Abstract
Purpose of review
Aspirin is one of the only proven therapeutic options for the prevention of preeclampsia, an important adverse pregnancy outcome with detrimental short- and long-term consequences to a woman’s health. The goal of this review is to provide information about the current recommendations for the use of aspirin to prevent preeclampsia and whether there is evidence for postpartum continuation.
Recent findings
Preeclampsia is linked to the development of future cardiovascular disease and adverse outcomes in women including stroke, ischemic heart disease, and heart failure. This is likely due to vascular dysfunction and inflammation as their shared pathophysiology. By decreasing vasoconstriction, aspirin targets these pathways, inhibiting cyclooxygenase-1 activity and thereby the synthesis of thromboxane A2. Low-dose aspirin use during pregnancy has been shown to decrease the frequency of preeclampsia and other adverse pregnancy outcomes such as fetal growth restriction and preterm birth.
Summary
Since adverse pregnancy outcomes and preeclampsia in particular significantly increase the risk for future cardiovascular disease, low-dose aspirin could have the potential to decrease onset and severity of adverse cardiac outcomes in young women. Improving cardiovascular indicators in reproductive-aged women, a demographic that unlike other populations (older, male) has experienced recent substantial increases in cardiovascular disease, has important public health implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women with a history of preeclampsia (PEC) have approximately double the risk of developing ischemic heart disease, venous thromboembolism, and stroke in the 5 to 15 years following pregnancy compared to women without PEC [1]. One of several adverse pregnancy outcomes (APOs), PEC is a disease that affects up to 8% of pregnancies and is a leading cause of maternal and fetal morbidity and mortality, with even higher rates and degrees of severity in African American women [2]. Women with PEC present with new-onset hypertension (systolic blood pressure of 140 mmHg or more or diastolic blood pressure of 90 mmHg or more on two occasions at least 4 h apart) after 20 weeks gestation and some combination of proteinuria and symptoms such as epigastric pain, right upper quadrant pain, visual disturbances, persistent headache, and renal, hepatic, and platelet lab abnormalities. PEC also frequently complicates the pregnancies of women with chronic hypertension. In the postpartum period, the risk for severe maternal complications from PEC including seizures, hepatic and renal dysfunction, pulmonary edema, stroke, myocardial infarction (MI), and coagulopathies persists. In 2011, the American Heart Association (AHA) recognized PEC as a risk factor for cardiovascular disease (CVD) and stroke [3]. Approaches to the prevention of CVD in women with a history of PEC are needed.
Recent studies clearly demonstrate the value of low-dose aspirin (LDA) during pregnancy to prevent or delay the onset of PEC, and aspirin is a mainstay of CVD prevention in adults. While systematic reviews and consensus statements have used doses ranging from 75 to 162 mg of aspirin daily, recommendations from the American College of Obstetrics and Gynecology (ACOG) for LDA use in pregnancy for the prevention of preeclampsia specify a dose of 81 mg/day, the only low dose preparation available in the USA [4]. Because both PEC and CVD share many common risk factors, aspirin may also be of benefit for CVD risk reduction for women with a history of PEC. Aspirin, a cyclooxygenase (COX) inhibitor, targets the proposed physiologic mechanisms of PEC-enhanced sensitivity to vasopressors, inflammation, and an imbalance in the production of vasoactive prostaglandins (TXA2 and prostacyclin). In terms of CVD prevention, aspirin’s acetylation of COX in platelets results in the key antithrombotic effect, thereby suppressing platelet aggregation without affecting important endothelial cell function.
It is clear that PEC can persist or worsen after delivery but there is presently no data supporting use of LDA postpartum either for treatment of PEC or CVD prevention. The continuation or initiation of PEC postpartum suggests that the underlying processes, including vascular dysfunction, persist in some women despite delivery of the placenta. Therefore, the postpartum continuation of LDA could mitigate the severity of disease, and blunt or prevent the changes associated with long-term CVD risk. However, the benefit of continuing LDA postpartum to minimize PEC or future CVD has not been assessed [5, 6]. Studies illustrate the value of aspirin for secondary prevention of CVD, but its use for primary prevention is controversial due to risk of gastrointestinal and, more rarely, intracranial bleeding. Nevertheless, three meta-analyses have demonstrated aspirin’s benefit of significantly decreasing risk of serious cardiovascular events, including non-fatal MI, stroke, or cardiovascular death [7,8,9] . In consideration of the aforementioned risks and benefits, several agencies, including the AHA, the American College of Chest Physicians, and the US Preventative Services Task Force (USPSTF), have concluded that aspirin is beneficial and safe for primary CVD prevention when used properly [10]. The USPSTF recommends initiating low-dose aspirin use for the primary prevention of CVD in adults aged 50 to 59 years who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years [11]. At present, we lack a targeted strategy to identify those individuals in whom LDA could potentially provide a mechanistic benefit to mitigate endothelial dysfunction and later-life complications including CVD in women with a history of PEC.
Preeclampsia is a vascular disease
Preeclampsia (PEC), a multisystem pregnancy-specific hypertensive disorder, affects 5–8% of pregnancies and is a leading cause of maternal morbidity and mortality accounting for approximately 10% of maternal deaths [12]. A less well-appreciated impact of PEC on women’s health is its association with later-life maternal vascular disease. A recent umbrella review in the British Medical Journal found significant associations between a history of PEC and increased risk of mortality and morbidity from composite CVD compared to those without a history of PEC [13••, 14]. Table 1 demonstrates effect sizes for cardiovascular outcomes associated with a history of PEC and recurrent PEC [13]. For CVD subtypes, PEC history was associated with increased likelihood of experiencing heart failure, fatal ischemic heart disease, and non-fatal stroke [15]. Furthermore, a history of recurrent PEC was associated with an even greater increase of composite CVD, coronary heart disease, heart failure, and cerebrovascular accident in comparison with an episode of PEC followed by a healthy pregnancy [16]. In an older meta-analysis with 198,252 preeclamptic women, it was concluded that in comparison to women with normotensive pregnancies, women with PEC have a 3.7-fold (95% CI, 2.70–5.05) relative risk for developing hypertension 14 years after pregnancy, a 2.16-fold (95% CI, 1.86–2.52) relative risk for ischemic heart disease after 12 years, and a 1.81-fold (95% CI, 1.45–2.27) relative risk of stroke after 10 years [1]. In addition, the severity of PEC is correlated with the severity of CVD later in life [17]. These data led the AHA to include PEC as a clinical risk factor for CV screening in 2011 [3, 18].
Shared pathophysiology between preeclampsia and cardiovascular disease
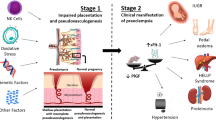
The pathophysiology and trajectory of post-PEC vascular disease may differ from classic ischemic disease, as endo/microvascular dysfunction may play a more central role than atherosclerosis and plaque rupture. This suggests that identification and preventative treatments may need to differ from those traditionally used for primary CVD prevention. In preeclampsia, the most probable initiating event is placental ischemia which leads to the release of factors that cause maternal vascular endothelial dysfunction [19]. Endothelial dysfunction results in generalized vasoconstriction and reduced blood to multiple organs leading to the systemic PEC phenotype [20,21,22,23] Although PEC is unique to human pregnancy, it shares biological and pathological similarities as well as many risk factors with CVD (Fig. 1) [25,26,27]. Endothelial dysfunction and inflammation are fundamental mechanisms for the initiation and progression of both atherosclerosis and PEC [27,28,29]. PEC is considered by many as either an early manifestation of CVD unmasked by the challenges and characteristics of pregnancy or, as described above, a risk factor for future CVD.
Cardiovascular disease in women and association with preeclampsia
Cardiovascular disease (CVD) is the leading cause of mortality for women and a significant contributor to morbidity as well [30]. For the past three decades, dramatic declines in heart disease mortality for both men and women have been observed. Recent data suggests stagnation in the improvements in incidence and mortality of coronary heart disease (CHD), specifically among younger women (< 55 years), making it critical that we understand the contributing factors as they may be modifiable [31]. CVD prediction models using traditional risk factors are less applicable in young adults [32] [33]. In the USA, the proportion of hospital admissions for acute MI for adults less than 55 years of age increased from 27 to 32% between the periods of 1995–1999 and 2010–2014, with the greatest change in women aged 35–54 [34]. Between 1996 and 2007 in Western Australia, hospital admissions in adults aged 35–54 for acute MI decreased by 0.2% in men while increasing by 4% in women [35]. On average, as early as 10 years after experiencing an adverse pregnancy outcome (APO)—examples include PEC, gestational diabetes mellitus, placental abruption, low birth weight, and preterm birth—young women could be susceptible to CVD events [36] [1]. Those who develop acute coronary syndromes have worsening indicators compared to men—longer hospital stays after admission, higher readmission rates, and higher mortality [37, 38].
Although many commonalities in CVD risk factors between men and women exist, there are several apparent differences. Traditional risk factors for CVD such as diabetes and smoking affect women disproportionately and may not be adequate to fully explain increased likelihood of CVD in later life [39, 40]. Furthermore, the contribution of female-specific risks such as reproductive risk factors—i.e., APOs and gynecologic/fertility complications such as recurrent pregnancy loss, premature ovarian insufficiency, polycystic ovarian syndrome, early menopause, endometriosis, and pelvic inflammatory disease—remains under recognized [41]. Women with such reproductive risks have often been found to experience early manifestations of vascular changes. At 1 year, women with PEC have six times the readmission risk for acute coronary syndromes, with a tendency to present with a more severe type of MI than women without PEC [42]. Endothelial dysfunction has been specifically linked with preeclampsia and other complications such as recurrent pregnancy loss and could persist beyond the adverse reproductive event, leading to further vascular complications and increased risk for future CVD [43]. Interestingly, biochemical risk factors for CVD including elevated concentrations of cholesterol, glucose, and triglycerides have been shown to remain many years beyond a hypertensive disorder of pregnancy [44].
How does aspirin target these shared pathways?
The World Health Organization estimates that over 75% of premature CVD is preventable, and that early risk factor amelioration is critical [45]. Primary prevention strategies for risk reduction through lifestyle changes/behavior modification, targeted pharmacotherapy with aspirin and statins, and aggressive treatment of hypertension have reduced CV mortality. Given the mechanistic overlap between PEC and CVD, there is biological plausibility for the use of these drugs in the postpartum period to modify ongoing risk. Early identification of increased CV and cerebrovascular risk may afford opportunities for health education, behavior modification, pharmacologic interventions, and tailored longitudinal care which may improve long-term health for mothers and their families.
Treatment: aspirin
Aspirin, endothelial function, and preeclampsia during pregnancy and early postpartum
By inhibiting cyclooxygenase-1 (COX-1) activity and thereby the synthesis of thromboxane A2 (TXA2), aspirin reduces vasoconstriction. Aspirin has also been shown to improve vasodilation through COX-independent mechanisms, such as the inhibition of a non-receptor tyrosine kinase known as proline-rich tyrosine kinase 2 (PKY2) in vascular smooth muscle cells. Exploratory clinical trials have also suggested that LDA (but not all salicylates) improves endothelium-dependent vasodilation by increasing the release of nitric oxide (NO) from the vascular endothelium and stimulating the activity of endothelial nitric oxide synthase (eNOS) [46]. Additionally, aspirin targets enhanced sensitivity to vasopressors, inflammation, and an imbalance in the production of vasoactive prostaglandins (TXA2 and prostacyclin) that leads to the vasoconstriction of small arteries and platelet activation [47,48,49] . The exact mechanisms of risk reduction with aspirin in patients predisposed to PEC are not fully understood but likely relate to its impact on endothelial reactivity.
Several studies have demonstrated the association between endothelial dysfunction and a high incidence of CV events [50]. A significant relationship between endothelial dysfunction and diastolic dysfunction, and hypertension has been previously described [51]. A decrease in the release of nitric oxide (NO) from the vascular and endocardial endothelium is involved in impaired vascular and myocardial relaxation [52]. Disease severity may affect the presence and magnitude of changes in flow-mediated dilation (FMD), a measure of endothelial function. FMD is a non-invasive endothelial function test that predicts CV event risk due to the physiology described above. FMD measures the change in artery diameter in response to reactive hyperemia. Baseline artery diameter and blood flow velocity are measured using duplex ultrasound. An occlusion cuff is inflated to stop blood flow to the lower arm for approximately 5 min. Ischemia in the tissue distal to the cuff causes the distal vessels to dilate, lowering vascular resistance. With release of the occlusion cuff, the reduction in downstream resistance dramatically increases blood flow to the arm. This endothelium responds to the resulting increase in shear stress by releasing vasodilators, including NO, which causes dilation in a healthy artery. In a patient with vascular dysfunction, dilation is reduced or absent and FMD is therefore low [53]. A recent meta-analysis concluded that for every 1% increase in brachial artery FMD, the relative risk of CV events was 0.87 (95% confidence interval 0.83 to 0.91) [54].
Similar to hypertension, systemic inflammation and endothelial activation play a role in PEC, resulting in the impairment of endothelium-dependent dilation. A FMD value ≤ 10% is consistent with the presence of endothelial dysfunction [50]. Recent work has revealed that aspirin induces NO release from vascular endothelium, due to acetylation of eNOS protein, thereby promoting vasodilation [55]. Several studies have shown that aspirin use at various doses by patients in early and advanced stages of hypertension improves vascular function [50].
The existing literature for endothelial function in pregnancy and the postpartum period demonstrates that FMD is lower in preeclamptic patients compared to normotensive women. Although FMD increases in women with PEC at about 4 to 6 weeks postpartum, it remains lower compared to women who were normotensive throughout their pregnancy for up to 3 years postpartum [43, 53, 56,57,58,59,60,61,62,63]. One study that examined women with PEC, including 47% of subjects with severe PEC, found persistence of endothelial dysfunction after 10–20 years [64]. However, there are limited studies in the literature investigating the impact of aspirin on the endothelium, particularly in patients with PEC who are pregnant or postpartum. Since endothelial dysfunction is critically important to the pathophysiology of PEC and linked to future CV risk and stroke in patients affected by this disease, it is necessary to better understand the role of aspirin during the postpartum period for mitigation of adverse outcomes.
Additional adverse pregnancy outcomes (APOs)
In a patient with one or more high risk factors for PEC, the incidence rate of this APO is 8% higher than in a pregnant woman without risk factors [4]. LDA, when given to women with moderate to high risk for PEC, reduces the frequency of PEC as well as other APOs (preterm birth, growth restriction) by approximately 10 to 20%. A meta-analysis from 2019 (encompassing 74 trials and more than 40,000 women, with 9 large trials recruiting more than 1000 women each) reported that LDA led to small-to-moderate benefits, including reductions in PEC (16 fewer per 1000 women treated), preterm birth (16 fewer per 1000 treated), small-for-gestational age newborns (seven fewer per 1000 treated), and fetal or neonatal death (five fewer per 1000 treated). Overall, administering antiplatelet agents to every 1000 women resulted in 20 fewer pregnancies with serious maternal and neonatal adverse outcomes [65].
These observed benefits of LDA use during pregnancy highlight the link between APOs such as PEC, spontaneous preterm birth (sPTB), and fetal growth restriction (FGR) and long-term CV risks, as well as the potential utility of LDA following APOs in addition to PEC. Although separate diagnoses, APOs are a group of interrelated disorders that share clinical features and risk factors with CVD, thereby suggesting a shared pathogenesis similar to PEC, involving inadequate placentation, inflammation, vasospasm, and maternal endothelial dysfunction [66,67,68]. The shallow trophoblast invasion and insufficient uterine spiral artery remodeling in a pregnancy complicated by an APO lead to defective placentation with features of malperfusion, ischemia, and inflammation, as well the production of anti-angiogenic proteins such as sFLt1 (soluble fms-like tyrosine kinase-1) and inflammatory cytokine proteins, which can be picked up in the maternal bloodstream [68, 69].
Due to this possible pathophysiology, women with a history of APOs—not only limited to PEC—have a higher risk of future development of CVD risk factors and overt CVD compared to women without any history of APOs. This includes progression to coronary heart disease, stroke, and heart failure [1, 36, 70, 71] . Just as with PEC, microvascular dysfunction, arterial stiffness, and myocardial dysfunction can emerge during other APOs such as sPTB and FGR and fail to improve postpartum due to continuing CV damage from inflammatory and anti-angiogenic mediators [72••]. This can lead to significant and premature CVD after an APO. Again, whether this is a singular pathway that is causative or unmasks a preexisting vulnerability resulting in CVD remains to be seen. As was discussed in detail with PEC, the use of LDA immediately postpartum and soon after recovery from an APO such as sPTB and FGR could be a potential key intervention in interrupting the progression from APO to CVD, while the disease process is still in its subclinical phase. However, the exact mechanism by which sPTB and FGR lead to CVD is less well understood [73, 74].
Standard dosage
Since pathologic features of PEC develop weeks before early disease is apparent, early therapy (ideally at the end of the first trimester and before 16 weeks) may be important. The evidence regarding initiation prior to 11 weeks is inconsistent. The majority of trials have initiated therapy before 28 weeks, and initiation after 16 weeks may still be effective. In the ASPRE trial, high-risk women were randomly assigned to receive 150 mg aspirin or placebo between 11 and 14 weeks, resulting in a significant reduction in odds of preterm PEC (< 37 weeks) with LDA therapy [5].
The optimal dose of aspirin is controversial, with systematic reviews and consensus statements using different amounts, ranging from 75 to 162 mg LDA. The USPSTF, Society for Maternal-Fetal Medicine (SMFM), and ACOG recommend 81 mg daily, which is commercially available in the USA and has proven efficacy [4, 24]. A higher dose of aspirin can be achieved by using one and a half or two 81 mg tablets. Groups such as the National Institute for Health and Care Excellence and World Health Organization suggest a 75 mg dose, while the Society of Obstetricians and Gynaecologists of Canada and International Federation of Gynecology and Obstetrics recommending 75 to 162 mg and 150 mg doses respectively [75,76,77]. Of note, there has been no head-to-head comparison or meta-analysis comparing doses less than 100 mg versus doses equal to or greater than 100 mg. A meta-analysis in 2017 by Roberge et al. suggested a dose-response effect of aspirin (50–150 mg) in early pregnancy as it relates to prevention of preeclampsia, severe preeclampsia, and FGR, with higher doses associated with greater risk reduction [78]. A subsequent meta-analysis in 2018 by authors of the aforementioned 2017 study involving a subgroup analysis of preterm preeclampsia and term preeclampsia concluded that aspirin at a daily dose of 100 mg or greater when initiated at 16 weeks or earlier reduced risk of preterm preeclampsia but not term preeclampsia [79]. In contrast, the beneficial effect of LDA was found to be consistent, whether treatment was started before or after 16 weeks, in a study by Meher et al. pooling individual data from 31 high-quality RCTs [65].
Contraindications
LDA has an excellent maternal/fetal safety profile in pregnancy with a low likelihood of serious maternal or fetal complications, or both, related to use. The short-term safety of the drug at low dose is well established for use in the second and third trimesters. The maternal and fetal risks brought into question as they relate to aspirin exposure in pregnancy will be not be discussed in this review, as our main focus is on the potential benefits of LDA use in the postpartum period in the setting of APO.
ACOG and SMFM currently recommend against LDA use solely for the indication of a prior unexplained stillbirth, FGR, or spontaneous preterm birth in the absence of risk factors for preeclampsia. Furthermore, LDA prophylaxis should not be used for prevention of early pregnancy loss [4].
Allergy (e.g., urticaria) and individual sensitivity should also be considered as potential causes for serious reactions such as anaphylaxis; therefore, patients with hypersensitivity to NSAIDs and salicylates should avoid aspirin due to significant cross-reactivity. Additionally, patients diagnosed with nasal polyps should not use aspirin, as this could result in life-threatening bronchoconstriction. A history of gastrointestinal bleeding, active peptic ulcers, other sources of bleeding (gastrointestinal or genitourinary), and hepatic dysfunction are considered relative contraindications to LDA.
Main drug interactions
Caution should be exercised and close monitoring should be implemented when using LDA with any drug that enhances its antiplatelet effect as well as agents with antiplatelet or anticoagulant properties. However, theoretically, at a lower dose of aspirin, these concerns regarding potential complications are much less significant. In terms of antihypertensives, salicylates may increase the nephrotoxic effect and decrease the therapeutic effect of angiotensin-converting enzyme inhibitors. Calcium channel blockers may enhance aspirin’s antiplatelet effect. Aspirin may diminish the diuretic effect of loop diuretics and loop diuretics may increase the serum concentration of salicylates.
Main side effects
The majority of systematic reviews of randomized controlled trials (RCTs) did not find an increase in hemorrhagic complications associated with LDA use in pregnancy. The previously mentioned 2019 meta-analysis by Duley et al. demonstrated that LDA may have slightly increased the risk of postpartum hemorrhage greater than 500 mL, but this was not based on high-quality evidence due to a concern for clinical heterogeneity in blood loss measurements [65].
Some advocate for discontinuation of LDA at 36 weeks or 5 to 10 days before expected delivery to decrease risk of bleeding during delivery; however, no adverse effects related to LDA at delivery have been demonstrated. ACOG suggests use of LDA until delivery, which should theoretically safely be able to be continued postpartum unless a complication such as postpartum hemorrhage or severe thrombocytopenia is noted. In the scenario of an obstetric complication that increases risk of bleeding, stabilization and/or resolution should be observed with consideration of use of LDA postpartum on a case-by-case basis. Of note, long-term daily aspirin use in non-pregnant adults (less than 300 mg/day for more than 5 years) has been associated with an increased risk of major gastrointestinal and cerebral bleeding events [80].
Additional side effects are those listed on product labeling, which include gastrointestinal (ulcers, gastritis, gastrointestinal erosion, heartburn, nausea, stomach pain, vomiting), hematologic (anemia, blood coagulation disorder, disseminated intravascular coagulation, hemorrhage, prolonged prothrombin time, thrombocytopenia), and hepatic (reversible hepatitis, hepatoxicity, increased serum transaminases) side effects. These adverse effects are dose related and thus should be rare at the low doses discussed in this article.
Special points
In addition to considering use of LDA postpartum in women with APOs to decrease risk for future CVD, identifying the characteristics of a subgroup of women who are most likely to respond to LDA treatment would improve targeted primary preventative efforts to decrease the development of CVD. This would also provide clearer insight into the known link between PEC, sPTB, FGR, and development of later-life CVD. LDA is affordable and widely available and has the potential to decrease maternal morbidity and mortality during the postpartum period and beyond by slowing down, stopping, or reversing CV dysfunction through structural, functional, and vascular mechanisms, and potentially lowering blood pressure.
Women with a history of APOs have a significantly greater relative risk of later-life CV events and death, as demonstrated by multiple studies worldwide [1, 81, 82]. Our country’s most expensive disease, cardiovascular disease (CVD), is the leading cause of death in women and rates of coronary heart disease are increasing in US women [30, 83, 84]. The possibility for LDA in the postpartum period to reduce vascular dysfunction associated with APOs and thereby severity of future CVD has the potential to change practice as well as impact a very important, wide-ranging, and costly public health issue.
Cost/cost-effectiveness
LDA is widely available and can be purchased relatively inexpensively, over the counter.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. https://doi.org/10.1136/bmj.39335.385301.BE.
Roberts JM, Druzin M, August PA, Gaiser RR, Bakris G, Granger JP, et al. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–31. https://doi.org/10.1097/01.AOG.0000437382.03963.88.
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–62. https://doi.org/10.1161/CIR.0b013e31820faaf8.
ACOG Committee Opinion No. 743. Low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):44–52. https://doi.org/10.1097/AOG.0000000000002708.
Rolnik D, Wright D, Poon L, O’Gorman N, Syngelaki A, et al. Aspirin versus placebo in pregnancies at high risk for preterm pre-eclampsia. N Engl J Med. 2017;377(7):613–22. https://doi.org/10.1056/NEJMoa1704559.
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–8. https://doi.org/10.1016/S0140-6736(07)60712-0.
Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107:1796–801. https://doi.org/10.1016/j.amjcard.2011.02.325.
Berger JS, Lala A, Krantz MJ, Baker GS, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials. Am Heart J. 2011;162:115–24. https://doi.org/10.1016/j.ahj.2011.04.006.
Seshasai SR, Wijesurjya S, Sivakumaran R, Nethercott S, Ergou S, Sattar N, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–16. https://doi.org/10.1001/archinternmed.2011.628.
Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy. 2012;32:1020–35. https://doi.org/10.1002/phar.1127.
Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836–45. https://doi.org/10.7326/M16-0577.
Hasegawa J, Sekizawa A, Tanaka H, Katsuragi S, Osato K, Murakoshi T, et al. Current status of pregnancy-related maternal mortality in Japan: a report from the maternal death exploratory committee in Japan. BMJ Open. 2016;6(3):e010304. https://doi.org/10.1136/bmjopen-2015-010304.
•• Okoth K, Chandan JS, Marshall T, Thomas N, Adderley N, et al. Association between the reproductive health of young women and CVD in later life: umbrella review. BMJ. 2020;371:m3502. https://doi.org/10.1136/bmj.m3502. Recent review summarizing large-scale research on reproductive health problems—gynecologic, fertility, and obstetric—and association with increased cardiovascular disease.
Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–79. https://doi.org/10.1161/CIRCULATIONAHA.118.036748.
Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. https://doi.org/10.1161/CIRCOUTCOMES.116.003497.
Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125:1642–54. https://doi.org/10.1111/1471-0528.15394.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. https://doi.org/10.1016/j.ahj.2008.06.042.
Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women. Stroke. 2014;45(5):1545–88. https://doi.org/10.1161/01.str.0000442009.06663.48.
Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. 2018;19(5):1496. https://doi.org/10.3390/ijms19051496.
Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):1243–9. https://doi.org/10.1161/01.HYP.0000188408.49896.c5.
Granger J, Alexander B, Llinas M, Bennett W, Khalil R. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147–60. https://doi.org/10.1038/sj.mn.7800137.
Noris M, Perico N, Remuzzi G. Mechanisms of disease: pre-eclampsia. Nat Clin Pract Nephrol. 2005:1(2). Doi: https://doi.org/10.1038/ncpneph0035.
Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21(1):4–14. https://doi.org/10.1111/micc.12079.
LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819. https://doi.org/10.7326/M14-1884.
Smith G, Walker M, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200(1):58.e51–8. https://doi.org/10.1016/j.ajog.2008.06.035.
Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175(2):189–202. https://doi.org/10.1016/j.atherosclerosis.2004.01.038.
Berends AL, De Groot CJM, Sijbrands EJ, Sie MPS, Benneheij SH, Pal R, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51(4):1034–41. https://doi.org/10.1161/HYPERTENSIONAHA.107.101873.
Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. https://doi.org/10.1056/NEJMra043430.
Lusis A. Atherosclerosis. Nature. 2000;407(6801):233–41. https://doi.org/10.1038/35025203.
Benjamin E, Virani S, Callaway C, Chamberlain A, Chang A, Cheng S. Heart Disease and Stroke Statistics--2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. https://doi.org/10.1161/CIR.0000000000000558.
Khan SU, Yedlpati SH, Lone AN, et al. A comparative analysis of premature heart disease- and cancer-related mortality in women in the USA, 1999–2018. Eur Heart J Qual Care Clin Outcomes. 2021. https://doi.org/10.1093/ehjqcco/qcaa099.
Nasir K, Michos ED, Blumenthal RS, Raggi P. Detection of high risk young adults and women by coronary calcium and National Cholesterol Education Program Panel III guidelines. J Am Coll Cardiol. 2005;46:1931–6. https://doi.org/10.1016/j.jacc.2005.07.052.
Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184:201–6. https://doi.org/10.1016/j.atherosclerosis.2005.04.004.
Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139:1047–56. https://doi.org/10.1161/CIRCULATIONAHA.118.037137.
Nedkoff LJ, Briffa TG, Preen DB, Sanfilippo FM, Hung J, Ridout SC, et al. Age- and sex-specific trends in the incidence of hospitalized acute coronary syndromes in Western Australia. Circ Cardiovasc Qual Outcomes. 2011;4:557–64. https://doi.org/10.1161/CIRCOUTCOMES.110.960005.
Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population based retrospective cohort study. Lancet. 2005;366:1797–803. https://doi.org/10.1016/S0140-6736(05)67726-4.
Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–45. https://doi.org/10.1016/j.jacc.2014.04.054.
Davis M, Diamond J, Montgomery D, Krishnan S, Eagle K, Jackson E. Acute coronary syndrome in young women under 55 years of age: clinical characteristics, treatment, and outcomes. Clin Res Cardiol. 2015;104:648–55. https://doi.org/10.1007/s00392-015-0827-2.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8. https://doi.org/10.1136/bmj.38678.389583.7C.
Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–7. https://doi.org/10.1136/bmj.316.7137.1043.
Humphries KH, Izadnegahdar M, Sedlak T, Saw J, Johnston N, Schenck-Gustafsson K, et al. Sex differences in cardiovascular disease - impact on care and outcomes. Front Neuroendocrinol. 2017;46:46–70. https://doi.org/10.1016/j.yfrne.2017.04.001.
Grand’Maison S, Pilote L, Schlosser K, Stewart DJ, Okano M, Dayan N. Clinical features and outcomes of acute coronary syndrome in women with previous pregnancy complications. Can J Cardiol. 2017;33:1683–92. https://doi.org/10.1016/j.cjca.2017.08.025.
Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49(1):90–5. https://doi.org/10.1161/01.HYP.0000251522.18094.d4.
Hermes W, Ket JCF, van Pampus MG, Franx A, Veenendaal MVE, Kolster C, et al. Biochemical cardiovascular risk factors after hypertensive pregnancy disorders: a systematic review and meta-analysis. Obstet Gynecol Surv. 2012;67:793–809. https://doi.org/10.1097/OGX.0b013e31827682fc.
World Health Organization (WHO). The challenge of cardiovascular disease – quick statistics. 2016.
Costa AC, Reina-Couto M, Albino-Teixeira A, Sousa T. Aspirin and blood pressure: effects when used alone or in combination with antihypertensive drugs. Rev Port Cardiol. 2017;36(7–8):551–67. https://doi.org/10.1016/j.repc.2017.05.008.
Dekker GA, Sibai BM. Low-dose aspirin in the prevention of preeclampsia and fetal growth retardation: rationale, mechanisms, and clinical trials. Am J Obstet Gynecol. 1993;168(1):214–27. https://doi.org/10.1016/s0002-9378(12)90917-5.
Friedman S. Preeclampsia: a review of the role of prostaglandins. Obstet Gynecol. 1988;71:122–37.
Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152(3):335–40. https://doi.org/10.1016/s0002-9378(85)80223-4.
Sahin T, Celikyurt U, Geyik B, Oner G, Kilic T, Bildirici U, et al. Relationship between endothelial functions and acetylsalicylic acid resistance in newly diagnosed hypertensive patients. Clin Cardiol. 2012;35(12):755–63. https://doi.org/10.1002/clc.22042.
Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens. 2001;19(3):415–20. https://doi.org/10.1097/00004872-200103000-00009.
Ma LN, Zhao SP, Gao M, Zhou QC, Fan P. Endothelial dysfunction associated with left ventricular diastolic dysfunction in patients with coronary heart disease. Int J Cardiol. 2000;72(3):275–9. https://doi.org/10.1016/s0167-5273(99)00203-x.
Weissgerber TL. Flow-mediated dilation: can new approaches provide greater mechanistic insight into vascular dysfunction in preeclampsia and other diseases? Curr Hypertens Rep. 2014;16(11):1–10. https://doi.org/10.1007/s11906-014-0487-z.
Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Card Imaging. 2010;26(6):631–40. https://doi.org/10.1007/s10554-010-9616-1.
Hermida RC, Ayala DE, Mojón A, Fernández JR. Ambulatory blood pressure control with bedtime aspirin administration in subjects with prehypertension. Am J Hypertens. 2009;22(8):896–903. https://doi.org/10.1038/ajh.2009.83.
Kuscu NK, Kurhan Z, Yildirim Y, Tavli T, Koyuncu F. Detection of endothelial dysfunction in preeclamptic patients by using color Doppler sonography. Arch Gynecol Obstet. 2003;268(2):113–6. https://doi.org/10.1007/s00404-002-0351-2.
Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K, et al. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens Res. 2014;37(2):145–50. https://doi.org/10.1038/hr.2013.131.
Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. J Am Med Assoc. 2001;285(12):1607–12. https://doi.org/10.1001/jama.285.12.1607.
Páez O, Alfie J, Gorosito M, Puleio P, De Maria M, Prieto N, et al. Parallel decrease in arterial distensibility and in endothelium-dependent dilatation in young women with a history of pre-eclampsia. Clin Exp Hypertens. 2009;31(7):544–52. https://doi.org/10.3109/10641960902890176.
Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25(11):2301–7. https://doi.org/10.1097/HJH.0b013e3282ef5fc0.
Hamad RR, Eriksson MJ, Berg E, Larsson A, Bremme K. Impaired endothelial function and elevated levels of pentraxin 3 in early-onset preeclampsia. Acta Obstet Gynecol Scand. 2012;91(1):50–6. https://doi.org/10.1111/j.1600-0412.2011.01238.x.
Goynumer G, Yucel N, Adali E, Tan T, Baskent E, Karadag C. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound. 2013;41(3):145–50. https://doi.org/10.1002/jcu.21962.
Tyldum EV, Backe B, Støylen A, Slørdahl SA. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand. 2012;91(5):566–73. https://doi.org/10.1111/j.1600-0412.2011.01282.x.
Valdés G. Preeclampsia and cardiovascular disease: interconnected paths that enable detection of the subclinical stages of obstetric and cardiovascular diseases. Integr Blood Press Control. 2017;10:17–23. https://doi.org/10.2147/IBPC.S138383.
Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev:2019(10). Doi: https://doi.org/10.1002/14651858.CD004659.pub3.
Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. https://doi.org/10.1016/S0140-6736(08)60074-4.
Labarrere CA, Althabe OH. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational-age infants. Br J Obstet Gynaecol. 1987;94:1113–6. https://doi.org/10.1111/j.1471-0528.1987.tb02302.x.
Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–7. https://doi.org/10.1016/j.placenta.2008.11.009.
Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–903. https://doi.org/10.1210/jc.2004-1955.
Silverberg O, Park AL, Cohen E, Fell DB, Ray JG. Premature cardiac disease and death in women whose infant was preterm and small for gestational age: a retrospective cohort study. JAMACardiol. 2018;3:247–51. https://doi.org/10.1001/jamacardio.2017.5206.
Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. https://doi.org/10.1136/bmj.326.7394.845.
•• Lane-Cordova A, Khan S, Grobman W, Greenland P, Shah S. Long-term cardiovascular risks associated with adverse pregnancy outcomes. J Am Coll Cardiol. 2019;73(16):2106–16. https://doi.org/10.1016/j.jacc.2018.12.092.Discussion regarding pathophysiology behind association of adverse pregnancy outcomes with increased cardiovascular risk.
Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–46. https://doi.org/10.1161/CIRCULATIONAHA.111.034884.
Heida KY, Velthuis BK, Oudijk MA, Reitsma JB, Bots ML, Franx A, et al. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: a systematic re-view and meta-analysis. Eur J Prev Cardiol. 2016;23:253–63. https://doi.org/10.1177/2047487314566758.
World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011.
National Institute for Health and Care Excellence. Hypertension in pregnancy: quality standard. Manchester (United Kingdom). 2013.
Magee L, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416–38. https://doi.org/10.1016/s1701-2163(15)30588-0.
Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–120.e6. https://doi.org/10.1016/j.ajog.2016.09.076.
Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293.e1. https://doi.org/10.1016/j.ajog.2017.11.561.
De Berardis G, Lucisano G, D’Ettorre A, Pellegrini F, Lepore V, Tognoni G, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–94. https://doi.org/10.1001/jama.2012.5034.
Mosca L, Hammond G, Mochari-Greenberger H, Towfghi A, Albert MA, Harvey-Berino J, et al. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127(11):1254–63. https://doi.org/10.1161/CIR.0b013e318287cf2f.
Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. https://doi.org/10.1016/j.jacc.2014.02.529.
Khavjou O, Phelps D, Leib A. Projections of cardiovascular disease prevalence and costs: 2015-2035. RTI Int 2016;(0214680):1–54.
Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–98. https://doi.org/10.1056/NEJMsa053935.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aleha Aziz declares that she has no conflict of interest.
Jacqueline Thompson declares that she has no conflict of interest.
Cynthia Gyamfi-Bannerman declares that she has no conflict of interest.
Mary D’Alton declares that she has no conflict of interest.
Ronald Wapner declares that he has no conflict of interest.
Natalie A. Bello declares that she has no conflict of interest.
Drs. Cynthia Gyamfi-Bannerman, Mary D’Alton, and Natalie A. Bello have received funding outside of this work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Reproductive Health and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Aziz, A., Thompson, J., Gyamfi-Bannerman, C. et al. The Evidence of Aspirin Use in Prevention of Adverse Pregnancy Outcomes (APOs): Should It Be Continued Long Term After an APO?. Curr Treat Options Cardio Med 23, 57 (2021). https://doi.org/10.1007/s11936-021-00936-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00936-z