index (BI). BI represents the purity of the brown color and is considered an important parameter associated with browning (Askari et al. 2008). For this purpose, the CIELAB parameter were converted to Hunterlab ones by CIE degree standard observer and D65 illuminant system (Anonymous 2008).
Total Anthocyanin Content
Sample preparation was conducted as described above in the case of color measurement. The total anthocyanin content of pomegranate juice was determined by a pH differential method using two buffer systems: potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M) (Lako et al. 2007; Cam et al. 2009). Briefly, 1 mL of pomegranate juice sample was mixed with 24 mL of the appropriate buffer and read against a water blank at 510 and 700 nm. Absorbance (A) was calculated as:
The total anthocyanin content of the samples (milligrams of cyanidin-3-glucoside per 100 mL of pomegranate juice) was calculated by the following equation:
where A is the absorbance; MW, molecular weight (449.2); DF, dilution factor (25); and MA, molar extinction coefficient of cyanidin-3-glucoside (26,900) (Cam et al 2009).
The visible spectra of samples were determined by scanning the absorbance between 350 and 700 nm using a Cecil CE 2502 UV–vis spectrophotometer (Cecil Ins., England). Quartz cuvettes with a 1-cm path length were used, and all measurements were carried out at room temperature (∼22 °C). Absorbance readings were made against distilled water as a blank.
Antioxidant Activity Determination
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Fluka (Buchs, Switzerland), and 2.5 mg of DPPH was dissolved in 100 mL methanol (≈0.0625 mmol L−1). This stock solution was prepared daily and kept in the dark at ambient temperature when not used. EC50 value is defined as the amount of sample necessary to decrease the initial DPPH˙concentration by 50%. EC50 was expressed as volume of sample (milliliter) to the amount of DPPH (grams).
EC50 was determined according to the method of Cam et al. 2009. Of appropriately diluted pomegranate juice samples, 0.1 mL was mixed with 3.9 mL of abovementioned DPPH solution. The control sample was prepared with the same volume of methanol. Absorbance at 515 nm was measured at different time intervals using a Cecil CE 2502 UV–vis spectrophotometer (Cecil Ins., England) until the reaction reached steady-state condition. The DPPH concentration in the reaction medium was calculated using calibration equation. The remaining amount of DPPH (percentage) at steady-state condition was calculated as follows:
where [DPPH′]of control sample and [DPPH′]t are the initial concentration of DPPH and the DPPH concentration at steady-state condition, respectively. The amount of remaining DPPH at steady state was plotted against the sample concentration to obtain.
Statistical Analysis
All experiments were conducted in triplicate, and an analysis of variance was performed. The least significant difference at p < 0.05 was calculated using Duncan’s Multiple Range Test to determine the significant differences in results using Minitab software (Minitab 15; Minitab Inc., Minneapolis, USA). The data were expressed as mean ± SD.
Results and Discussions
Effect of Operational Pressure on Concentration Rate
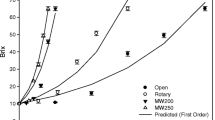
Figure 2 illustrates the total soluble solid (TSS) concentrations of pomegranate juice versus time using two evaporation methods under different operational pressures. These results show that the elapsed time to reach the final concentration (42° Brix) is shorter for the samples processed at lower operational pressures.
The changes in the soluble solid concentration of the samples followed a first-order reaction model (Table 1, Fig. 3). The first-order concentration rate constant (k) was calculated by the following equation:
where C 0 is the initial soluble solid content, and C t is the soluble solid content after t minutes of concentrating at a given pressure (or temperature).
The desired level of concentration was obtained using a conventional heating after 140, 127, and 109 min at 100, 38.5, and 12 kPa, respectively. This result shows that the operational time can be reduced depending on governing pressure. In the case of microwave heating, the required times were 118, 95, and 75 min, respectively, for the three operational pressures. Similar results were found in the literature (Maskan 2006). The larger elapsed time and the smaller effect of pressure in the case of conventional heating could be related to the common rotary evaporator procedure used here. The difference between the boiling point of processed samples and the operative liquid (oil) could not be more than 20 °C; consequently, it would induce significant differences in heat transfer at different temperatures.
The temperature changes of juices during evaporation are shown in Fig. 4. As can be seen, the temperature of juices increased during concentration. This can be explained by an increase in the soluble solid concentration of juices, which increases the boiling point of liquids. However, the boiling point depends on the heating method as the boiling points of microwave-heated products are evidently higher than conventionally heated ones. This is due to the superheating phenomenon that takes place during microwave heating of some kinds of liquids such as water.
The phenomenon is more evident towards the end of the evaporation processes when there is a higher soluble solid concentration and lower water content. This reduces the specific heat of the products, and the temperature of the concentrated juice undergoes a greater change.
Effect of Pressure and Concentration Technique on Quality Attributes
Total Anthocyanin Content
The stability of anthocyanins during thermal processing has been well documented by several researchers (Scalzo et al. 2008; Kirka et al. 2007; Wang and Xu 2007). The anthocyanin content of pomegranate juice was calculated to be 0.086 g/kg (fresh weight), expressed in cyanidin-3-glucoside. Thermal degradation of the anthocyanins from the pomegranate juice followed first-order reaction kinetics at 12, 38.5, and 100 kPa (Table 2 and Fig. 5), and the degradation of the anthocyanins over the whole pressure range is also well described using the Eq. 6. However, in this case, C is referred to anthocyanin content.
These results are in agreement with those from previous investigations, which reported a first-order reaction model for the degradation of monomeric anthocyanins from the different products (Culpepper and Caldwell 1927; Kirka et al. 2007; Yang et al. 2008). There is an obvious difference between the degradation rates of the anthocyanins depending on the heating method (Table 2).
As shown in Table 2, the degradation rate of the anthocyanins is affected by temperature, pressure, and concentration, which is a function of process time. Roughly, a 5% decrease in anthocyanin content occurs during concentration, as previously reported (Wang and Xu 2007). Since the anthocyanin content was not reduced to 50% during this experiment, the t 0.5 (the time required for the degradation of 50% of the total anthocyanins, which is usually used for the definition of anthocyanin loss) could not be calculated for the samples during concentration, except for the samples processed at atmospheric pressure using the conventional method. The different heating methods lead to different results for anthocyanin degradation, as observed in Table 2. The degradation rate of anthocyanins in a microwave is smaller than for conventional heating method. The constants (k) of anthocyanin degradation rate in microwave heating were calculated as 0.02, 0.06, and 0.09 (for 12, 38.5, and 100 kPa, respectively), which are smaller compared to those obtained for conventional heating (Table 2); these results are in agreement with those reported by Scalzo et al. (2008).
Color Parameters
The changes in the color values (L*, a*, and b*) were measured during thermal concentration for both the microwave and conventional methods. There were obvious changes in the L* and a* values, confirming the degradation of visual color components of the pomegranate juices. The value of L* decreased in all cases during concentration, but the final values were affected by the operational pressure, especially in the case of conventional heating (Fig. 6). In that case, the higher pressure leads to a longer process time and lower L* values. Concentration of pomegranate juices using microwave power leads to a decrease in the L* value, but there is an obvious difference between the samples produced using the different heating methods. The final L* values of the samples concentrated using conventional heating were about 22, 18, and 10 for 12, 38.5, and 100 kPa, respectively. However, in the case of microwave heating, the final values were the same, about 21 for all cases. This indicates that the color changes were affected by other factors more than by the increase of soluble solids. Short process time in microwave heating led to a lower decrease in the lightness of the concentrated samples.
Figure 7 shows the changes in the value during the concentration process of pomegranate juices by the conventional and microwave methods. At atmospheric pressure, this parameter decreased with time for both methods. At lower pressure, a different trend over time was observed. The a* value increased in the initial stages due to the concentration of pigments. At higher concentrations, the pigment degradation was dominant, and the a* value decreased. This phenomenon was more obvious in the case of microwave concentration. Therefore, the final a* values at lower pressure were higher than that of fresh pomegranate juice (Fig. 7). This indicates that microwave heating, especially at lower pressure, could preserve the visual characteristics of concentrated juices.
As a result of changes in the color parameters, the BI was changed during the process. Two different behaviors were observed: small increases in the BI were observed at lower concentrations (TSS <30%), and then after this point, the BI increased rapidly and reached its final value. This indicates that a browning reaction such as Maillard or caramelization reaction is enhanced at lower moisture content and higher temperatures. This effect is more evident for the conventional heating method. The final BI values obtained by the conventional method were higher than those from microwave heating (Fig. 8). Figure 8 demonstrates that the extent of color degradation increases with soluble solid concentration. There have been some reports of the effects of sugar and its degradation products, including the acceleration of anthocyanin breakdown and enhancement of nonenzymatic browning reactions during thermal concentration (Cemeroglu et al. 1994; Suh et al. 2003; Bchir et al. 2010).
Antioxidant Activity
Antioxidant activity of concentrated samples is expressed as EC50 values (lower values indicate more powerful antioxidant capacity) in Fig. 9a, b. As Fig. 9 shows, applying higher pressures led to the degradation of antioxidant capacity of pomegranate juice for both conventional and microwave heating methods. Investigation on the effect of heating method on the degradation of antioxidant activity showed that applying microwave instead of conventional heating method could conserve better the antioxidant activity of pomegranate juice. Results obtained from different heating procedures were fitted using following equation (Eq. 6). However, C is corresponding to antioxidant activity.
As shown in Table 3, the antioxidant capacity of pomegranate decreased with pressure and temperature. The different heating methods lead to different results for the degradation of antioxidant capacity of pomegranate juice, as observed in Table 3. The degradation rate in a microwave is smaller than for conventional heating method, which is in agreement with results obtained for the degradation of anthocyanins in this study. Similar to anthocyanin degradation, the degradation rate of the antioxidant capacity is affected by pressure as well as heating method, which is a function of process time. Increasing the pressure of process from 12 to 100 kPa increased the degradation rate by 41% when conventional method was applied. However, this phenomenon is more conspicuous in the case of microwave heating. The rise of pressure from 12 to 100 kPa caused an increase in the degradation rate of antioxidant activity about 60%.
Conclusion
The effect of microwave and conventional rotary heating on evaporation rate, optical properties, anthocyanin content, and antioxidant activity of pomegranate juice was investigated. Results showed that the required time to obtain a designed concentration was affected by operational pressure as well as heating method. The lowest time was obtained when microwave heating at low pressure was applied. The obtained results showed that the anthocyanin content was increased when lower operational pressures (or lower temperatures) and microwave heating were used. Antioxidant capacity of the samples was ruled by anthocyanin content, so a similar behavior was observed when different pressures and heating techniques were applied. Results also showed that the degradation rate of anthocyanin (and consequently antioxidant capacity) of juice was more dependent on process time than temperature (or pressure). The more rapid the process, the better preserved antioxidant capacity. Investigation of the visual parameters of the samples showed that the lightness was decreased in all cases, but this effect was more obvious in conventional heating. On the other hand, the redness of the samples was well preserved under microwave heating and lower operational pressure. The BI of the samples was increased during all treatments, but applying conventional heating increased the BI more rapidly due to the eventual browning reactions and solid concentration during process.
References
Alvarez, L. D., & Dorantes, L. P. (2005). Blanching using microwave processing. In H. Schubert & M. Regier (Eds.), Microwave processing of foods. Cambridge: Woodhead Publishing Ltd.
Anonymous. (2006). Statistical book of agricultural of Iran. Tehran: Iranian Statistical Centre.
Anonymous. (2008). Hunter Lab versus CIE 1976 L*A*B*. In The basics of color perception and measurement (pp. 70–71). Road Reston: Hunter Associate Laboratory Inc.
Askari, G. R., Emam-Djomeh, Z., & Mousavi, S. M. (2008). Investigation of the effects of microwave treatment on the optical properties of apple slices during drying. Drying Technology, 26, 1362–1368.
Bchir, B., Besbes, S., Karoui, R., Attia, H., Pagout, M., & Blecker, C. (2010). Effect of air-drying conditions on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food and Bioprocess Technology. doi:10.1007/s11947-010-0469-3.
Bridle, P., & Timberlake, C. F. (1997). Anthocyanins as natural food colours—selected aspects. Food Chemistry, 58(1–2), 103–109.
Cam, M., Hisil, Y., & Durmaz, G. (2009). Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chemistry, 112, 721–726.
Cemeroglu, B., Velioglu, S., & Isik, S. (1994). Degradation kinetics of anthocyanins in sour cherry juice and concentrate. Journal of Food Science, 59, 1216–1218.
Culpepper, C. W., & Caldwell, J. S. (1927). The behavior of the anthocyanin pigments in canning. Journal of Agricultural Research, 35(2), 107–132.
Erle, U. (2005). Drying using microwave processing. In H. Schubert & M. Regier (Eds.), Microwave processing of food (pp. 142–151). Cambridge: Woodhead Publications.
Hertog, M. G. L., Sweetnam, P. M., Fehily, A. M., Elwood, P. C., & Kromhout, D. (1997). Antioxidant flavonols and ischaemic heart disease in a welsh population of men. The caerphilly study. The American Journal of Clinical Nutrition, 65, 1489–1494.
Kirka, A., Ozkan, M., & Cemeroglu, B. (2007). Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chemistry, 101, 212–218.
krokida, M. K., & Maroulis, Z. B. (1999). Effect of microwave drying on some quality properties of dehydrated products. Drying Technology, 17(3), 449–466.
Lako, J., Trenerry, V. C., Wahlqvist, M., Wattanapenpaiboon, N., Sotheeswaran, S., & Premier, R. (2007). Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chemistry, 101, 1727–1741.
Markakis, P. (1982). Anthocyanins as food additives. In P. Markakis (Ed.), Anthocyanins as food colors (pp. 245–253). New York: Academic.
Maskan, M. (2006). Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: colour degradation and kinetics. Journal of Food Engineering, 72, 218–224.
Mousavinejad, G., Emam-Djomeh, Z., Rezaei, K., & Haddad Khodaparast, M. H. (2009). Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chemistry, 115, 1274–1278.
Mujumdar, A. S. (2000). Drying technology in agricultural and food science (pp. 61–98). Plymouth: Science Publishers, Inc. pp. 253–286.
Perez-Vicente, A., Serreno, P., Abellan, P., & Garcia-Viguera, C. (2004). Influence of packaging material on pomegranate juice colour and bioactive compounds, during storage. Journal of the Science of Food and Agriculture, 84, 639–644.
Scalzo, R., Genna, A., Branca, F., Chedin, M., & Chassaigne, H. (2008). Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chemistry, 107, 136–144.
Suh, H. J., Noh, D. O., Kang, C. S., Kim, J. M., & Lee, S. W. (2003). Thermal kinetics of color degradation of mulberry fruit extract. Die Nahrung, 47, 132–135.
Sumner, M. D., Elliot-Eller, M., Weidner, G., Daubenmier, J. J., Chew, M. H., Marlin, R., et al. (2005). Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. The American Journal of Cardiology, 96, 810–814.
Torregiani, D., Forni, E., Guercilena, I., Maestrelli, A., Bertolo, G., Archer, G. P., et al. (1999). Modification of glass transition temperature through carbohydrates additions: effect upon colour and anthocyanin pigment stability in frozen strawberry juices. Food Research International, 32, 441–446.
Vadivambal, R., & Jayas, D. S. (2010). Non-uniform temperature distribution during microwave heating of food materials—a review. Food and Bioprocess Technology, 3, 161–171.
Wang, W. D., & Xu, S. H. Y. (2007). Degradation kinetics of anthocyanins in blackberry juice and concentrate. Journal of Food Engineering, 82, 271–275.
Yang, Z., Zhenxin Gu, Y., Fan, G., & Chen, Z. (2008). Thermal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.) cob. Innovative Food Science & Emerging Technologies, 9, 341–347.
Acknowledgment
This research was founded by the Vice-Chancellor for Research, University of Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Practical Applications
Pomegranate fruit (Punica granatum L.) is a popular edible fruit native to Iran. The edible parts of pomegranate fruits can be consumed fresh or used for the preparation of fresh juice, canned beverages, jelly, jam, and paste and also for flavoring and coloring beverage products. Due to their seasonal production, many processes such as cold storage, concentration, reducing to paste, or drying are used to conserve pomegranates or their juice. Conventional thermal processing seems to cause significant damages to the quality attributes and nutritional values of pomegranate juice. Compared with conventional heating, microwave heating has the following advantage such as better appearance and higher nutritional value.
Rights and permissions
About this article
Cite this article
Yousefi, S., Emam-Djomeh, Z., Mousavi, S.M.A. et al. Comparing the Effects of Microwave and Conventional Heating Methods on the Evaporation Rate and Quality Attributes of Pomegranate (Punica granatum L.) Juice Concentrate. Food Bioprocess Technol 5, 1328–1339 (2012). https://doi.org/10.1007/s11947-011-0603-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0603-x