Abstract
Purpose of Review
A diagnosis of type I or type 2 diabetes confers heightened risk for virtually every obstetric and perinatal complication, with the incidence of superimposed preeclampsia representing a particularly high-risk scenario. Over the past three decades, studies have investigated the role of aspirin in preeclampsia prevention, yielding some promising results for certain at-risk groups, yet unconvincing evidence of benefit among women with pre-pregnancy diabetes. The purpose of this review is to present the current evidence base for aspirin use in pregnancy as a means of mitigating preeclampsia risk in the setting of pregestational type I or type 2 diabetes.
Recent Findings
Meta-analysis data examining low-dose aspirin for preeclampsia prevention in at-risk and low-risk women has demonstrated modest benefit, but subanalyses of cohorts with diabetes have failed to demonstrate a beneficial effect. Evidence is emerging that indicates a benefit only among women who initiate aspirin therapy prior to 16 weeks’ gestation, and uncertainty exists surrounding the effective dose.
Summary
In light of equipoise surrounding the potential role of aspirin for prevention of preeclampsia in women with diabetes, current research is targeted at determining clinical efficacy of aspirin in this high-risk obstetric population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregestational diabetes, type 1 and type 2, affects 4 per 1000 pregnancies in Ireland. A global increase in the prevalence of diabetes has, however, been observed in recent years. Pre-existing diabetes confers a substantial burden of obstetric risk compared to pregnancy among women without diabetes and therefore this condition merits specialist multidisciplinary input before and during pregnancy in order to optimise maternal and neonatal outcomes.
Preeclampsia is known to occur more commonly in pregnancies complicated by pregestational diabetes, and the consequences of this multisystem disorder may be of greater magnitude in this high-risk obstetric population. This review will explore the role of low-dose aspirin in preeclampsia-prevention among women with type I and type II diabetes.
Preeclampsia
Preeclampsia is widely defined as onset of hypertension and proteinuria, with or without associated symptoms, after 20 weeks of gestation. The diagnostic criteria for preeclampsia, as defined by the American College of Obstetricians and Gynecologists, are presented in Table 1. At the earliest opportunity in pregnancy, women with diabetes should undergo baseline testing for the vascular sequelae of diabetes, including determination of the presence or extent of diabetic nephropathy, via quantification of 24-h urinary protein or urinary protein to creatinine ratio. During normal pregnancy, glomerular filtration rate and renal plasma flow increase by up to 65 and 85%, respectively [1]. Nephropathy can worsen during pregnancy due to this increased renal load and in particular in the event that superimposed preeclampsia evolves. The American College of Obstetricians and Gynecologists (ACOG) has advised that physicians should increase surveillance in those patients who have creatinine > 1.5 mg/dL or proteinuria > 3 g/day as they are most at risk of progression to end stage renal disease [2]. The diagnosis of preeclampsia can be particularly challenging among women with pre-existing nephropathy, such that early pregnancy determination of proteinuria status is of paramount importance.
While the precise etiologic basis for preeclampsia remains poorly understood, a number of putative mechanisms have been proposed, including abnormal vascular damage and immunomodulatory mechanisms [2]. Pregestational diabetes remains a significant risk factor for the development of preeclampsia. The risk of developing preeclampsia in type 1 diabetes has been quoted up to 15%, compared to up to 7% of the general population [3, 4]. Among women with pre-existing diabetic nephropathy the risk of superimposed preeclampsia may reach 50% [5].
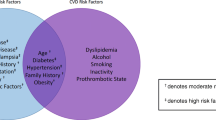
Recognised risk factors for preeclampsia include advanced maternal age, multifetal gestation, family history of preeclampsia and body mass index (BMI) > 35 kg/m2. The National Institute for Clinical Excellence (NICE) recommends that women with these risk factors should take low dose aspirin [6]. Several professional groups and societies also list pregestational diabetes as a risk factor for preeclampsia, including NICE, the American College of Obstetricians and Gynecologists (ACOG) [1], and the Royal College of Physicians of Ireland (RCPI) [7] and state that aspirin should be considered for those women at risk.
The combination of diabetes and preeclampsia places a pregnancy at heightened risk for hypoxia and stillbirth. Placental dysfunction, due to disordered early placental development, is central to the disease process. Early placental disease is followed months later by clinical manifestations of preeclampsia which reflect widespread endothelial dysfunction resulting in vasoconstriction, ischaemia and increased vascular permeability [8]. While not all adverse perinatal outcomes in diabetes are attributed to placental dysfunction, any therapy that offers the potential to optimise placentation in this group deserves close attention.
Aspirin for Prevention of Preeclampsia
Aspirin is a non-steroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic effects. Aspirin also inhibits cyclo-oxygenase isoforms 1 and 2 (COX1/COX2) by irreversible acetylation, thus inhibiting the biosynthesis of prostaglandins and thromboxane from arachidonic acid [9]. The antiplatelet effects of aspirin relate to its inhibition of the production of platelet thromboxane A2, a prothrombotic vasoconstrictor. This inhibitory effect is cumulative on repeat dosing, with complete suppression of platelet thromboxane synthesis estimated to occur within a few days after daily dosing of 20 to 50 mg and more rapid inhibition occurring with larger doses of 150–300 mg [9]. Much higher doses are required for anti-inflammatory, analgesic and antipyretic effects (3.6–4 g daily).
Low-dose aspirin has been investigated for the prevention of preeclampsia owing to its negative effect on thromboxane production. An imbalance between prostacyclin and thromboxane plays a role in the development of preeclampsia and is believed to result from shallow placental invasion and ischaemia that occur shortly after implantation, very early in the first trimester of pregnancy [10].
Studies on the role of aspirin in the prevention of preeclampsia in high-risk women began when 102 women at high risk of developing preeclampsia were recruited by Beaufils [11]. This study demonstrated that preeclampsia was significantly reduced in the aspirin group compared with the untreated group. Since then, numerous randomised trials were conducted to assess the effect of aspirin in the prevention of preeclampsia in high-risk women and they have yielded conflicting results. Initial studies suggested a protective effect [11,12,13,14,15]. Subsequently, some larger studies failed to identify a benefit to aspirin therapy [16,17,18]. Because of discrepancy in the results of the trials that could be attributed to differences in inclusion criteria, timing of initiation of administration of aspirin and different dosages of aspirin, the CLASP trial (Collaborative Low Dose Aspirin in Pregnancy) tried to answer this clinical question definitively, and in doing so concluded that the overall reduction of PE attributed to low-dose aspirin therapy was only 12% [17].
The first meta-analysis in the efficacy of aspirin in the prevention of PE in high-risk patients was published by Duley in 2001. It included 39 trials and over 30,000 patients. [19] The observed reduction in PE incidence was modest (15%) with the use of aspirin (32 trials, 29,331 women); (RR = 0.85, 95% CI 0.78–0.92). Duley correctly reports that most large studies of the efficacy of aspirin for preventing and treating PE are disappointing, and that aspirin is shown to have little beneficial effect. Of note, most patients in these trials were recruited in the second and third trimesters of pregnancy. It is plausible that recruitment late in gestation may be responsible for the failure to observe a benefit to aspirin therapy in reducing the risk of preeclampsia. Trophoblast invasion occurs mainly in the first and second trimesters and is most active in the first; the defects in trophoblast invasion associated with PE are certainly present from about 16 weeks’ gestation. It therefore seems that most patients were recruited after the primary pathology developed, which may explain the limited impact of aspirin.
Since then, more than 11 meta-analyses and systematic reviews have been published on aspirin and many of them have been based on relatively small studies.
A meta-analysis by Askie [20] in 2007 based on 27 trials on 31,678 women concluded that aspirin is effective in preventing preeclampsia, although a 10% reduction in preeclampsia incidence was too modest to warrant routine use in all women. The authors concluded that, despite a very large dataset, ‘the evidence base for particular groups of high-risk women remains limited’. Of note, pre-existing diabetes was identified in 905 randomised women in this meta-analysis and the authors calculated a RR for PE of 0.76 (95% CI 0.56 to 1.04), failing to demonstrate a statistically significant effect. No information is provided on gestational age at recruitment for this sub-analysis, but very few studies were included that recruited women < 16 weeks. Two thirds of the overall population were recruited after 20 weeks’ gestational age. However, if started early in pregnancy, the treatment may be effective [21, 22]. The authors noted a more marked benefit when aspirin treatment started before 20 weeks of gestation and when the dosage was ≥ 75 mg/day. The rationale for this is that the second wave of trophoblast invasion occurs at approximately 14 weeks’ gestation by induced oxidative stress due to an increase in oxygen availability. At this point the maternal arterial circulation commences [23].
In a meta-analysis of individual-participant data from the trials in Askie’s meta-analysis, the effect of aspirin was not affected by the gestational age at the onset of therapy [24]. However, other meta-analyses by Roberge and Bujold showed aspirin started at or before 16 weeks of gestation resulted in reducing the rates of preeclampsia, fetal-growth restriction, and perinatal death by half, whereas aspirin started after 16 weeks of gestation did not have a significant benefit [25, 26]. In addition, the beneficial effect of aspirin that was started at or before 16 weeks of gestation was dose dependent, with a greater reduction in the incidence of preeclampsia being associated with a daily dose of aspirin of 100 mg or more [27••].
The Aspirin for Evidence-Based Preeclampsia Prevention Trial (ASPRE) is a recent, multicenter, randomised, double blinded placebo study in this field that studied the impact of using aspirin at 150 mg daily starting between 11 and 14 weeks’ gestation in pregnancies deemed to be at high risk for the development of preeclampsia based on a first trimester serum screening test. The primary objective of this study was to evaluate the effect of a prophylactic low-dose aspirin administered in the first trimester of pregnancy on the incidence of delivery with preeclampsia before 37 weeks of gestation in women identified at high risk using combined screening for detection of PE. The secondary objectives were to study the effects of aspirin on early onset preeclampsia (delivery before 34 weeks of gestation), the incidence of intrauterine growth restriction (IUGR), fetal death, perinatal death, admission to neonatal intensive care, a composite measure of neonatal morbidity and mortality and placental abruption. The authors found that the occurrence of preterm PE was significantly reduced by aspirin. Preterm PE occurred in 13 of 798 participants (1.6%) in the aspirin group, as compared with 35 of 822 (4.3%) in the placebo group. However, it is worth noting that just 7% (124/1776) of patients in the ASPRE study had pregestational diabetes.
Most recently, Hoffmann and colleagues have lead a multicentre placebo-controlled randomised trial (the ASPIRIN Study [28]) in low income countries that sought to investigate the impact of aspirin 81 mg daily, initiated in the first trimester among nulliparous low-risk women, on the incidence of preterm birth. A significant reduction in preterm birth was reported in the aspirin group (11% incidence of preterm birth < 37 weeks’ gestation, versus 13% incidence in control group), with no observed impact on preeclampsia incidence. The applicability of such studies to the diabetes population is unclear.
Aspirin for Prevention of Preeclampsia in the Setting of Pregestational Diabetes
Only two randomised controlled trials of aspirin therapy in pregnancy have specifically recruited women with pregestational diabetes.
A large multicenter Maternal-Fetal Medicine Units (MFMU) Network study [29] in the United States investigated the role of aspirin in prevention of preeclampsia for high-risk women. These included women with a history of preeclampsia, chronic hypertension and pregestational diabetes mellitus. A total of 471 women with pregestational diabetes were included. Although this study overall did not demonstrate a difference in the incidence of preeclampsia between aspirin and placebo groups, women were recruited in the 2nd trimester (mean gestational age at recruitment 18 weeks ± 4 weeks). It is plausible that any effect of aspirin on placentation and outcome may only be observed if the aspirin is initiated in the first trimester of the pregnancy.
The Estudo Colaborativo para Prevenção da Pré-eclampsia com Aspirina (ECPPA) trial in Brazil recruited women considered to be high risk for preeclampsia, including 62 women with diabetes. Participants were recruited between 12 and 32 weeks gestation and found there was no significant difference in perinatal outcome between the aspirin or placebo- treated group in any category of high risk women [18].
New and Interesting Findings
Since the original MFMU trial was conducted [29], secondary analyses performed on this cohort more recently have indicated more promising findings. Where the original study demonstrated no difference in pregnancy outcome between aspirin and placebo- treated women, a secondary analysis in 2015 including only those women who were less than 17 weeks of gestation at the time of recruitment revealed that late-onset preeclampsia (greater than or equal to 34 weeks of gestation at the time of diagnosis), could be reduced by 29% [30]. There was no reduction in early onset preeclampsia. This observation lends weight to the contention that aspirin therapy may have a role in risk-modification for preeclampsia only if commenced early in gestation.
While many of the studies in this area have sought to analyse the benefits of aspirin use in pregnancies at high risk for preeclampsia, very few have looked at the potential for aspirin-induced harm in this group. We know that aspirin is a safe drug for use in pregnancy when commenced at a low dose (60–150 mg). A large US cohort study included over 50,000 pregnant women of whom approximately 15,000 had aspirin exposure in early pregnancy [31]. After adjusting for differences between exposed and non-exposed pregnancies there was no association between exposure and congenital anomalies. Similarly, a United States Preventive Services Task Force (USPSTF) report on low-dose aspirin for prevention of preeclampsia identified no increased risk of placental abruption (11 trials [23,332 women]; relative risk [RR], 1.17; CI, 0.93–1.48), postpartum haemorrhage (nine trials [22,760 participants]; RR, 1.02; CI, 0.96–1.09), or mean blood loss (five trials, [2478 women]; RR not reported). [32]
However evidence has emerged that the use of aspirin may lead to large for gestational age infants and the associated complications for both mothers and babies [33]. In a secondary analysis of the Caritis data, Adkins and colleagues hypothesised that aspirin would improve fetal growth in pregnancies with known vascular complications of diabetes at highest risk for poor fetal growth. However, they demonstrated that any observed increase in fetal growth was confined to women with pregestational diabetes who did not have known vascular complications. They also found that contrary to what has been observed in the non-diabetes population, the incidence of small-for-gestational-age was not reduced with aspirin use in those with vascular complications of diabetes. Indeed, aspirin-randomised women with nonvascular diabetes had more large-for-gestational-age births than those treated with placebo (40.2 vs 26.6%; P = .005). This observation is particularly concerning in light of the well-recognised and substantial perinatal morbidity associated with hyperglycaemia-induced fetal macrosomia.
The dose of aspirin used in randomised trials ranges from 50 mg to 150 mg. However, for some women, particularly those with type 2 diabetes-related obesity, the commonly-used doses under 150 mg may be too low to exert a full effect. Recent work by Kenny et al. (in non-pregnant patients) indicates that 20% of patients in Ireland with established coronary artery disease are inadequately ‘protected’ by aspirin, as evidenced by thromboxane level which indicated platelet aggregation of greater than 20% [34]. Age, hypertension and weight were noted to be contributing factors for this inadequate aspirin response.
IRELAnD Study
A large multicentre trial, the IRELAnD Study (investigating the Role of Early Low-dose Aspirin in Diabetes), has been undertaken by our research group to investigate the role of low dose aspirin commenced in the first trimester of pregnancies complicated by pregestational diabetes. This is a double blinded placebo-controlled study of 150 mg of aspirin commenced in the first trimester of pregnancy. This trial has commenced recruitment in 7 tertiary-level perinatology centres in Ireland, all with a dedicated Obstetric Diabetes service. Women are recruited between 11 and 13 + 6 weeks of gestation, with a viable, singleton pregnancy. Exclusion criteria include aspirin hypersensitivity current aspirin use or if other risk factors present that necessitates treatment with aspirin during the pregnancy, active or recurrent peptic ulceration. A recruitment target of 280 to each arm of the study has been set and the primary outcome is a composite measure of placental dysfunction including preeclampsia, preterm birth less than 34 weeks, birthweight below the 10th centile and perinatal mortality. The IRELAnD Study is designed to answer the question of whether aspirin should be universally prescribed to women with type 1 and type 2 diabetes in the first trimester of pregnancy [35•].
Conclusion
Despite the dearth of studies providing robust evidence for the use of aspirin in women with pregestational diabetes, many centres routinely prescribe aspirin in this population. In the absence of convincing evidence in favour of routine aspirin administration to all women with diabetes, randomised trial evidence of a benefit in pregnancy outcome should be secured before its widespread use in other populations is interpreted as being applicable to diabetes. Conclusive evidence of aspirin effect should emerge with the IRELAnD trial, the outcome of which will lead to a definitive clinical solution.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25.
Gabbe S, Niebyl J, Galan H, Jauniaux E, Landon M, Simpson J, Driscoll D. Obstetrics: normal and problem pregnancies. 6th ed: Elsevier Saunders. Philadelphia, Pennsylvania, United States; 2012.
Sibai BM, Caritis SN, Hauth JC, MacPherson C, VanDorsten JP, Klebanoff M, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. The National institute of Child health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;183(6):1520–4.
Garner P. Type I diabetes mellitus and pregnancy. Lancet. 1995;346(8968):157–61.
Reece EA, Coustan DR, Gabbe MD, Steven G. Diabetes in women: adolescence, pregnancy, and menopause. 3rd ed. Lippincott Williams & Wilkins, Wolters Kluwer, Philadelphia, Pennsylvania, United States; 2004.
NICE. Hypertension in pregnancy: diagnosis and management. NICE, NICE; 2019.
Gynaecologists RIoOa. The management of hypertension in pregnancy. Clinical Practice Guideline. Dublin: RCPI; 2016.
Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99.
Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e89S–e119S.
Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152(3):335–40.
Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet. 1985;1(8433):840–2.
Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986;1(8471):1–3.
Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med. 1989;321(6):351–6.
Uzan S, Beaufils M, Breart G, Bazin B, Capitant C, Paris J. Prevention of fetal growth retardation with low-dose aspirin: findings of the EPREDA trial. Lancet. 1991;337(8755):1427–31.
Hauth JC, Goldenberg RL, Parker CR Jr, Philips JB 3rd, Copper RL, DuBard MB, et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol. 1993;168(4):1083–91 discussion 91-3.
Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–8.
CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet. 1994;343(8898):619–29.
ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. ECPPA (Estudo Colaborativo para Prevencao da Pre-eclampsia com Aspirina) Collaborative Group. Br J Obstet Gynaecol. 1996;103(1):39–47.
Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659. https://doi.org/10.1002/14651858.CD004659.pub2.
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–8.
Vainio M, Kujansuu E, Iso-Mustajarvi M, Maenpaa J. Low dose acetylsalicylic acid in prevention of pregnancy-induced hypertension and intrauterine growth retardation in women with bilateral uterine artery notches. BJOG. 2002;109(2):161–7.
Chiaffarino F, Parazzini F, Paladini D, Acaia B, Ossola W, Marozio L, et al. A small randomised trial of low-dose aspirin in women at high risk of pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):142–4.
Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54(2–3):303–12.
Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol. 2017;216(2):121–8.e2.
Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491–9.
Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–14.
•• Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–20.e6 While other trials had been carried out which lead to conflicting evidence as to whether aspirin should be commenced or not, this meta-analysis showed that aspirin is effective but must be commenced early in pregnancy in order to have an effect.
Hoffman MK, Goudar SS, Kodkany BS, Metgud M, Somannavar M, Okitawutshu J, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):285–93.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–5.
Moore GS, Allshouse AA, Post AL, Galan HL, Heyborne KD. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU High-Risk Aspirin Study. J Perinatol. 2015;35(5):328–31.
Slone D, Siskind V, Heinonen OP, Monson RR, Kaufman DW, Shapiro S. Aspirin and congenital malformations. Lancet. 1976;1(7974):1373–5.
Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695–703.
Adkins K, Allshouse AA, Metz TD, Heyborne KD. Impact of aspirin on fetal growth in diabetic pregnancies according to White classification. Am J Obstet Gynecol. 2017;217(4):465.e1–5.
Dea K. National evaluation of the aspirin response. Ir J Med Sci. 2013;182:359–92.
• Finnegan C, Dicker P, Fernandez E, Tully E, Higgins M, et al. Investigating the role of early low-dose aspirin in diabetes: a phase III multicentre double-blinded placebo-controlled randomised trial of aspirin therapy initiated in the first trimester of diabetes pregnancy. Contemp Clin Trials Commun. 2019;16:100465 This trial will definitively answer the clinical question as to whether these women should receive aspirin during pregnancy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Catherine Finnegan and Fionnuala M Breathnach each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Pregnancy
Rights and permissions
About this article
Cite this article
Finnegan, C., Breathnach, F.M. The Role of Aspirin for Preeclampsia Prevention in Women with Diabetes. Curr Diab Rep 20, 76 (2020). https://doi.org/10.1007/s11892-020-01365-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-020-01365-1