Abstract
Purpose of Review
Obesity is highly prevalent and is associated with bone fragility and fracture. The changing nutrient availability to bone in obesity is an important facet of bone health. The goal of this article is to summarize current knowledge on the effects of carbohydrate and dietary fat availability on bone, particularly in the context of other tissues.
Recent Findings
The skeleton is a primary site for fatty acid and glucose uptake. The trafficking of carbohydrates and fats into tissues changes with weight loss and periods of weight gain. Exercise acutely influences nutrient uptake into bone and may affect nutrient partitioning to bone. Bone cells secrete hormones that signal to the brain and other tissues information about its energetic state, which may alter whole-body nutrient trafficking.
Summary
There is a critical need for studies to address the changes that metabolic perturbations have on nutrient availability in bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maintaining a healthy musculoskeletal system is a critical component of wellness across the lifespan. Nutrition and physical activity are lifestyle factors that contribute to bone quality and quantity, where bone characteristics are altered by the coordinated activity of osteocytes (mechanosensitive bone maintenance cells), osteoblasts (bone-forming cells), and osteoclasts (bone-resorbing cells). Chronic overnutrition and physical inactivity leads to obesity and has a prevalence of epidemic proportions in children, adolescents, and adults [1]. Obesity is associated with metabolic dysfunction, reduced mobility [2, 3], and fracture in youth [4]. In older adults, obesity is linked with a lower risk of fracture at certain skeletal sites [5] (Table 1), but these studies utilized cohorts who were raised during a time in which childhood obesity was rare (i.e., born before 1975). When Generation Xers and Millennials reach older age (> 60 years), cohort studies may reveal a different association between obesity and fracture rates in adults because of the higher proportion of individuals who developed obesity prior to achieving peak bone mass. Since obesity is often associated with physical inactivity and a relatively high intake of fat and sugar, nutritional and exercise management is recommended for improving multiple health outcomes [6, 7].
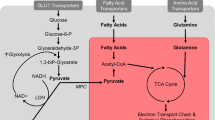
Bone is a metabolically active organ with its own macronutrient requirements to support healthy bone remodeling [22, 23, 24••]. Glucose is a major energy source for bone, and the skeleton is one of the primary sites for the uptake of fatty acids [25]. Bone appears to have mechanisms in place to communicate or regulate its energetic state, as bone-derived factors help regulate aspects of systemic energy metabolism, such as glucose homeostasis [23, 26, 27, 28]. For bone to store or utilize energy substrates, they must be trafficked to and taken up by bone cells. Nutrient availability to bone is an integral aspect of how diet affects bone health, and this may be influenced by obesity or weight change (Table 1). The purpose of this narrative review is to provide a unique perspective on how nutrition influences bone health.
Association Between Western Diets and Bone Health
Studies have evaluated the relationship of Western dietary patterns and obesity prevalence with bone health in different ages, cultures, and countries [8, 9]. In North America, Australia, and Europe, long-term consumption of high-fat and high-sucrose diets has been found to be inversely associated with bone quality in humans, such as a decrease in bone mineral density (BMD) and increased bone fragility and fracture prevalence [8, 29]. Health studies in Asia report positive associations in dietary patterns and BMD as Western dietary habits have merged into lifestyles with increasing prevalence [30, 31]. People in China who regularly consume fish, vegetables, and carotenoid have lower incidence of osteoporosis and fractures, when compared to people who consume little to no fish, vegetables, and carotenoid [30]. In addition, postmenopausal women on a Mediterranean diet were found to have superior femoral neck, lumbar spine, and total hip BMD, and a reduction in the risk of hip fracture [32, 33, 34]. In contrast, those who prefer Western-style diets with land-based animal meat, saturated fats, and sugars have increased fracture risk associated with excess adiposity [29, 35]. One of the consequences of obesity is hyperglycemia. Human studies have suggested that skeletal fragility is strongly associated with persistent hyperglycemia [10, 11•]. This is contrary to animal studies in which increases in bone volume fraction and strength have been noted with high sugar intake, although this increase in bone strength may be explained by gains in body weight [36, 37].
There are several major lines of thought explaining how a high-fat diet (HFD) can decrease bone parameters. First is the induction of adipogenesis at the expense of osteoblastogenesis in mesenchymal stem cells, particularly when there is a shortage of n3 fatty acids, which results in reduced bone formation [18]. Second, excess lipid accumulation in osteoblasts and osteocytes negatively influences bone remodeling [19•, 20]. Other important factors include the potential effect of HFD on inflammatory mechanisms, and the association between HFD and high sedentary time [38, 39, 40]. Persistent hyperglycemia also induces an inflammatory response that may decrease BMD and impair bone turnover and mineralization through a decrease in osteoblast migration and mitochondrial biogenesis [12, 13]. What has not been fully established is whether the development of metabolic dysfunction or obesity is a requirement for HFD to have a negative association with bone parameters (Table 2).
Preclinical studies have also demonstrated the effect of HFD with and without obesity on bone health. Animal models under prolonged exposure to a HFD recapitulate an obese state in humans [16, 18, 21, 48]. Dietary fat intake has been positively and negatively associated with osteoclast activity and osteoclastogenesis in bone-remodeling processes [49, 50]. HFD and obesity detrimentally affect bone structure, strength, and mass to a greater extent in skeletally immature mice when compared to mature mice [14, 15]. In addition, mice fed a HFD have increased bone marrow adiposity (BMA) when compared to mice fed a low-fat diet (LFD) or regular-chow diet [18, 21]. There have also been observations of a decreased mineral to matrix ratio and increased crystallinity in male and female rats on a HFD when compared to LFD-fed rats [16]. However, one study found an increase in femur size and whole bone mechanical properties in mice on a HFD [51]. These conflicting results may point to a HFD reducing the microscale bone quality and not whole bone geometry and strength, thus generating the rationale for more studies to investigate microscale bone outcomes. One of the major limitations to preclinical models of obesity in bone health is the use of diets that contain 60% of their kilocalories from fat. These diets do not translate to human dietary conditions. The type of fatty acid in the diet could be driving the resulting effect on bone outcomes independently from the amount of fat consumed. Studies that included diets rich in fat have led to both increases and reductions in BMD, depending on the type of fatty acid content [52, 53]. Diet product numbers and therefore fatty acid types are not always disclosed in published work, but it is critically important for studies to disclose the product numbers of diets used to improve the reproducibility and interpretation of results.
Glucose Uptake and Utilization in Bone
Glucose is essential for osteoblast differentiation and bone formation [26, 46•, 54, 55]. Short-term administration of high-concentration glucose is associated with enhanced bone differentiation and mineralization [13]. Osteoblasts express GLUT1, GLUT3, and GLUT4 [41], indicating that glucose uptake occurs through insulin-independent and insulin-dependent mechanisms [56]. GLUT4 expression increases up to fivefold when primary mouse osteoblasts undergo further osteogenic induction [41]. Glucose uptake and RUNX2, an indispensable transcription factor in osteoblast differentiation, have been shown to have synergistic effects in osteoblastogenesis and bone formation throughout life [57]. Hence, osteoblastogenesis could be compromised if glucose uptake and utilization is impaired [57, 58]. Glucose uptake into bone can be altered with metabolic perturbations. One study demonstrated that glucose uptake into bone is suppressed in mice lacking the insulin receptor in osteoblasts and osteocytes, as well as after being fed a HFD, which may have been due to resistance to insulin-stimulated glucose uptake in bone. It is unknown how quickly glucose uptake in bone is reduced with obesity, relative to other tissues. Glucose uptake into bone was enhanced in the presence of insulin in low-fat diet–fed mice [45].
Multiple studies have demonstrated that bone glucose uptake increases with acute exercise, indicating a short-term need for heightened nutrient availability. For example, an increase in intensity from low to moderate cycling was shown to increase glucose uptake in femoral bone marrow in healthy young men [46•]. Glucose uptake in bone was increased during knee extension exercise [47]. More studies are needed to determine how long glucose uptake in bone remains elevated after exercise and if this is directly impacting bone formation and mineralization by osteoblasts.
It is well established that weight loss improves glucose disposal and hyperlipidemia status [59, 60, 61, 62]. During and after diet-induced weight loss, the liver, skeletal muscle, and adipose tissue undergo metabolic changes that improve nutrient trafficking and can increase appetite, lower energy expenditure, and promote weight regain (reviewed in [63]). In the liver, carbohydrate and lipid metabolism improves, glucose production is decreased, and there is an increase in trafficking of glucose to lipid storage as opposed to glycogen pools [63, 64, 65]. Skeletal muscle facilitates the clearance of excess carbohydrates, thus increasing glucose uptake. This preference for glucose as fuel is associated with the suppression of fat oxidation [66] (reviewed in [63, 65]), and consequently leaves surplus nutrients to be stored. The induction of lipogenesis by insulin in adipose tissue is restored, and there is a reduction in the average size of adipocytes and an increase in adipocyte number that become prepared to store excess energy [67]. Together, these changes enhance rapid utilization of nutrients, shuttle glucose effectively into tissues, and therefore decrease circulating glucose. The role of bone in nutrient uptake during weight loss remains unknown. Bone is highly dependent on glucose for energy, but the increase in glucose uptake in the liver and skeletal muscle may deplete the glucose availability for bone when carbohydrate intake is very low.
Fat Trafficking and Utilization in Marrow and Bone
Fat trafficking and utilization in bone are most frequently thought of in terms of marrow adiposity, where the marrow cavity is an adipose depot [68]. Marrow cavities are mainly filled with hematopoietic cells at birth, but with aging and menopause, marrow adiposity gradually occupies as much as 70% of long bone cavities [69, 70, 71]. Appendicular skeletal regions are the main adipose storage sites, accounting for over 10% of total fat volume in adults [72•]. Progression of bone marrow adiposity (BMA) occurs during obesogenic conditions, where chylomicrons are cleared from circulation into bone marrow, mediated by perisinusoidal macrophages from endothelial cells [73•]. When radiolabeled chylomicron remnants were intravenously injected into mice, the skeleton (marrow and cortical bone) had the second highest uptake of those remnants [17]. A study found that diet-induced obesity increases the marrow cavity and BMA [74], thus supporting the notion that more lipid is being transported to the bone. On the other hand, BMA volume and adipocyte area have been found to be increased in a calorie-restricted diet compared to a normal diet, which was followed by reductions in trabecular thickness and cortical area fraction [75••]. Diet-induced weight loss decreases BMA in animals and humans [76, 77, 78]. Surgical weight loss and, more specifically, sleeve gastrectomy increases BMA [79, 80]. Although the role of BMA in bone health is unclear, observed reductions in BMA should coincide with the decreases in BMD during weight loss, due to the lack of nutrient availability. Curiously, people who experience anorexia nervosa often have a high BMA [81], adding to the lack of clarity regarding the function of BMA during caloric restriction. The purpose of marrow adiposity is uncertain, but potential functions are as follows.
One potential function of marrow fat includes acting as a local energy source to support the energy requirements of bone turnover or blood cell production, as exercise attenuates BMA accumulation and reduces BMA volume in obese mice [21, 74, 75••]. Also, the marker of fatty acid uptake, CD36, is increased in mice exercising on a normal diet but is reduced in mice exercising on a calorie restriction [75••], thus pointing to the skeleton’s ability to shift nutrient uptake in a low-nutrient environment. Despite evidence that BMA is negatively associated with bone microarchitecture and BMD [68], one study indicated that excessive suppression of BMA in caloric restriction was associated with enhanced bone resorption [75••], suggesting that BMA is important for maintaining optimal bone health. Another potential function of bone marrow is to protect osteoblasts, osteocytes, and osteoclasts from excess lipid accumulation. The expansion of BMA from nutrient excess to the degree that ectopic adiposity is accumulating in osteoblasts and osteocytes may explain why an increase in BMA is often accompanied by bone loss. The consequences of prolonged high marrow adiposity are not known and could result in more lipolysis that mimics an insulin-resistant state, along with the release of inflammatory cytokines. The regulation of BMA may differ from other fat depots. Like other depots, obesity is associated with increased BMA. Unlike other depots, marrow adiposity can remain high during periods of prolonged negative energy imbalance, such as in anorexia nervosa [81], suggesting that marrow adipose is resistant to depletion of lipid.
Fat trafficking to osteoblasts, osteoclasts, and osteocytes needs to be considered as well, as cortical bone is a major destination for dietary fat [25]. Fat is an essential energy source for all bone cells [42•, 43, 44, 82]. The ability to oxidize fat may also prevent excess accumulation of lipid. Studies have observed an excessive accumulation of lipids in bone tissue that leads to lipotoxicity that induces apoptosis in osteocytes and affects osteoblast differentiation and function [19•, 83]. Fatty acid delivery was investigated in transgenic mice that lacked central regulators of fatty acid transport and exhibited impairments of fatty acid uptake in the tibia and femur when compared to wild-type mice [25]. Aside from evidence that cortical bone and bone marrow had a similar fatty acid profile [25], it is unclear whether there is a preferential trafficking to marrow versus bone. It is also unclear whether nutrient trafficking can be altered by exercise or diet, but data related to fat trafficking in other tissues yield clues. For example, one study investigating the influence of a HFD on dietary fat trafficking found a preferential disposition to store dietary fat in adipose tissue in obesity-prone rats. Obesity-prone rats were able to clear tracer more rapidly from the plasma and had a decreased 14CO2 production following a HFD, suggesting a lowered oxidation of dietary fat. In obesity-prone females on a HFD, there was a decrease in dietary fat tracer oxidation in both the liver and skeletal muscle. This was followed by an increase in triglyceride content in the liver and a decrease in dietary fat tracer in the muscle. Lastly, there was an increase in the tracer in whole-body adipose tissue following HFD, but to a greater extent in obesity-prone rats [84]. These results indicate that an introduction of a HFD alters the way nutrients are trafficked between tissues, and a genetic predisposition to obesity may further amplify these changes. Therefore, the excess circulating dietary fat as a consequence from a HFD could end up in the bone and in turn influence bone turnover.
With weight loss, skeletal muscle enhances the suppression of fat oxidation [63]. It is undetermined whether weight loss suppresses fat oxidation/lipolysis in bone or bone marrow. If so, then this would likely suppress bone formation, possibly accelerating bone resorption and leading to the bone loss that is frequently observed. So like muscle, the loss of bone mass may preserve the energetic state of the whole body in a way that protects body weight and promotes weight regain. Adipose appears to send signals to the brain that indicate its energetic state [67], and growing evidence that bone is an endocrine organ indicates that bone may do the same.
Bone: an Endocrine Organ with the Power to Regulate Nutrient Availability
The skeleton serves as an endocrine organ that helps regulate nutrient availability and uptake into other tissues [22, 85]. Studies thus far have examined three skeleton-secreted proteins that appear to have endocrine-like functions. Sclerostin, a glycoprotein produced by osteocytes, inhibits bone formation. Interestingly, sclerostin appears to act in an endocrine-like manner and is associated with diet-induced obesity. Specifically, there was a reduction of white adipose tissue and improvements in glucose handling, fatty acid oxidation, and reduced adipocyte de novo fatty acid synthesis in both sclerostin-deficient mice and sclerostin-neutralizing antibody treated mice on a HFD [28].
Recent studies proposed a new osteocyte-dependent mechanism to attenuate the accumulation of fat mass in response to loading, resulting from the observation of reduced body weight and fat mass in animals that received implanted weighted capsules compared to unweighted capsules [86]. The depletion of osteocytes prevented persistent weight loss in mice. Although an osteocyte-mediated reduction of food intake was the proposed means of weight loss, food intake and relative (body weight) VO2 were only reported in osteocyte-replete animals at 1 time point, and energy balance was never reported. Therefore, it is unclear if osteocyte depletion independently influenced energy expenditure, energy storage, and/or food intake because of the removal of the energy required to detect and respond to loading. It is possible that a weighted capsule creates enough discomfort to reduce appetite. Weight loss was also observed in humans that wore a high-load (11% of body weight) vest for 8 h a day when compared to humans that wore a low-load (1% of body weight) vest [87]. However, rats exposed to simulated hyper gravity did not lose body weight [88]. Importantly, abruptly inserting a weighted capsule in a rodent or a vest on a human creates a form of overload exercise, which can reduce appetite in males and some females [89]. Having a heavier load on the skeleton may increase the energy demand on the osteocytes which may alter nutrient trafficking to the bone and marrow. Further studies are needed to determine the relationship between bone loading, appetite, and energy balance.
Osteoblasts secrete osteocalcin (OCN) and lipocalin-2 (LCN2); these proteins serve different roles as potential hormones to regulate energy balance. Experiments in lean and obese mice demonstrated that bone-derived LCN2 can reduce appetite, improve glucose intolerance, and increase circulating insulin levels [23]. Another study suggests that increases in LCN2 levels in obese mice may counteract metabolic dysregulation and reduce fat mass [24••]. OCN has been shown to stimulate pancreatic islet β-cell proliferation, enhance insulin production and secretion from the pancreas, improve insulin sensitivity in mice, improve glucose tolerance, and prevent obesity in wild-type mice fed a HFD, and this has been reviewed extensively [26, 27]. The question remains why OCN plays a role in endocrine functions similar to insulin, which points to the idea that OCN may be assisting the skeleton to communicate its energetic state or protect its nutrient availability.
Conclusion
Obesity-related overconsumption of food has a negative impact on bone quality. Fat and glucose oxidation are necessary to provide the energy required for bone formation and remodeling processes. However, ectopic lipid accumulation and chronic hyperglycemia in osteoblasts and osteocytes due to chronic overfeeding impair bone formation and adaptations to mechanical loading. These changes may begin with relative differences in nutrient trafficking to bone and other tissues, but bone has been largely omitted from nutrient trafficking studies. Exercise may affect the capacity to take up and oxidize glucose and fat in bone cells, but more studies are needed to support this notion. Further investigations are needed to understand how mechanical and metabolic perturbations alter nutrient trafficking to bone and marrow and subsequently affect glucose and fat oxidation and storage in bone.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ahmadi N, Sadr SM, Mohammadi MR, Mirzaei M, Mehrparvar AH, Yassini Ardekani SM, Sarebanhassanabadi M, Nilforoshan N, Mostafavi SA. Prevalence of abdominal obesity and metabolic syndrome in children and adolescents: a community based cross-sectional study. Iran J Public Health. 2020;49(2):360–8.
Liu AW, Song SO, Hayashi T, Sato KK, Kahn SE, Leonetti DL, Fujimoto WY, Boyko EJ. Change in CT-measured abdominal subcutaneous and visceral but not thigh fat areas predict future insulin sensitivity. Diabetes Res Clin Pract. 2019;154:17–26.
Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–9.
Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471(4):1199–207.
Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–73.
Rodriguez-Gomez I, Manas A, Losa-Reyna J, Rodriguez-Manas L, Chastin SFM, Alegre LM, et al. Associations between sedentary time, physical activity and bone health among older people using compositional data analysis. PLoS ONE. 2018;13(10):e0206013.
Tamme R, Jurimae J, Maestu E, Remmel L, Purge P, Mengel E, et al. Physical activity in puberty is associated with total body and femoral neck bone mineral characteristics in males at 18 years of age. Medicina (Kaunas). 2019;55(5).
Kim HY, Jung HW, Hong H, Kim JH, Shin CH, Yang SW, Lee YA. The role of overweight and obesity on bone health in Korean adolescents with a focus on lean and fat mass. J Korean Med Sci. 2017;32(10):1633–41.
Zeng FF, Wu BH, Fan F, Xie HL, Xue WQ, Zhu HL, Chen YM. Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab. 2013;98(6):2347–55.
Wolfel EM, Jahn-Rickert K, Schmidt FN, Wulff B, Mushumba H, Sroga GE, et al. Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality. Bone. 2020;140:115556.
• Merlo K, Aaronson J, Vaidya R, Rezaee T, Chalivendra V, Karim L. In vitro-induced high sugar environments deteriorate human cortical bone elastic modulus and fracture toughness. J Orthop Res. 2020;38(5):972-83. This paper observed reductions in elastic modulus and fracture toughness in human cortical bone specimens incubated with ribose compared to bone samples that were not treated.
Yang L, Liu J, Shan Q, Geng G, Shao P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys Res Commun. 2020;522(2):471–8.
Pahwa H, Khan MT, Sharan K. Hyperglycemia impairs osteoblast cell migration and chemotaxis due to a decrease in mitochondrial biogenesis. Mol Cell Biochem. 2020;469(1-2):109–18.
Inzana JA, Kung M, Shu L, Hamada D, Xing LP, Zuscik MJ, Awad HA, Mooney RA. Immature mice are more susceptible to the detrimental effects of high fat diet on cancellous bone in the distal femur. Bone. 2013;57(1):174–83.
Wee NKY, Enriquez RF, Nguyen AD, Horsnell H, Kulkarni R, Khor EC, Herzog H, Baldock PA. Diet-induced obesity suppresses cortical bone accrual by a neuropeptide Y-dependent mechanism. Int J Obes (Lond). 2018;42(11):1925–38.
Sherk VD, Heveran CM, Foright RM, Johnson GC, Presby DM, Ferguson VL, MacLean PS. Sex differences in the effect of diet, obesity, and exercise on bone quality and fracture toughness. Bone. 2021;145:115840.
Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PCN, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–7.
Picke AK, Sylow L, Moller LLV, Kjobsted R, Schmidt FN, Steejn MW, et al. Differential effects of high-fat diet and exercise training on bone and energy metabolism. Bone. 2018;116:120–34.
• Al Saedi A, Bermeo S, Plotkin L, Myers DE, Duque G. Mechanisms of palmitate-induced lipotoxicity in osteocytes. Bone. 2019;127:353-9. This paper observed decreases in RANKL, DKK1, and sclerostin expression in MLO-Y4 cells treated with palmitic acid compared to cells not treated. These conditions replicate what cells may experience in an environment exposed to an excess high-fat diet.
Al Saedi A, Myers DE, Stupka N, Duque G. 1,25(OH)2D3 ameliorates palmitate-induced lipotoxicity in human primary osteoblasts leading to improved viability and function. Bone. 2020;141:115672.
McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N, et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20–31.
Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017;132:1–8.
Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, Lanzano P, Deng L, Leibel RL, Rubin M, Nickolas T, Chung W, Zeltser LM, Williams KW, Pessin JE, Kousteni S. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–90.
•• Mosialou I, Shikhel S, Luo N, Petropoulou PI, Panitsas K, Bisikirska B, et al. Lipocalin -2 counteracts metabolic dysregulation in obesity and diabetes. J Exp Med. 2020;217(10). Bone is emerging as an endocrine organ and this paper demonstrates how reducing lipocalin-2 expression in mice leads to metabolic dysfunction, increases in fat mass, and glucose intolerance.
Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F, et al. Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. Int J Mol Sci. 2017;18(6).
Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105(13):5266–70.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114(52):E11238–E47.
Nguyen HH, Wu F, Oddy WH, Wills K, Winzenberg T, Jones G. Associations between dietary patterns and osteoporosis-related outcomes in older adults: a longitudinal study. Eur J Clin Nutr. 2021;75(5):792–800.
Qiu R, Cao WT, Tian HY, He J, Chen GD, Chen YM. Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS ONE. 2017;12(1):e0168906.
Regu GM, Kim H, Kim YJ, Paek JE, Lee G, Chang N, et al. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30-75 years using data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008-2011). Nutrients. 2017;9(9).
Silva TRD, Martins CC, Ferreira LL, Spritzer PM. Mediterranean diet is associated with bone mineral density and muscle mass in postmenopausal women. Climacteric. 2019;22(2):162–8.
Perez-Rey J, Roncero-Martin R, Rico-Martin S, Rey-Sanchez P, Pedrera-Zamorano JD, Pedrera-Canal M, et al. Adherence to a Mediterranean diet and bone mineral density in Spanish premenopausal women. Nutrients. 2019;11(3).
Malmir H, Saneei P, Larijani B, Esmaillzadeh A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2018;57(6):2147–60.
Mu M, Wang SF, Sheng J, Zhao Y, Wang GX, Liu KY, Hu CL, Tao FB, Wang HL. Dietary patterns are associated with body mass index and bone mineral density in Chinese freshmen. J Am Coll Nutr. 2014;33(2):120–8.
Minematsu A, Nishii Y, Sakata S. High-fat/high-sucrose diet results in higher bone mass in aged rats. Bone Rep. 2018;8:18–24.
Tian L, Wang C, Xie Y, Wan S, Zhang K, Yu X. High fructose and high fat exert different effects on changes in trabecular bone micro-structure. J Nutr Health Aging. 2018;22(3):361–70.
Hafner H, Chang E, Carlson Z, Zhu A, Varghese M, Clemente J, et al. Lactational high-fat diet exposure programs metabolic inflammation and bone marrow adiposity in male offspring. Nutrients. 2019;11(6).
Zhang Z, Lin T, Meng Y, Hu M, Shu L, Jiang H, Gao R, Ma J, Wang C, Zhou X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism. 2021;119:154767.
Sherk VD, Jackman MR, Higgins JA, Giles ED, Foright RM, Presby DM, et al. Impact of exercise and activity on weight regain and musculoskeletal health post-ovariectomy. Med Sci Sports Exerc. 2019;51(12):2465–73.
Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, Riddle RC, Clemens TL. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology. 2016;157(11):4094–103.
• Rendina-Ruedy E, Guntur AR, Rosen CJ. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6(3):250-8. This paper provides evidence lipid uptake is required for osteoblasts in order to function properly.
Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24(2):e6–10.
Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, Choi HS. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51(5):892–9.
Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004.
• Heinonen I, Kemppainen J, Fujimoto T, Knuuti J, Kalliokoski KK. Increase of glucose uptake in human bone marrow with increasing exercise intensity. Int J Sport Nutr Exerc Metab. 2019;29(3):254-8. Bone marrow adipose is often thought of only as an ectopic fat reservoir. This paper observed an increase in bone marrow glucose uptake in males exposed to moderate-intensity exercise compared to low-intensity exercise. This refutes the reservoir idea and shows a change in nutrient intake under increased energy demands.
Heinonen I, Kemppainen J, Kaskinoro K, Langberg H, Knuuti J, Boushel R, Kjaer M, Kalliokoski KK. Bone blood flow and metabolism in humans: effect of muscular exercise and other physiological perturbations. J Bone Miner Res. 2013;28(5):1068–74.
Rojas JM, Bolze F, Thorup I, Nowak J, Dalsgaard CM, Skydsgaard M, Berthelsen LO, Keane KA, Søeborg H, Sjögren I, Jensen JT, Fels JJ, Offenberg HK, Andersen LW, Dalgaard M. The effect of diet-induced obesity on toxicological parameters in the polygenic Sprague-Dawley rat model. Toxicol Pathol. 2018;46(7):777–98.
Montalvany-Antonucci CC, Zicker MC, Ferreira AVM, Macari S, Ramos-Junior ES, Gomez RS, Pereira TSF, Madeira MFM, Fukada SY, Andrade I Jr, Silva TA. High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J Nutr Biochem. 2018;59:93–103.
Montalvany-Antonucci CC, Duffles LF, de Arruda JAA, Zicker MC, de Oliveira S, Macari S, Garlet GP, Madeira MFM, Fukada SY, Andrade I Jr, Teixeira MM, Mackay C, Vieira AT, Vinolo MA, Silva TA. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone. 2019;125:112–21.
Silva MJ, Eekhoff JD, Patel T, Kenney-Hunt JP, Brodt MD, Steger-May K, Scheller EL, Cheverud JM. Effects of high-fat diet and body mass on bone morphology and mechanical properties in 1100 advanced intercross mice. J Bone Miner Res. 2019;34(4):711–25.
Wang Y, Dellatore P, Douard V, Qin L, Watford M, Ferraris RP, Lin T, Shapses SA. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res. 2016;36(7):742–50.
Zhan Q, Tian Y, Han L, Wang K, Wang J, Xue C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020;11(8):7048–60.
Niu Y, Yang Z, Li X, Zhang W, Lu S, Zhang H, Chen X, Zhu L, Xing Y, Ning G, Qin L, Su Q. Association of osteoprotegerin with impaired glucose regulation and microalbuminuria: the REACTION study. BMC Endocr Disord. 2015;15:75.
Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33(6):1052–65.
Hahn TJ, Westbrook SL, Sullivan TL, Goodman WG, Halstead LR. Glucose transport in osteoblast-enriched bone explants: characterization and insulin regulation. J Bone Miner Res. 1988;3(3):359–65.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Iezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91.
Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T, Nefyodova E, Cardoen R, Mathieu C, van der Schueren B, Confavreux CB, Clemens TL, Maes C. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128(3):1087–105.
Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41(7):1526–34.
Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, Ghigo E, Bo S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):17–33.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biologyʼs response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R581–600.
Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1117–29.
MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: a daunting complexity. Obesity (Silver Spring). 2017;25(Suppl 1):S8–S16.
Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587(Pt 20):4949–61.
MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16(Suppl 1):45–54.
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71.
Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10–9.
Ricci C, Cova M, Kang YS, Yang A, Rahmouni A, Scott WW Jr, Zerhouni EA. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177(1):83–8.
Sieron D, Drakopoulos D, Loebelenz LI, Schroeder C, Ebner L, Obmann VC, et al. Correlation between fat signal ratio on T1-weighted MRI in the lower vertebral bodies and age, comparing 1.5-T and 3-T scanners. Acta Radiol Open. 2020;9(1):2058460120901517.
• Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, et al. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun. 2020;11(1):3097. This paper observed differences between bone marrow adipose tissue (BMA) and white and brown adipose tissue in glucose metabolism, thus providing support to the hypothesis of BMA contributing to whole body energy uptake.
• Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264(30):17931-8. This paper is one of the first studies that demonstrates chylomicron uptake into the bone marrow, providing evidence that bone is a metabolically active organ.
Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin CT, Rubin J. Exercise decreases marrow adipose tissue through ss-oxidation in obese running mice. J Bone Miner Res. 2017;32(8):1692–702.
•• McGrath C, Sankaran JS, Misaghian-Xanthos N, Sen B, Xie Z, Styner MA, et al. Exercise degrades bone in caloric restriction, despite suppression of marrow adipose tissue (MAT). J Bone Miner Res. 2020;35(1):106-15. This paper observed increases in bone fatty acid uptake in calorie-restricted mice when compared to mice on a regular diet.
Spurny M, Jiang Y, Sowah SA, Schubel R, Nonnenmacher T, Bertheau R, et al. Changes in bone marrow fat upon dietary-induced weight loss. Nutrients. 2020;12(5).
Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front Endocrinol (Lausanne). 2016;7:102.
Bosy-Westphal A, Later W, Schautz B, Lagerpusch M, Goele K, Heller M, Glüer CC, Müller MJ. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring). 2011;19(7):1503–10.
Kim TY, Schwartz AV, Li X, Xu K, Black DM, Petrenko DM, Stewart L, Rogers SJ, Posselt AM, Carter JT, Shoback DM, Schafer AL. Bone marrow fat changes after gastric bypass surgery are associated with loss of bone mass. J Bone Miner Res. 2017;32(11):2239–47.
Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90.
Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36.
Levental KR, Surma MA, Skinkle AD, Lorent JH, Zhou Y, Klose C, et al. Omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci Adv. 2017;3(11):eaao1193.
Al Saedi A, C AG, D EM, Hayes A, Duque G. Rapamycin affects palmitate-induced lipotoxicity in osteoblasts by modulating apoptosis and autophagy. J Gerontol A Biol Sci Med Sci. 2020;75(1):58-63.
Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2006;291(5):E1083–91.
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–19.
Jansson JO, Palsdottir V, Hagg DA, Schele E, Dickson SL, Anesten F, et al. Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc Natl Acad Sci U S A. 2018;115(2):427–32.
Ohlsson C, Gidestrand E, Bellman J, Larsson C, Palsdottir V, Hagg D, et al. Increased weight loading reduces body weight and body fat in obese subjects - a proof of concept randomized clinical trial. EClinicalMedicine. 2020;22:100338.
Turner RT, Branscum AJ, Wong CP, Iwaniec UT, Morey-Holton E. Studies in microgravity, simulated microgravity and gravity do not support a gravitostat. J Endocrinol. 2020;247(3):273–82.
Foright RM, Presby DM, Sherk VD, Kahn D, Checkley LA, Giles ED, Bergouignan A, Higgins JA, Jackman MR, Hill JO, MacLean PS. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav. 2018;188:86–93.
Funding
NIH KL2 TR002534 (VS), ASBMR Rising Star Award (VS)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any further conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
This article was prepared while Vanessa Sherk, PhD, was employed at the University of Colorado. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition, Exercise and Lifestyle
Rights and permissions
About this article
Cite this article
Bermudez, B., Ishii, T., Wu, YH. et al. Energy Balance and Bone Health: a Nutrient Availability Perspective. Curr Osteoporos Rep 21, 77–84 (2023). https://doi.org/10.1007/s11914-022-00765-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00765-4