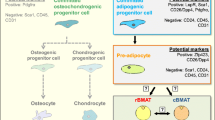
Abstract
Purpose of Review
Beyond aging, senescent cells accumulate during multiple pathological conditions, including chemotherapy, radiation, glucocorticoids, obesity, and diabetes, even earlier in life. Therefore, cellular senescence represents a unifying pathogenic mechanism driving skeletal and metabolic disorders. However, whether senescent bone marrow adipocytes (BMAds) are causal in mediating skeletal dysfunction has only recently been evaluated.
Recent Findings
Despite evidence of BMAd senescence following glucocorticoid therapy, additional evidence for BMAd senescence in other conditions has thus far been limited. Because the study of BMAds presents unique challenges making these cells difficult to isolate and image, here we review issues and approaches to overcome such challenges, and present advancements in isolation and histological techniques that may help with the future study of senescent BMAds.
Summary
Further insights into the roles of BMAd senescence in the pathogenesis of skeletal dysfunction may have important basic science and clinical implications for human physiology and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone marrow adipocytes (BMAds) have become an area of intense research interest. Although known for many years to increase with aging and in response to obesity, it has become increasingly clear that BMAds also accumulate in the context of several diseases, including osteoporosis, anorexia, cancer, and diabetes [1]. Other circumstances where BMAds accumulate include after skeletal unloading, in response to inflammation, and following several treatments such as radiation, chemotherapy, and corticosteroids [1]. A better understanding of the biology of BMAds will be important toward the development of therapeutic approaches to treat several skeletal and metabolic disorders.
The study of BMAds has been technically challenging, although due in large part to lineage tracing methods, advanced imaging protocols, and specialized BMAd isolation techniques, the field has moved forward in recent years at an accelerated pace. For example, these approaches have been utilized to provide preliminary evidence that at least a subset of BMAds can undergo cellular senescence, which is a process whereby cells remain viable and metabolically active yet cease dividing and acquire distinct phenotypic alterations in response to DNA and cell damage accumulation and various stressors [2]. Senescent cells have recently emerged as promising therapeutic targets for several diseases and pathological conditions [2], including bone loss [3,4,5]. Therefore, the extent to which BMAds undergo senescence in the context of various diseases and pathologies and the roles of BMAd senescence in the pathogenesis of bone loss and skeletal dysfunction have important basic science and clinical implications for human physiology and disease.
Cellular senescence is defined as an essentially permanent cell fate that includes a series of multi-step progressive and phenotypically diverse cellular phases resulting in loss of proliferative capacity [2]. Originally described by Hayflick and Moorhead [6], cellular senescence is characterized by growth arrest, tumor suppressor activation, upregulation of cyclin-dependent kinase inhibitors (CDKi’s), chromatin alterations, resistance to apoptosis, and often increased protein synthesis [2]. The protein secretome of senescent cells can consist of a complex, broad spectrum of proinflammatory cytokines, chemokines, interleukins, matrix degrading metalloproteases, ceramides, and nucleotides (e.g., nuclear DNA, mitochondrial DNA, and microRNAs), collectively termed the senescence-associated secretory phenotype (SASP). The SASP can act in a local paracrine or potentially also systemic manner to cause detrimental inflammation, immune cell activation, stem cell dysfunction, apoptosis, and fibrosis, thus driving tissue damage and spreading senescence to neighboring cells [2].
Several triggers can induce cellular senescence, such as DNA damage, telomere dysfunction, oncogene activation, cell damage, mitochondrial dysfunction, metabolic stressors, reactive oxygen species (ROS), inflammatory factors, radiation, chemotherapy, and some types of extracellular vesicles (EVs) [2]. Frequently, in the cases of ROS, inflammation, and EVs, these signals originate from other senescent cells that spread the phenotype. These triggers result in activation of downstream senescence pathways that include p16INK4a/RB and p53/p21CIP1 [7, 8], although the relative contributions of each of these effector pathways to induce the senescent cell fate can vary depending on the initial type(s) of senescence-inducing stimuli. While classically associated with aging, where senescent cells accumulate across several different tissues [2], it is now becoming clear that cellular senescence can be triggered in vivo by several different types of environmental stressors – e.g., radiation [9,10,11], chemotherapy [12], glucocorticoids [13••] obesity [14,15,16], and diabetes [17,18,19], independent of age. Therefore, cellular senescence represents a unifying pathogenic mechanism driving several diseases and pathological conditions, even earlier in life [2].
Characterizing Senescent Cells
Because there is not a single universal marker to identify a senescent cell, there is now a consensus that multiple criteria should be applied to properly characterize senescent cells [20]. These criteria include, but are not limited to: (i) increased expression of chronic senescence effectors (i.e., p16INK4a or p21Cip1); (ii) macromolecular damage and morphological alterations (e.g., senescence-associated distension of satellites [SADS] – i.e., large-scale unraveling of peri-centromeric satellite DNA) [21], telomere-associated foci [TAF] – i.e., sites of DNA damage [λH2AX] co-localized with telomeres) [22]; (iii) increased anti-apoptotic factors (e.g., BCL2 or BCL-xL); (iv) growth arrest (e.g., low Ki67), and (iv) upregulated SASP. To fulfill these criteria, several methods have been developed for detecting and monitoring the accumulation and/or clearance of senescent cells, including real-time quantitative PCR (rt-qPCR), time-of-flight mass cytometry (CyTOF), immunohistochemistry (IHC), and immunofluorescence (IF), among other markers [20]. For example, multiplexed protein profiling via CyTOF has been utilized to permit combinations of these markers (e.g., p16 + , Ki67-, BCL2 +) to identify senescent cells in vivo with high fidelity [23]. In addition, immunoassays or multiplex profiling platforms can be used to measure the SASP when appropriate [20]. However, SASP factors can vary across tissues, may differ in the local microenvironment versus systemically, and can be diverse among different types of senescent cells as well as in response to various senescence-inducing stimuli, which contributes to its complexity, plasticity, challenge to measure, and array of diverse effects on various biological processes [2].
Senescence of Post-Mitotic Cells
As noted earlier, given that cellular senescence occurs in response to numerous different types of inducing stimuli, senescent cells can accumulate at sites of pathology at any time during life, but these cells have most notably been documented in the context of aging across tissues. Most cells in mammals are post-mitotic and recent studies have established that senescence is not limited to proliferating cells but also occurs in terminally differentiated cells, including cardiomyocytes, hepatocytes, myofibers, neurons, and osteocytes [14, 16, 24,25,26]. In addition, as summarized in more detail below, recent studies have identified features of cellular senescence in various types of adipocytes.
Senescence with Obesity and Type 2 Diabetes
Senescence markers have been known for many years to accumulate in preadipocytes (also termed adipocyte progenitors or adipose-derived stem cells) in multiple contexts [27], but emerging evidence demonstrates that senescence can also occur in mature post-mitotic adipocytes of mice on high-fat diet [28] and obese humans [29]. Senescent cells also accumulate prematurely in adipocytes of mice and humans with type 2 diabetes (T2D) [30,31,32] and in other tissues subject to diabetic complications such as liver, brain, and pancreas [14, 16, 18]. In addition, the pathophysiological consequences of T2D, including elevated glucose levels and hyperlipidemia, can cause accumulation of senescent cells and attract macrophages to adipose tissue depots to potentially amplify the burden of cellular senescence, not only locally in adipose tissue, but also systemically in multiple other tissues prone to dysfunction in T2D including the heart, vasculature, kidney, skeletal muscle, and retina [2]. Preadipocytes that undergo cellular senescence can contribute to insulin resistance in metabolic tissues by releasing SASP factors (e.g., activin A, IL-6, and TNFα) that impede insulin signaling pathways and that attract, anchor, and activate immune cells, thereby mechanistically linking senescence to metabolic syndrome [15, 27, 33]. In humans, adipocyte size has been correlated with senescence markers in subcutaneous fat biopsies [34], suggesting that findings in mice are likely to have translational relevance to help inform clinical studies.
Alterations in Bone Marrow Adipocytes
Obesity and T2D are also associated with the accumulation of BMAds. In contrast to the decline in bone mineral density (BMD) that occurs in both sexes with old age, resulting from imbalanced bone resorption relative to formation, aging is associated with increased accumulation of BMAds [35]. Indeed, this reciprocal relationship between BMD and BMAds exists in both animals and humans with old age, when bone marrow stromal cells (BMSCs) differentiate more favorably toward BMAd’s, which thereby infiltrate osteoporotic bone at axial and appendicular skeletal sites.
Although the mechanisms underlying these observations remain incompletely understood, it has been postulated that fundamental aging mechanisms may cause the lineage fate of mesenchymal stem cells (MSCs) to be altered from their default osteoblastogenesis program toward favoring an adipogenic fate [36]. This notion is supported by the observation that osteoblast numbers on bone surfaces decline with aging, whereas BMAds increase. However, the causes of this reciprocal change in lineage allocation are likely multifactorial, as BMAds may originate from multiple sources, whereas the decline in osteoblasts may be due to deficient pools of MSC progenitors or defects in the programs that activate or promote their proper differentiation [37]. It is also noteworthy that BMAd senescence could occur in the context of both metabolic dysfunction and bone loss conditions as mentioned in previous studies [38,39,40]; however, BMSCs are defined as a more broad-encompassing cell population, capable of forming, e.g., bone and cartilage, in addition to adipocytes. Therefore, BMAds are a more specific cell population that can be isolated and studied, as described below. Interestingly, in the context of metabolic dysfunction and bone loss conditions, senescence and the SASP of bone-resident cells in bone marrow and bone itself, including but not limited to senescent BMAds, may contribute toward the theory whereby BMSCs differentiate more favorably towards BMAds as opposed to osteoblasts. However, it still unclear whether this shift in lineage allocation leads to an increase in the accumulation of senescent BMAds. Nevertheless, therapeutic approaches that causes the selective death of senescent cells (i.e., “senolysis”) have been shown in mice to modulate the bone marrow niche to simultaneously increase osteoblast numbers on bone surfaces and reduce BMAds, thus preserving bone mass, at least in mice [41••].
Clearing Senescent Cells to Prevent Skeletal Aging
The selective elimination of senescent cells can be accomplished in mice using both genetic and pharmacological approaches. For example, global clearance of p16+-senescent cells has been achieved using the p16-INK-ATTAC (i.e., p16Ink4a-linked apoptosis through targeted activation of caspase) transgenic mouse model [42], which harbors a p16Ink4a-driven suicide transgene that activates an apoptosis cascade in p16+-senescent cells, thereby resulting in their selective killing in response to administration of a synthetic drug (AP20187). Clearance of senescent cells in old p16-INK-ATTAC mice has been shown to prevent multiple aspects of skeletal aging [41••]. Indeed, following four months of AP20187 treatment in old p16-INK-ATTAC mice, the selective removal of p16+-senescent cells resulted in preservation of bone microarchitecture at both trabecular and cortical skeletal sites due to reduced osteoclasts, increased osteoblasts, improved bone formation rates, and significantly reduced BMAds [41••].
Consistent with these findings in old p16-INK-ATTAC mice, treating old mice intermittently (i.e., once monthly) with the senolytic cocktail, dasatinib plus quercetin (D + Q), a pharmacological approach that selectively kills senescent cells [43], resulted in concordant findings [41••]. Interestingly, the significant increase in osteoblasts and reductions in BMAds following the global clearance of senescent cells [41••], is consistent with reversal of the aforementioned lineage allocation changes in which MSCs transition from commitment to BMAds, as is typical with skeletal aging [37], toward favoring osteoblastogenesis. These findings contrast with the specific removal of senescent osteocytes in old mice, which has been shown to prevent bone loss at the spine (trabecular skeletal site), but not the femur (cortical bone site), by improving bone formation predominantly on periosteal bone surfaces without affecting osteoclasts or BMAds [44•]. These findings are consistent with studies demonstrating that BMAds are an important source of RANKL [45, 46].
Senescence in Response to Radiation and Chemotherapy
Global clearance of senescent cells has also been shown to have beneficial skeletal effects in the context of radiotherapy- [9,10,11] and chemotherapy-induced [12] bone loss. Although both radiation and chemotherapy are effective treatments in slowing cancer, in both of these settings, cellular senescence in the bone microenvironment is a subsequent bystander resulting from cell damage that includes DNA breaks and chromatin disruption [5]. For example, administration of focal radiation therapy (FRT) to young mice has been shown to cause bone loss as well as senescence of both osteoblasts and osteocytes [9]. Global clearance of senescent cells using either p21-ATTAC [11] or intermittent treatment with D + Q [9, 10] has been shown to preserve bone in FRT-treated mice by preserving bone formation and reducing BMAds. Consistent with these observations, bone loss and increases in BMAds resulting from chemotherapy treatment (e.g., doxorubicin [DOX]) in young mice are associated with the accelerated accumulation of bone-resident senescent cells, and this bone loss can be prevented by globally clearing senescent cells in young adult DOX-treated p16-INK-ATTAC mice following AP20187 administration [12]. However, this study did not report whether BMAds were altered in response to senolysis.
Glucocorticoid Therapy-Induced Senescence
It should be noted that none of the aforementioned studies in mice where senescent cells were eliminated reported measurements of cellular senescence markers specifically in BMAds. Therefore, this will be an important direction of future studies that aim to establish a potential causal role of BMAd senescence in the pathogenesis of age-, radiation-, or chemotherapy-induced bone loss, among other contexts. For example, another setting that has been associated with accelerated bone loss in young adult mice as well as the premature accumulation of senescent cells in the bone microenvironment is glucocorticoid therapy [13••, 47]. Indeed, chronic glucocorticoid therapy is known to be associated with decreased BMD, secondary osteoporosis, and increased fracture risk in humans [48]. Recently, Liu et al. [13••] reported in young adult mice treated with glucocorticoids, that a subset of BMAds (identified as perilipin + cells) developed features of cellular senescence, including increased levels of the DNA damage marker, γH2AX, and accumulation of SADS – a hallmark characteristic feature of senescent cells [21]. Interestingly, senescent BMAds in glucocorticoid-treated mice develop a SASP that causes senescence of neighboring cells in the bone microenvironment [13••].
Challenges in Studying Senescence of Bone Marrow Adipocytes
Despite this recent evidence of BMAd senescence in response to glucocorticoid therapy [13••], additional evidence demonstrating that BMAds can become senescent under other conditions has thus far been limited. In part, this is due to fact that the study of BMAds presents unique challenges that make these cells difficult to isolate and image. Thus, to overcome these challenges, advancements in both isolation and histological techniques require continued development and refinement. Herein, we refer predominantly to murine (mouse) samples because to our knowledge, cellular senescence of BMAds has not been reported in humans, although we acknowledge that both the concepts and methodology we describe may be applicable to humans in future studies. It should be noted that BMAds in humans are heterogeneous and that their isolation from human samples varies based on both origin and skeletal site [49, 50]. As noted above, to our knowledge, cellular senescence of BMAds isolated from humans has not been reported in the literature, thus herein we focus predominantly on mice. However, an important limitation of studying BMAds in mice is that it can be challenging to isolate these cells from various skeletal sites as previous studies have done from human samples [49, 50]. Thus, this issue needs to be investigated in both mice and humans more rigorously in future studies.
Unlike many other cell types in the bone marrow microenvironment, BMAds are particularly vulnerable to damage during conventional isolation methods, which typically involve numerous washes and centrifugation steps that do not adversely affect most cells. However, because BMAds contain a large lipid droplet that constitutes most of the cell’s structure, they are prone to rupturing under the stress of washing and centrifugation, which can lead to cellular destruction. To overcome this challenge, the isolation of BMAds is performed using a density gradient technique, which leverages the low-density nature of these cells when suspended in water-based buffers such as phosphate buffered saline to facilitate their isolation. During this process, as shown schematically in Fig. 1, lipid-heavy cells gently rise to the surface, whereas other cells slowly settle to the bottom of the tube. To protect the floating layer containing the BMAds, the subsequent cell pellet and infranatant are carefully removed using a metal syringe. This procedure can be repeated multiple times until the infranatant becomes clear and the cell pellet is no longer visible. With this resulting purified population of BMAds, downstream applications can then be performed. To ensure BMAd purity, several groups have isolated both the BMAds as well as the resulting infranatant for analyses using, e.g., rt-qPCR or RNAseq to measure expression of adipocyte- versus stem cell-specific genes. These studies have established that adipocyte genes are specific only to the BMAd population. For example, Suchacki et al. isolated human BMAds from which RNA was extracted to perform rt-qPCR thereby permitting comparison of differences in gene expression and protein profiles of various types of peripheral adipocytes (i.e., brown, beige, and white) in comparison to BMAds [51]. Other approaches to analyze BMAds typically involve conventional imaging techniques.
Bone Marrow Adipocyte Whole Cell and Nuclei Isolation. (1) Murine bones are excised at the ends and briefly centrifuged to release bone marrow cells. The cells are then resuspended in wash buffer and centrifuged at low speed to avoid cellular damage. (2) Bone marrow adipocytes (BMAds) gradually rise to the supernatant due to their lower density. After sufficient incubation, the cell pellet and infranatant are removed and discarded, carefully so not to disturb the floating BMAd layer. The adipocytes are then washed lightly, centrifuged, and allowed to float. This step is repeated three times to ensure that the BMAds are unharmed and that a pure population is obtained. (3) Once the cell pellet is no longer present and the infranatant is clear, the floating layer of BMAds can be carefully collected for downstream applications, including preparation for IHC techniques or lysed for nucleic acid extraction. (4) The isolation process continues with the extraction of nuclei by gently lysing the cells in nuclear extraction buffer (NEB), followed by centrifugation. This step is repeated to ensure complete lysis and removal of cellular debris. (5) After the second centrifugation, the remaining pellet, which contains purified intact nuclei, is ready for various applications (similar to that of the whole cells). (6) For instance, these nuclei can be cytocentrifuged onto glass slides for IHC techniques, such as a senescence-associated distension of satellite (SADS) or telomere-associated foci (TAF) assays
Indeed, traditionally BMAds have been studied using in vivo bone sections with methods such as histomorphometry and IHC assays. It is also important to use immunofluorescence to check for the presence of immune cells (CD45 +). Although these are considered standard practices, these techniques tend to be associated with significant challenges, particularly in the identification of BMAd nuclei. In sectioned bone samples, a prominent circular gap within the bone marrow often indicates the space previously occupied by BMAds. These spaces are easily identified via brightfield imaging or by using specific markers (e.g., perilipin) via fluorescent microscopy. However, some analyses focus on the cell nucleus, rather than the cell membrane, which can be challenging to identify when the BMAd nuclei tend to blend in with those of neighboring cells, making accurate identification both technically difficult and time-consuming. To overcome this challenge, the BMAds can be directly isolated, as described above, and then gently dried onto hydrophobic coated microscope slides. Once on the slide, this purified cell population enables a more detailed examination of the BMAd morphology, beyond the confines of the bone marrow microenvironment and its numerous neighboring cell types. As shown in Fig. 2, to confirm the effectiveness of the initial isolation, slides can be stained with fluorescent neutral lipid dye (BODIPY) and DAPI to identify the adjacent BMAd nuclei [52], whereas impure populations contain several free floating nuclei that are not associated with an adjacent lipid droplet.
Isolated Bone Marrow Adipocytes. Isolation of whole bone marrow adipocytes allows for an in-depth observation of cell morphology. Cells were stained with BODIPY 493/503 (neutral lipids) dye and DAPI to identify the bone marrow adipocyte (BMAd) structures, highlighting the singular lipid droplet (green) and the adjacent nucleus (blue). The lipid droplet constitutes most the cell’s volume, resulting in the nucleus being displaced toward the cell periphery
Despite these advancements, the technique still poses several challenges. For example, even when BMAds are carefully isolated, seeded, and fixed, they remain more fragile than other cells. Histological techniques that place significant stress on the cells or involve hybridization can lead to rupture of the lipid droplet due to exposure of high temperatures and chemicals. This presents a significant obstacle in key assays relevant to senescence research, as assays such as the SADS approach [21] require a hybridization step.
An alternative approach noted earlier – i.e., the TAF assay, which is a robust and specific method for identifying various types of senescent cells [22], has several advantages for application to the identification and characterization of senescent BMAds. Using a modified TAF protocol that involves isolating and seeding only the nuclei of BMAds onto a slide, thereby bypassing the issues associated with the lipid droplet, the isolation steps can be followed as described above (Fig. 1). However, a secondary isolation needs to be performed to obtain pure BMAd nuclei, after which, these nuclei can then be centrifuged onto a glass slide, allowing them to be processed and analyzed by TAF like any other cell type. By excluding the lipids and other cellular components, this approach effectively prevents the non-specific binding and autofluorescence issues commonly encountered with attempts to study whole BMAds. Therefore, this innovative approach enables TAF and other in situ hybridization (-ISH) analyses to be conducted on BMAds (Fig. 3).
Telomere-Associated Foci (TAF) in BMAd Nuclei. The telomere-associated foci (TAF) assay has emerged as a gold standard in identifying cells that have undergone senescence, with a senescent cell displaying three or more TAF. A TAF is when DNA damage (green) occurs at the site of a telomere (red), which creates a colocalization defined as a TAF (yellow arrows). Using a combination of immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) techniques, TAF can be easily quantified using microscopy and imaging software. In this case, the isolated nuclei are stained with γ-H2AX (green) to identify points of DNA damage, then hybridized with a fluorescent labelled TelC telomere probe, containing repeating CCCTAA leading strand (red). Traditionally a TAF can be identified by an overlap of these two labels using numerous Z-stacks, in which the overlap creates a yellow pigment from the red and green overlay. However, with improvements in imaging technology, we show this overlap in a three-dimensional space, which permits for precise identification and quantification of TAF
The successful isolation of both entire BMAds and their nuclei has significantly broadened the experimental possibilities with this challenging cell type. For example, these advancements permit the application of rt-qPCR for targeted gene expression analysis as well as next generation sequencing (NGS) bioinformatic applications. In addition, techniques like single-nuclei RNA-sequencing or inCITE-seq, which have been recently utilized in white and brown adipocyte studies [53], can now be adapted for BMAds. These techniques will aid in the comparisons of the SASP signatures of senescent BMAds versus peripheral senescent adipocytes, and will help determine the potential functional consequences of these differences on, for example, insulin sensitivity, which will be an important avenue for future investigations. The isolation of these cells also extends to phenotypical analyses, thus making it feasible to investigate cellular structure and analyze protein or mRNA expression using techniques such as IHC and in situ hybridization (-ISH). These methods, previously limited in their application to BMAds, can now be employed to permit deeper characterization of senescent BMAds.
Conclusion
Despite recent evidence of BMAd senescence following glucocorticoid therapy, additional evidence for BMAd senescence in other conditions has thus far been limited. Further refinements in the methodological approaches to isolate and image senescent BMAds will be important toward better understanding the potential roles of BMAd senescence in pathogenesis of skeletal dysfunction in the context of several skeletal and metabolic disorders.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
de Paula FJA, Rosen CJ. Marrow Adipocytes: Origin, Structure, and Function. Annu Rev Physiol. 2020;82:461–84.
Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263–75.
Farr JN, Khosla S. Cellular senescence in bone. Bone. 2019;121:121–33.
Farr JN, Kaur J, Doolittle ML, Khosla S. Osteocyte Cellular Senescence. Curr Osteoporos Rep. 2020;18(5):559–67.
Khosla S, Farr JN, Monroe DG. Cellular senescence and the skeleton: pathophysiology and therapeutic implications. J Clin Invest. 2022;132(3):e154888.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621.
Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93(24):13742–7.
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212–22.
Chandra A, Lagnado AB, Farr JN, Monroe DG, Park S, Hachfeld C, Tchkonia T, Kirkland JL, Khosla S, Passos JF, Pignolo RJ. Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss. J Bone Miner Res. 2020;35(6):1119–31.
Chandra A, Lagnado AB, Farr JN, Schleusner M, Monroe DG, Saul D, Passos JF, Khosla S, Pignolo RJ. Bone Marrow Adiposity in Models of Radiation- and Aging-Related Bone Loss Is Dependent on Cellular Senescence. J Bone Miner Res. 2022;37(5):997–1011.
Chandra A, Lagnado AB, Farr JN, Doolittle M, Tchkonia T, Kirkland JL, LeBrasseur NK, Robbins PD, Niedernhofer LJ, Ikeno Y, Passos JF, Monroe DG, Pignolo RJ, Khosla S. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell. 2022;21(5): e13602.
Yao Z, Murali B, Ren Q, Luo X, Faget DV, Cole T, Ricci B, Thotala D, Monahan J, van Deursen JM, Baker D, Faccio R, Schwarz JK, Stewart SA. Therapy-Induced Senescence Drives Bone Loss. Cancer Res. 2020;80(5):1171–82.
Liu X, Gu Y, Kumar S, Amin S, Guo Q, Wang J, Fang CL, Cao X, Wan M. Oxylipin-PPARgamma-initiated adipocyte senescence propagates secondary senescence in the bone marrow. Cell Metab. 2023;35(4):667-684.e6. This was the first study to establish that glucocorticoid therapy causes the premature accumulation of senescent bone marrow adipocytes in mice.
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691.
Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, von Zglinicki T, LeBrasseur NK, Campisi J, Tchkonia T, Kirkland JL. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3): e12950.
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019;29(5):1061-1077.e8.
Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A. Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell Metab. 2019;29(5):1045-1060.e10.
Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of beta Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019;30(1):129-142.e4.
Eckhardt BA, Rowsey JL, Thicke BS, Fraser DG, O'Grady KL, Bondar OP, et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight. 2020;5(9):e135236.
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular Senescence: Defining a Path Forward. Cell. 2019;179(4):813–27.
Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol. 2013;203(6):929–42.
Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708.
Doolittle ML, Saul D, Kaur J, Rowsey JL, Vos SJ, Pavelko KD, Farr JN, Monroe DG, Khosla S. Multiparametric senescent cell phenotyping reveals targets of senolytic therapy in the aged murine skeleton. Nat Commun. 2023;14(1):4587.
Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38(5):e100492.
Zhang X, Habiballa L, Aversa Z, Ng YE, Sakamoto AE, Englund DA, Pearsall VM, White TA, Robinson MM, Rivas DA, Dasari S, Hruby AJ, Lagnado AB, Jachim SK, Granic A, Sayer AA, Jurk D, Lanza IR, Khosla S, Fielding RA, Nair KS, Schafer MJ, Passos JF, LeBrasseur NK. Characterization of cellular senescence in aging skeletal muscle. Nat Aging. 2022;2(7):601–15.
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res. 2016;31(11):1920–9.
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56.
Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–7.
Espinosa De Ycaza AE, Sondergaard E, Morgan-Bathke M, Carranza Leon BG, Lytle KA, Ramos P, Kirkland JL, Tchkonia T, Jensen MD. Senescent cells in human adipose tissue: A cross-sectional study. Obesity (Silver Spring). 2021;29(8):1320–7.
Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes. 2015;64(7):2289–98.
Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10):1835–41.
Palmer AK, Tchkonia T, Kirkland JL. Senolytics: potential for alleviating diabetes and its complications. Endocrinol. 2021;162(8):bqab058.
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4: e12997.
Gustafson B, Nerstedt A, Smith U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun. 2019;10(1):2757.
Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300.
Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–9.
Sfeir JG, Drake MT, Khosla S, Farr JN. Skeletal Aging. Mayo Clin Proc. 2022;97(6):1194–208.
Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J Bone Miner Res. 2018;33(6):1154–65.
Figeac F, Tencerova M, Ali D, Andersen TL, Appadoo DRC, Kerckhofs G, Ditzel N, Kowal JM, Rauch A, Kassem M. Impaired Bone Fracture Healing in Type 2 Diabetes Is Caused by Defective Functions of Skeletal Progenitor Cells. Stem Cells. 2022;40(2):149–64.
Ali D, Figeac F, Caci A, Ditzel N, Schmal C, Kerckhofs G, Havelund J, Faergeman N, Rauch A, Tencerova M, Kassem M. High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: Evidence from ovariectomized mice. Aging Cell. 2022;21(12): e13726.
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–9. This was the first demonstration that systemic removal of senescent cells reduces bone marrow adipocytes in old mice.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Farr JN, Saul D, Doolittle ML, Kaur J, Rowsey JL, Vos SJ, et al. Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J Clin Invest. 2023;133(8):e162519. This study established that the specific elimination of senescent osteocytes in old mice does not alter bone marrow adipocytes.
Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z, et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Invest. 2021;131(2):e140214.
Hariri H, Kose O, Bezdjian A, Daniel SJ, St-Arnaud R. USP53 Regulates Bone Homeostasis by Controlling Rankl Expression in Osteoblasts and Bone Marrow Adipocytes. J Bone Miner Res. 2023;38(4):578–96.
Liu X, Chai Y, Liu G, Su W, Guo Q, Lv X, Gao P, Yu B, Ferbeyre G, Cao X, Wan M. Osteoclasts protect bone blood vessels against senescence through the angiogenin/plexin-B2 axis. Nat Commun. 2021;12(1):1832.
Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease, N Engl J Med. 2011;365(1):62–70.
Lucas S, Tencerova M, von der Weid B, Andersen TL, Attane C, Behler-Janbeck F, Cawthorn WP, Ivaska KK, Naveiras O, Podgorski I, Reagan MR, van der Eerden BCJ. Guidelines for Biobanking of Bone Marrow Adipose Tissue and Related Cell Types: Report of the Biobanking Working Group of the International Bone Marrow Adiposity Society. Front Endocrinol (Lausanne). 2021;12: 744527.
Attane C, Esteve D, Moutahir M, Reina N, Muller C. A protocol for human bone marrow adipocyte isolation and purification. STAR Protoc. 2021;2(3): 100629.
Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, Sinton MC, Ramage LE, McDougald WA, Lovdel A, Sulston RJ, Thomas BJ, Nicholson BM, Drake AJ, Alcaide-Corral CJ, Said D, Poloni A, Cinti S, Macpherson GJ, Dweck MR, Andrews JPM, Williams MC, Wallace RJ, van Beek EJR, MacDougald OA, Morton NM, Stimson RH, Cawthorn WP. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun. 2020;11(1):3097.
Attane C, Esteve D, Chaoui K, Iacovoni JS, Corre J, Moutahir M, Valet P, Schiltz O, Reina N, Muller C. Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism. Cell Rep. 2020;30(4):949-958.e6.
Benitez GJ, Shinoda K. Isolation of adipose tissue nuclei for single-cell genomic applications. J Vis Exp. 2020;(160):10.3791/61230.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants: P01 AG062413 (J.N.F., S.K.), R21 AG065868 (J.N.F., S.K.), R01 DK128552 (J.N.F.).
Funding
This work was supported by National Institutes of Health (NIH) grants P01 AG062413 (J.N.F., S.K.), R01 AG062413 (S.K.), R21 AG065868 (J.N.F., S.K.), R01 DK128552 (J.N.F.)
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
There were no animal or human studies involved in this review.
Disclosures
The authors do not have a relevant financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Froemming, M.N., Khosla, S. & Farr, J.N. Marrow Adipocyte Senescence in the Pathogenesis of Bone Loss. Curr Osteoporos Rep 22, 378–386 (2024). https://doi.org/10.1007/s11914-024-00875-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-024-00875-1