Abstract
Purpose of Review
Bone marrow adipose tissue (BMAT) is currently considered as a unique and typical fat depot, able to modulate the metabolism of bone cells that share the same microenvironment, with putative subsequent impact on skeletal health. The aim of the present review is to update knowledge related to the molecular phenotype of the BMAT adipocytes.
Recent Findings
Although sharing white and brown adipose tissue-like features, BMAT exhibits its own specific properties. It may consist of two sub-populations of adipocytes, ensuring different metabolic functions and presenting distinct lipidomic and genetic profiles. Current evidence highlights the dynamic lipid composition of BMAT, varying according to pathophysiological situations.
Summary
Since several studies are now demonstrating an alteration of BMAT lipid composition in bone diseases associated with a loss of bone integrity, the investigation of the qualitative aspect of marrow adiposity is currently overtaking its quantitative assessment. A better knowledge of the BMAT lipid content could enlarge the therapeutic potential for bone diseases such as osteoporosis and osteonecrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last two decades of research provided emerging evidence that bone marrow adipose tissue (BMAT), also called yellow marrow as opposed to the hematopoietic red marrow, is a unique fat depot. However, although remarkable advances have been performed in the understanding of its developmental origin, metabolism and function, the physiological roles of BMAT as well as its contribution to the etiology of bone diseases remain to be clarified.
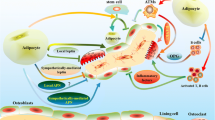
Bone marrow adipocytes have been previously considered as inert space filling bystanders but this concept has radically changed. Currently, it is widely accepted that BMAT constitutes a metabolically active depot that contributes in modulating the bone marrow microenvironment, with putative subsequent impact on skeletal health [1, 2]. An excessive expansion of BMAT is definitely associated with the loss of bone quality observed in aging, unloading, and in pathological conditions such as osteoporosis, type 1 diabetes, and anorexia nervosa [3•].
Through the release of cytokines, adipokines, and free fatty acids into the bone marrow microenvironment, bone marrow adipocytes may indeed influence the biology of their neighboring cells. Depending on the pathophysiological context, BMAT could be a source of energy for bone cells, or conversely, when present in excess, it could disturb surrounding bone cell function and, therefore, interferes with skeletal homeostasis [3•].
At birth, marrow adipocytes are extremely sparse, but their exponential accumulation into the bone marrow is promptly observed during childhood and is followed by their gradual accretion throughout the adult life [4]. BMAT follows a centripetal development, from distal to proximal extremities of the bones, showing a similar pattern in all vertebrates. In humans, the middle phalanges of the toes are fully converted to BMAT by 1 year of age, while in 7-year-old humans, the entirety of feet and hand bones is filled by BMAT [4, 5] In 25-year-old humans, BMAT occupies about 70% of the bone marrow volume of the appendicular skeleton, the hematopoietic red bone marrow being restricted to the axial skeleton, ribs, and sternum [5].
The aim of the present review is to update the knowledge related to the molecular phenotype of BMAT.
Is BMAT Equivalent to White, Brown, or Beige Adipose Tissue?
In order to understand the function and the regulation of the BMAT, many investigators tried to classify bone marrow adipocytes among the two well-known different subtypes of adipose tissue. This is either white adipose tissue (WAT) which takes over the body energy storage and supply, and participates to the inflammatory process or brown adipose tissue (BAT), which dissipates energy in the form of heat in order to maintain the body temperature [6].
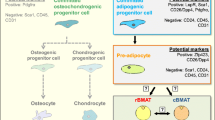
In contrast to the extramedullary adipocytes, lineage tracing studies performed in mice have shown that bone marrow adipocytes express Osterix, a transcriptional factor indispensable for osteoblast differentiation in mammals [7]. This observation suggests that bone marrow adipocytes and osteoblasts arise from a common progenitor cell, which is distinct from the progenitor of the adipocytes constituting the WAT and the BAT. It is now generally accepted that bone marrow adipocytes originate from skeletal stem cells (SSC), which are multipotent progenitor cells able to differentiate into several cell types such as chondrocytes, osteoblasts, and adipocytes [8, 9]. Nevertheless, bone marrow adipocytes present some similarities with the adipocytes located in the WAT and in the BAT.
Although displaying a smaller diameter than white adipocytes, the histological appearance of bone marrow and white adipocytes is quite similar. However, the BMAT does not present the homogeneous organization observed in WAT, since bone marrow adipocytes are scattered within the bone marrow and nested among the hematopoietic cells. Moreover, the accumulation of BMAT in the bone marrow is due to a combination of hypertrophy and hyperplasia of adipocytes whereas the enlargement of WAT is mostly the result of cellular hypertrophy, hyperplasia occurring uniquely in case of extreme obesity [10,11•]. BMAT also differs from WAT with respect to the impact of the nutritional status. Unlike WAT, the amount of BMAT is not strictly associated with body mass index (BMI) or with body fat. For example, in humans, obesity is not always linked to an increase of medullar adiposity [12, 13], whereas patients suffering from anorexia nervosa exhibit an excess of marrow adipocytes contrasting with the leanness of the subjects [14]. In vitro studies have revealed that the ability to secrete cytokines such as IL-6, IL-8, and macrophage-colony stimulating factor (M-CSF) is similar between human SSC-derived adipocytes, primary adipocytes isolated from bone marrow and adipocytes isolated from the WAT [15,16••]. By contrast, several studies have shown that human marrow adipocytes produce low quantities of TNFα and IL-1β compared to WAT [16••,17], although in a murine model, these cytokines are secreted by marrow adipocytes in larger amounts than by epididymal adipocytes (WAT) [18]. Of note, in mice, marrow adipocytes are characterized by a high expression of inflammatory genes and a weak expression of adipogenic genes [18].
More recently, characteristics specific for BAT were attributed to the adipocytes of the BMAT. In mice, marrow adipocytes express thermogenic genes such as deiodinase 2 (Dio2) and the peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1α) [19]. Nevertheless, the expression of these genes is reduced with aging and the uncoupling protein 1 (UCP-1) is scarcely present compared to BAT. However, another study showed the presence of UCP-1 in marrow adipocytes derived from lumbar vertebrae of 3 weeks-old mice as well as from vertebrae of cold-exposed healthy humans [20]. Some authors also suggest that BMAT may exhibit properties specific to beige (or bright) adipocytes, which derive from a subtype of white adipocytes that are able to reversibly differentiate in brown adipocytes and to express UCP-1 [21].
In conclusion, regarding its expression of specific marker genes, its anatomical distribution and its response to the nutritional status, it turns out that BMAT is considered as a unique and typical fat depot, exhibiting its own specific properties while possessing some WAT- and BAT-like features as well.
Is BMAT Constituted by Two Sub-populations of Adipocytes?
Inspired by the study of Tavassoli et al. suggesting that adipocytes located in the red bone marrow are phenotypically different from those situated in the yellow marrow [22], Scheller and colleagues recently used osmium tetroxide staining combined with μCT visualization to demonstrate that in mice, marrow adipocytes may exist in two sub-populations, constituting a regulated and a constitutive type of BMAT (rBMAT and cBMAT, respectively). These types of BMAT are located in specific areas of the bone and revealed distinct temporal development as well as lipidomic profiles [23••].
The adipocytes of the cBMAT are formed early during development, present with a large size and are highly enriched in unsaturated lipids. Such an enrichment may be associated with an elevated expression of several desaturase enzymes, including the two isoforms of stearoyl-CoA desaturase, SCD-1 and -2, and of fatty acid desaturase, FADS-1 and -2. The adipocytes of the rBMAT are characterized by a smaller diameter and contain high levels of saturated lipids.
cBMAT and rBMAT might also follow a preferential distribution within the bone marrow. cBMAT seems mostly located in the distal area within the yellow marrow, whereas rBMAT may be found essentially in the proximal area, among the red marrow enriched in hematopoietic cells, and could be characterized by a high bone turnover. Extrapolating this BMAT distribution in humans, the shift in marrow fat composition described in diseases associated with high fracture risk, such as osteoporosis [24, 25•] and type 2 diabetes [26], as discussed below, and characterized by a rise of saturated lipids and a decrease of unsaturated lipids in the BMAT, could be linked to a prevailing development of rBMAT. Taking these findings together, the existence of cBMAT and rBMAT is still under debate, both in humans and in mice.
Does BMAT Present a Specific Secretory Profile?
When compared to white and brown adipocytes, the secretory profile of bone marrow adipocytes remains largely unexplored [6]. However, since co-culture experiments have revealed that marrow adipocytes exert paracrine effects that are deleterious for osteoblasts [27, 28] while beneficial for osteoclasts [29••], an increased number of studies now focuses on the characterization of the BMAT secretory profile.
Abdallah et al. have recently shown that mouse bone marrow adipocytes inhibit osteoblastic differentiation of bone marrow SSC by blocking BMP2-induced osteoblastogenesis and by activating the proinflammatory NF-κB signaling pathway [30•]. Moreover, marrow adipocytes secrete frizzled-related protein 1 (sFRP-1), an inhibitor of the Wnt signaling pathway and it was shown in co-culture experiments that depletion of sFRP-1 abolishes this anti-osteoblastic effect [31].
Interestingly, in humans, marrow adipocytes display the particularity to express RANKL and OPG, and the RANKL/OPG ratio increases following dexamethasone treatment [32, 33], a situation close resembling that observed in mature osteoblasts. In mice, RANKL expression rises while OPG is reduced throughout adipogenesis and aging, an observation further supporting the link between adipogenesis and osteoclastogenesis [34]. This is reinforced by the recent findings of Fan et al. demonstrating that murine marrow adipocytes, unlike extra-medullar adipocytes, secrete RANKL and that deletion of the parathyroid hormone receptor gene rises RANKL production, further supporting osteoclastogenesis [35].
Leptin and adiponectin are two well-studied adipokines produced by the WAT. Both are also secreted by marrow adipocytes, although to a lesser extent, and it has been shown that bone cells express the receptors of these two adipokines [16••, 19]. Released by white adipocytes in response to triglycerides storage and cell hyperplasia, leptin regulates body mass by modulating food intake and energy expenditure [36]. It also controls the skeletal metabolism by exerting two opposite actions: locally, leptin stimulates bone formation [37, 38] whereas via its sympathetic activity, the adipokine favors bone resorption by suppressing osteoblastic activity [39]. Nevertheless, human epidemiological studies attempting to correlate peripheral leptin levels and bone mass or fracture risk remain inconclusive [40, 41].
Adiponectin can also modulate bone metabolism by two opposite mechanisms which partially counteract the actions of leptin [42]. Locally, adiponectin blocks osteoblastic proliferation while it induces bone formation via an inhibitory effect on the sympathetic tone. In human and murine models, caloric restriction drastically increases adiponectinemia and is associated with BMAT accumulation despite scarce extramedullary fat depots [43]. The inhibition of BMAT expansion via Wnt10b overexpression is sufficient to abolish hyperadiponectinemia. Additionally, in a rabbit model of caloric restriction in which BMAT does not display expansion, the circulating level of adiponectin is not increased [43]. Altogether, these observations suggest that circulating adiponectin largely originates from marrow adipocytes, and that BMAT is required for caloric restriction-induced hyperadiponectinemia. In contrast to leptin, adiponectin seems to be a reliable marker of bone integrity. Prior clinical studies described that elevated circulating adiponectin levels are correlated with low bone mineral density (BMD) in both genders [44] and with a higher fracture risk in men [41].
Chemerin, a more recently identified adipokine, is also secreted by marrow adipocytes. Its pro-adipogenic and anti-osteoblastic effects were highlighted in mice by the group of Muruganandan et al. [45]. They established that chemerin promotes adipocyte function and differentiation whereas it negatively regulates osteoblastogenesis.
More recently, Martin et al. demonstrated that human bone marrow adipocytes influence the medullar microenvironment through the secretion of extracellular vesicles containing adipogenic mRNAs (i.e., PPARγ, CEBPα, and CEBPδ mRNA) [46•], which could be incorporated and translated by osteoblasts, supporting the idea that marrow adipocytes may modulate the osteoblastic phenotype [47].
As discussed above (see section “Is BMAT Equivalent to White, Brown, or Beige Adipose Tissue?”), adipocytes of the BMAT also secrete a wide range of cytokines such as IL-1β, IL-6, IL-8, and TNFα, depending on the animal species studied. In addition to the release of cytokines and adipokines, in vitro co-culture experiments have shown that human SSC-derived adipocytes release free fatty acids [48]. Depending on their type, they may differently affect skeletal health (see below).
Lipid Composition of BMAT
The concept that not only the amount but also the nature of the lipids contained in marrow adipocytes may be relevant for skeletal health originates from in vitro studies showing the deleterious impact of saturated fatty acids on the bone forming cells and on theirs progenitors, the SSC [48, 49]. Conversely, unsaturated fatty acids, particularly the monounsaturated ones, are beneficial for osteoblasts and prevent lipotoxicity [50•,51]. Interestingly, fatty acids seem to exert opposing actions on osteoclasts, the bone-resorbing cells; the saturated fatty acid palmitate is not toxic for osteoclasts and enhances osteoclastogenesis [29••, 52] whereas the monounsaturated fatty acid oleate counteracts palmitate-induced osteoclast differentiation [29••]. Thus, it was proposed that depending on the nature of the lipids present in the BMAT, bone marrow adipocytes could either prevent or support bone remodeling and, therefore, could impact BMD and skeletal integrity.
Since the conversion of red hematopoietic bone marrow to yellow fatty bone marrow was firstly described by histomorphometric studies of human iliac crest biopsies [53, 54], the progress of medical imaging now allows to distinguish the two types of bone marrow non-invasively. Magnetic resonance imaging (MRI) is currently the method of choice to carry out these studies and recent biochemical data obtained by magnetic resonance (MR)-spectroscopy support the above hypothesis [55, 56]. The non-invasive adiposity assessment by MR-spectroscopy is based on the presence or absence of single or double hydrogen bonds in a bone marrow volume of interest and allows quantification of the saturated and unsaturated fractions of bone marrow fat [57]. Using that method, Patsch et al. showed for the first time that BMAT of type 2 diabetes patients with prevalent fragility fractures is depleted in unsaturated fatty acids and enriched in saturated fatty acids, when compared to control subjects [26]. However, no differences were detected in the total marrow fat content between the two groups. Type 2 diabetes patients are characterized by the paradoxical combination of a normal or increased BMD and a rise in fracture risk. Indeed, bone strength is not only determined by BMD but also depends strongly on bone microarchitecture [58]. Along the same lines, the recent pilot study of Bredella et al. demonstrated that, when compared to non-diabetic obese controls, the femoral neck of type 2 diabetes subjects displaying morbid obesity is characterized by a higher BMD although its BMAT content is elevated and impoverished in unsaturated lipids [59•]. Bredella’s research group also used MR-spectroscopy to investigate the BMAT composition in patients suffering from anorexia nervosa, a metabolic disease characterized by an extremely low BMI combined with an excessive BMAT development and a poor bone quality [60]. They demonstrated that the saturation level in the fatty acids BMAT was inversely correlated with BMD in anorexic subjects [61]. However, despite its higher total lipid content, the BMAT of the anorexic subjects displays a similar unsaturation index compared to normal-weight control.
Very recently, data obtained by high-resolution magic angle spinning (HRMAS) MR-spectroscopy of ex vivo punctures of the iliac crest, revealed that the BMAT of osteoporotic and osteopenic patients is characterized by low levels of unsaturated and high levels of saturated fatty acids [25•], which is in accordance with the in vivo MR-spectroscopy study conducted by Yeung et al. [24].
In light of these findings, some authors suggested that lipid composition, rather than lipid quantity, of the BMAT is correlated with bone integrity and may serve as a biomarker for bone quality [55].
To precisely characterize the nature of the lipids composing the interstitial compartment surrounding bone marrow cells, the composition of the bone marrow interstitial fluid has gained considerable interest. For this purpose, bone marrow samples obtained from the iliac crest are nowadays centrifuged and the supernatant, referred to as the bone marrow supernatant fluid (BMSF), is collected and analyzed. It is anticipated that the lipid concentration and composition of the BMSF will reflect better the physiological lipid profile of the marrow microenvironment than values found in blood, and consequently, will be more representative for the activity of marrow adipocytes. Miranda et al. showed that the fatty acid composition of the BMSF differed from that of the circulation, displaying an enrichment in saturated fatty acids and a reduction in unsaturated fatty acids [62••]. They also observed modifications of the fatty acid composition of the BMSF obtained from osteoporotic women suffering from hip fracture. The content of saturated fatty acids decreased whereas the unsaturated ones were on the rise, suggesting that the fatty acid profile of the BMSF is dynamic and directly related to the local release of marrow adipocytes-derived lipids. The authors proposed that following a hip fracture, the metabolism of BMAT adipocytes is modified to provide energy to osteoblasts for injury repair, or to suppress excessive inflammation [56].
Also characterized by excessive BMAT accumulation, osteonecrosis of the femoral head (ONFH) is a painful disorder attributed to necrosis of the osteomedullary elements of the hip [63]. We analyzed the BMSF isolated from those patients and we revealed major modifications of their bone marrow microenvironment compared to healthy volunteers [64••]. In osteonecrotic patients, the amounts of saturated, monounsaturated, and polyunsaturated fatty acids of the BMSF are severely increased; the concentration of the saturated fatty acid palmitate rises by 50%, whereas the concentrations of the monounsaturated fatty acid oleate and of the polyunsaturated fatty acid linoleate tripled compared to BMSF of healthy volunteers. Interestingly, prior studies correlated an increased food intake or blood value of linoleate with a higher fracture risk in elderly and osteoporotic subjects [65, 66]. Of note, the study of Gillet et al. also highlighted the presence of cis-vaccenic acid in the BMSF of osteonecrotic patients whereas this fatty acid is absent in the BMSF of control subjects [64••]. An epidemiological study performed in Inuit women revealed that bone strength is negatively correlated with the content of saturated fatty acids and cis-vaccenic acid in the erythrocyte membrane phospholipids, and conversely, bone strength is positively correlated with their oleic acid content [67]. Finally, the modifications of the lipid profile observed in the BMSF of patients suffering from ONFH were associated with dysfunctions of SSC, suggesting that marrow adipocyte enlargement plays a role in the pathogenesis of the disease [64••].
Despite the growing interest in the physiological and pathological roles of BMAT, analyses of the BMSF are still sparse and, up to now, no study has been published related to the BMSF assessment of obese or type 2 diabetes subjects.
Conclusions
Through the growing interest of investigators, the secretory and lipidomic profiles of the BMAT have become partially established during the last decade. The use of animal models has allowed for great progress in understanding the multiple functions of bone marrow adipocytes. However, in the context of the study of bone-fat interactions, one should keep in mind that a large diversity of bone responses may be observed depending on the animal models used. Data should thus be interpreted cautiously [68] and, as notably highlighted by Lecka-Czernik et al., genetic background, age, gender, animal species, and duration of the study are essential criteria to consider [69••]. As alluded to before, in mice, two sub-populations of adipocytes presenting distinct bone distribution, function and lipidomic profiles may constitute the BMAT.
In humans, advanced imaging techniques and BMSF assessment allow for indirect evaluation of the adipose lipid content. Current evidence reveals a dynamic lipid composition of BMAT, varying according to physiological and pathological situations. Having highlighted the impact of excessive BMAT enlargement on skeletal health, the research field is now focusing on the characterization of the qualitative aspects of marrow adiposity.
However, several questions are still unresolved. What is the physiological lipid composition of the BMAT? In which circumstances and upon which modifications of its composition does BMAT become pathological? Why is the increase of BMAT beneficial for bone formation during childhood? Why is the presence of excessive BMAT deleterious for skeletal integrity in adults?
In that respect, a better knowledge of BMAT and all its aspects will improve the understanding of the pathophysiological mechanisms of bone diseases characterized by an excessive accumulation of BMAT, such as osteoporosis and ONFH, and therefore, allow the development of new therapeutic approaches.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. J Clin Endocrinol Metab. 2015;100(10):3613–21. https://doi.org/10.1210/jc.2015-2338.
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016;27(6):392–403. https://doi.org/10.1016/j.tem.2016.03.016.
• Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–7. This work discusses the complexity of the relationship of marrow adipose tissue to other fat depots and explores its role in modulation of metabolic homeostasis, haemopoiesis, and osteogenesis. https://doi.org/10.1016/S2213-8587(14)70007-5.
Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. 2007;37(3):185–94. https://doi.org/10.1053/j.semnuclmed.2007.01.002.
Scheller EL, Troiano N, JN VH, Bouxsein MA, Fretz JA, Xi Y, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014;537:123–39. https://doi.org/10.1016/B978-0-12-411619-1.00007-0.
Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510(7503):76–83. https://doi.org/10.1038/nature13477.
Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:1–6.
Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci World J. 2012;2012:793823. https://doi.org/10.1100/2012/793823.
Bianco P, Robey PG. Skeletal stem cells. Development. 2015;142(6):1023–7. https://doi.org/10.1242/dev.102210.
Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2(4):239–54. https://doi.org/10.1046/j.1467-789X.2001.00042.x.
• Lecka-Czernik B, Rosen CJ. Skeletal integration of energy homeostasis: translational implications. Bone. 2016;82:35–41. This review summarizes the knowledge of common determinants in bone and adipose function as well as the translational implications of recent work in this field. https://doi.org/10.1016/j.bone.2015.07.026.
Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity. 2011;19:49–53.
Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. 2012;97(4):1337–46. https://doi.org/10.1210/jc.2011-2605.
Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. https://doi.org/10.1210/jc.2008-2532.
Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Pénicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. John Libbe. Eur Cytokine Netw. 2000;11:634–9.
•• Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41(6):558–66. This study characterizes human bone marrow adipocytes for their morphological, molecular, and immunophenotypic properties and defines their secretory profile. https://doi.org/10.1016/j.exphem.2013.02.005.
Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Pénicaud L, Casteilla L. European cytokine network. Eur Cytokine Netw. 2000;11:634–39.
Liu L-F, Shen W-J, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:12. https://doi.org/10.1186/1471-2164-12-212.
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52. https://doi.org/10.1016/j.bone.2011.06.016.
Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16(3):394–406. https://doi.org/10.1016/j.cmet.2012.08.001.
Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659–67. https://doi.org/10.1038/ncb2740.
Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100(1):16–8.
•• Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6. This work examines marrow adipose tissue (MAT) formation and regulation during development and following cold exposure using mouse models and defines the characteristics of the constitutive and regulated MAT. https://doi.org/10.1038/ncomms8808.
Yeung DKW, Griffith JF, Antonio GE, Lee FKH, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22:279–85.
• Li X, Shet K, Xu K, Rodríguez JP, Pino AM, Kurhanewicz J, et al. Unsaturation level decreased in bone marrow fat of postmenopausal women with low bone density using high resolution magic angle spinning (HRMAS) (1)H NMR spectroscopy. Bone. 2017;105:87–92. This study quantifies marrow adipose tissue (MAT) unsaturation profile of marrow samples from post-menopausal women using ex vivo high-resolution magnetic angle spinning proton NMR spectroscopy and investigates the relationship between MAT composition and bone mineral density. https://doi.org/10.1016/j.bone.2017.08.014.
Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28(8):1721–8. https://doi.org/10.1002/jbmr.1950.
Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26(5):485–9. https://doi.org/10.1016/S8756-3282(00)00252-0.
Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation. 2010;80(1):40–5. https://doi.org/10.1016/j.diff.2010.04.004.
•• Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, Gong S, Khan S, Van Dyke T, et al. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J Bone Miner Res. 2014;29(5):1183–95. This work shows for the first time that increased palmitate levels affect bone health by altering precursor cell differentiation and enhancing receptor activator of NF-kB ligand (RANKL)-stimulated osteoclastogenesis. In contrast, oleate does not enhance osteoclast differentiation and inhibits palmitate-induced osteoclastogenesis. https://doi.org/10.1002/jbmr.2150.
• Abdallah BM. Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling. J Biomed Sci J Biomed Sci. 2017;24(1):11. This work demonstrates that marrow adipocytes exert a paracrine inhibitory effect on osteoblast differentiation from bone marrow MSC by blocking BMP signaling in a mechanism mediated by adipokines-induced NF-κB pathway activation. https://doi.org/10.1186/s12929-017-0321-4.
Taipaleenmäki H, Abdallah BM, AlDahmash A, Säämänen AM, Kassem M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp Cell Res Elsevier Inc. 2011;317(56):–745.
Hozumi A, Osaki M, Goto H, Sakamoto K, Inokuchi S, Shindo H. Bone marrow adipocytes support dexamethasone-induced osteoclast differentiation. Biochem Biophys Res Commun. 2009;382(4):780–4. https://doi.org/10.1016/j.bbrc.2009.03.111.
Goto H, Osaki M, Fukushima T, Sakamoto K, Hozumi A, Baba H, et al. Human bone marrow adipocytes support dexamethasone-induced osteoclast differentiation and function through RANKL expression. Biomed Res. 2011;32(1):37–44. https://doi.org/10.2220/biomedres.32.37.
Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289(24):16699–710. https://doi.org/10.1074/jbc.M114.547919.
Fan Y, Hanai J, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017;25(72):–661.
Cock T-A, Auwerx J. Leptin: cutting the fat off the bone. Lancet. 2003;362(9395):1572–4. https://doi.org/10.1016/S0140-6736(03)14747-2.
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8. https://doi.org/10.1210/endo.140.4.6637.
Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15. https://doi.org/10.1677/joe.0.1750405.
Ducy P, Amling M, Takeda S, Priemel M. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. https://doi.org/10.1016/S0092-8674(00)81558-5.
Jürimäe J, Jürimäe T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23. https://doi.org/10.1007/s00774-008-0861-5.
Barbour K, Zmuda J, Boudreau R, Strotmeyer E, Horwitz M, Evans R, et al. Adipokines and the risk of fracture in older adults. J. Bone Miner. Res. 2011;26:1568–76. https://doi.org/10.1002/jbmr.361.
Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through foxo1. Cell Metab. 2013;17(6):901–15. https://doi.org/10.1016/j.cmet.2013.04.009.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–75. https://doi.org/10.1016/j.cmet.2014.06.003.
Araneta MRG, von Mühlen D, Barrett-Connor E. Sex differences in the association between adiponectin and BMD, bone loss, and fractures: the Rancho Bernardo Study. J Bone Miner Res. 2009;24(12):2016–22. https://doi.org/10.1359/jbmr.090519.
Muruganandan S, Roman AA, Sinal CJ. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res. 2010;25(2):222–34. https://doi.org/10.1359/jbmr.091106.
• Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015;16:10. This work shows, for the first time, RNA transfer between human MSC-derived adipocytes and osteoblasts through extracellular vesicles.
Morris E V, Edwards CM. Bone marrow adipose tissue: a new player in cancer metastasis to bone. Front Endocrinol. (Lausanne). 2016;7:90.
Wang D, Haile A, Jones LC. Dexamethasone-induced lipolysis increases the adverse effect of adipocytes on osteoblasts using cells derived from human mesenchymal stem cells. Bone. 2013;53:520–30.
Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155(1):108–16. https://doi.org/10.1210/en.2013-1712.
• Gillet C, Spruyt D, Rigutto S, Dalla Valle A, Berlier J, Louis C, et al. Oleate abrogates palmitate-induced lipotoxicity and Proinflammatory response in human bone marrow-derived mesenchymal stem cells and osteoblastic cells. Endocrinology. 2015;156(11):4081–93. This study shows that physiological concentrations of the saturated fatty acid (FA) palmitate and the monounsaturated FA oleate differently modulate cell death and function in human bone cells and proposes that FA could influence skeletal health. https://doi.org/10.1210/en.2015-1303.
Fillmore N, Huqi A, Jaswal JS, Mori J, Paulin R, Haromy A, et al. Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival. PLoS One. 2015;10:1–17.
Oh S-R, Sul O-J, Kim Y-Y, Kim H-J, Yu R, Suh J-H, et al. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51(5):892–9. https://doi.org/10.1194/jlr.M800626-JLR200.
Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. https://doi.org/10.1097/00003086-197110000-00021.
Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferrán MJ. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17(1):34–7.
Devlin MJ. Bone marrow composition, diabetes, and fracture risk: more bad news for saturated fat. J Bone Miner Res. 2013;28(8):1718–20. https://doi.org/10.1002/jbmr.2013.
Pino AM, Miranda M, Figueroa C, Rodriguez JP, Rosen CJ. Qualitative aspects of bone marrow adiposity in osteoporosis. Front Endocrinol (Lausanne). 2016;7:1–6.
Karampinos DC, Ruschke S, Dieckmeyer M, Diefenbach M, Franz D, Gersing AS, et al. Quantitative MRI and spectroscopy of bone marrow. J Magn Reson Imaging. 2017;47:332-53.
Lecka-czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia. 2017;60(7):1163–9. https://doi.org/10.1007/s00125-017-4269-4.
• Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone. 2017;97:38–42. This work demonstrates that marrow adipose tissue may serve as an imaging biomarker of skeletal health and metabolic risk in type 2 diabetic patients and adults with morbid obesity.
Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923–9. https://doi.org/10.1210/jc.2012-4153.
Bredella MA, Fazeli PK, Daley SM, Miller KK, Rosen CJ, Klibanski A, et al. Marrow fat composition in anorexia nervosa. Bone. 2014;66(204):199.
•• Miranda M, Mar Ia Pino A, Fuenzalida K, Rosen CJ, Seitz GA, Rodr Iguez JP. Characterization of fatty acid composition in bone marrow fluid from postmenopausal women: modification after hip fracture. J Cell Biochem. 2016;117(10):2370–6. This work demonstrates that the fatty acid (FA) composition of the human bone marrow supernatant fluid is enriched in saturated FA and decreased in unsaturated FA as compared to blood plasma, but this relationship switched in women who suffered a hip fracture. https://doi.org/10.1002/jcb.25534.
Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8(3):201–9. https://doi.org/10.1007/s12178-015-9277-8.
•• Gillet C, Dalla Valle A, Gaspard N, Spruyt D, Vertongen P, Lechanteur J, et al. Osteonecrosis of the femoral head: lipotoxicity exacerbation in MSC and modifications of the bone marrow fluid. Endocrinology. 2017;158:490–502. This study demonstrates that the fatty acid concentration and composition are modified in the bone marrow microenvironment of osteonecrotic patients and proposes that marrow adipocytes enlargement could play a role in the pathogenesis of the disease by affecting bone remodelling.
Farina E, Kiel D. Plasma phosphatidylcholine concentrations of PUFA are differentially associated with hip bone mineral density and hip fracture in older adults: the framingham osteoporosis study. J. Bone Miner. Res. 2012;27:1222–30.
Orchard TS, Ing SW, Lu B, Belury MA, Johnson K, Wactawski-Wende J, et al. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J Bone Miner Res. 2013;28(3):505–15. https://doi.org/10.1002/jbmr.1772.
Paunescu AC, Ayotte P, Dewailly E, Dodin S. Saturated and monounsaturated fatty acid status is associated with bone strength estimated by calcaneal ultrasonography in Inuit women from Nunavik (Canada): a cross-sectional study. J Nutr Health Aging. 2014;18(7):663–71. https://doi.org/10.1007/s12603-014-0498-0.
Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow fat and bone-new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–45. https://doi.org/10.1210/jc.2012-3634.
•• Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. 2015;410:35–41. This study concerns the fat and bone cross-talk and underlines the impact of factors such as genetic background, age, gender, and animal species on bone response to obesity and high fat diet. https://doi.org/10.1016/j.mce.2015.01.001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Both authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical collection on Molecular Biology of Bone Marrow Fat Adiposity
Rights and permissions
About this article
Cite this article
Gillet, C., Rasschaert, J. Phenotype of Bone Marrow Adipose Tissue: Specificities of the Anatomical Distribution, Secretory Profile, Lipid Content, and Response to Nutritional Status. Curr Mol Bio Rep 4, 8–15 (2018). https://doi.org/10.1007/s40610-018-0086-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-018-0086-x