Abstract
Purpose of Review
Calcium and vitamin D supplementation is recommended for patients at high risk of fracture and/or for those receiving pharmacological osteoporosis treatments. Probiotics are micro-organisms conferring a health benefit on the host when administered in adequate amounts, likely by influencing gut microbiota (GM) composition and/or function. GM has been shown to influence various determinants of bone health.
Recent Findings
In animal models, probiotics prevent bone loss associated with estrogen deficiency, diabetes, or glucocorticoid treatments, by modulating both bone resorption by osteoclasts and bone formation by osteoblast. In humans, they interfere with 25-hydroxyvitamin D levels, and calcium intake and absorption, and slightly decrease bone loss in elderly postmenopausal women, in a quite similar magnitude as observed with calcium ± vitamin D supplements. A dietary source of probiotics is fermented dairy products which can improve calcium balance, prevent secondary hyperparathyroidism, and attenuate age-related increase of bone resorption and bone loss.
Summary
Additional studies are required to determine whether probiotics or any other interventions targeting GM and its metabolites may be adjuvant treatment to calcium and vitamin D or anti-osteoporotic drugs in the general management of patients with bone fragility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supplements of vitamin D ± calcium are recommended for osteoporotic patients with low calcium intake or absorption, vitamin D insufficiency, or under pharmacological treatment for osteoporosis [1]. Intakes of 800–1000 mg/day of calcium and 800 IU of vitamin D are recommended in the general management of patients with osteoporosis [2•]. However, the efficacy of calcium and vitamin D treatment on fracture risk reduction and hence its role in osteoporosis treatment have been challenged over the last decade. Calcium supplements associated with vitamin D treatment, not calcium supplementation alone, are associated with a modest reduction in fracture risk. Adverse events of calcium supplementation include mainly gastrointestinal symptoms and renal stones. Higher cardiovascular risk resulting from calcium supplementation at appropriate doses has not been confirmed by current evidence. In addition, high loading dose of vitamin D may increase the risk of fall and fracture and is no more recommended [3,4,5].
Probiotics are live micro-organisms which, when given in adequate amounts, meaning able to trigger the targeted effect, confer a health benefit to the host [6••]. Their adequacy and strength depend on food processing and matrix, strain specificity, and the targeted effect. Probiotics are available as yogurt, milk-based foods, powder, capsules, or solutions like ice cream and beer. The probiotic bacteria concentration for every gram is approximately 10e7 to 10e8, with a serving size of 100 to 200 mg. Probiotics usually administered include Lactobacillus, Bifidobacterium, Escherichia, Enterococcus, and Bacillus subtilis, as well as yeast like Saccharomyces. Gut microbiota (GM) is more and more recognized as an important determinant of bone health. Its composition changes in relation with age [7, 8], sex [9], diet [10, 11], living conditions, geography [8, 12], diseases requiring or not antibiotics treatment, and various drugs [13, 14]. Within dietary intakes, pre- and probiotics are also major determinants of GM composition and function.
In this paper, the contribution of probiotics to bone health and how they may interfere in osteoporosis management are discussed.
Evidence for a Role of Gut Microbiota in Bone Metabolism
Germ-Free Animals
Germ-free (GF) mice with a C57BL/6 genetic background, characterized by the absence of GM, have higher bone mass and better microstructure, with higher relative bone volume, cortical area, and trabecular number. When these mice are recolonized with a normal GM by 3 weeks of age, trabecular BMD and cortical area are lower than in GF mice controls. These differences in the bone phenotype are associated with change in osteoclasts number and activity, since osteoclast number is reduced while bone formation rate is maintained in GF animals. In contrast, osteoclast precursors are increased in GF mice recolonized with a normal GM. These data indicate that in the absence of GM, bone mass and microstructure are better in relation with a decreased bone resorption [15]. Normal GM also supports growth and bone development in Balb/c and CB6F1 mice [16, 17], indicating that the mouse genetic background influences how the GM affects bone physiology.
Antibiotics
Low doses of antibiotics in early life change fecal microbiome and the expression of genes implicated in carbohydrate metabolism, with increased short-chain fatty acid production and in hepatic lipid metabolism [18]. They have been used as growth promoters in poultry and cattle industry [19]. Subtherapeutic doses of various antibiotics, such as low dose penicillin from birth on or from weaning, modulate BMD in female mice, suggesting that intestinal microbiota alterations during a critical development window exert lasting metabolic consequences [20]. Tetracycline administration is also associated with higher bone strength [21] and has been shown to prevent OVX-induced bone loss [22].
Effects of Probiotics Administration on Bone
The effect on bone health of the direct administration of some bacteria to the gastrointestinal tract, i.e., probiotics use, has been tested in intervention studies in animal and human.
In animal models (Table 1)
OVX-induced bone loss in mice is prevented by various probiotics including Lactobacillus reuteri [24], Lactobacillus paracasei prevent OVX-mediated bone loss [27, 31], and Lactobacillus helveticus fermented milk [30]. Bifidobacterium longum partially prevent OVX-induced bone loss in rats, without significantly affecting bone strength [32]. In these models, the decrease of osteoclastic bone resorption with probiotics is associated with a decrease of pro-inflammatory cytokines (TNFα, IL-1β) and RANKL expression osteoclastic bone resorption [24, 31]. Other models showed a benefit of probiotics on inflammation observed in various conditions in bone. In a rat model of collagen-induced arthritis, Lactobacillus casei decreases both TNF-alpha and IL6 production, and increases the anti-inflammatory cytokine IL-10 [34]. In rodents, Bacillus subtilis reduces periodontitis-stimulated bone loss [35]. Saccharomyces cerevisiae blunts alveolar bone loss, by decreasing the expression of IL-1β, TNFα, and IL-10 [36].
The effects of probiotics administration on bone were not only observed in high-bone turnover conditions. Bacillus longum combined with the prebiotic yacon flour increases bone mineral content in rats [33]. Lactobacillus reuteri also increases vertebral and femoral BMD in male mice, but not in female mice [23], and prevents bone loss in type-1 diabetes [25] and trabecular bone loss in glucocorticoid-treated mice [26]. No benefit of Lactobacillus rhamnosus on glucocorticoid-induced bone loss was observed in the same study [26]. In diabetic mice and glucocorticoid-treated mice, two models characterized by low bone turnover pattern with low bone formation, Lactobacillus reuteri treatment prevented the TNFα suppression of Wnt10b in bone which has been implicated in the decrease of osteoblast activity and osteoporosis activity in these two conditions [25, 26].
In humans
Oral supplementation with bile salt hydrolase-active Lactobacillus reuteri increases circulating 25-hydroxyvitamin D levels, without effect any on other fat-soluble vitamins, and reduces cholesterol and the absorption of non-cholesterol sterols in hypercholesterolemic adults [37]. Probiotics also improve vitamin D levels in women with gestational diabetes [38] or after bariatric surgery to a higher extent than those observed in controls in parallel to weight loss [39, 40]. The mechanisms of this effect on vitamin D remain unclear, and may involve increased intestinal production of lactic acid, synthesis of 7-dehydrocholesterol, and higher expression and activity of vitamin D receptors.
In a 6-month randomized controlled trial, healthy 1- to 6-year old children receiving milk fortified with 5 × 10e8 Lactobacillus reuteri have a greater weight and height monthly gain [41].
Five randomized placebo-controlled trials have assessed the effects of probiotics on bone metabolism in healthy postmenopausal women (Table 2). Different amounts of various strains were administered for 6 or 12 months. Some decrease in bone resorption markers was observed in 3 of the trials, and a benefit on BMD in 4 of them. In one study, lactic acid bacteria were combined with isoflavone, so that the specific contribution of probiotic on the benefit on lumbar spine, femoral neck, and trochanter BMD (1.2 to 2.1% positive difference as compared with the placebo group) is difficult to individualize [43]. The 3 other studies used various probiotic strains (Lactobacillus reuteri, Bacillus subtilis, or a combination of 3 Lactobacillus strains) which prevented bone loss at the distal tibia, at the lumbar spine, or at the hip, respectively [44, 45, 47]. The magnitude of the effect (≤ 1 percentage point difference versus placebo at 12 months) was however much lower than those observed with anti-resorptive drugs used in osteoporosis treatment, but of the same order compared with calcium ± vitamin D (Fig. 1) [48]. None of these studies was designed to test the effect on incident fractures.
The effects of probiotics (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and Lactobacillus plantarum DSM 15313) on changes in spine BMD relative to placebo in the study of Jansson et al. [46] (X), compared with the effect of various agents tested in other studies (O). Adapted from Hauselmann and Rizzoli [48], with permission from the publisher. The size of the symbols (O) is proportional to the number of patients evaluated at the end of the studies. Displacement of the dot on the right and below the equality line reflects the magnitude of treatment effects
In a double-blind placebo-controlled clinical trial including 417 elderly patients with an acute distal radius fracture, Lactobacillus casei Shirota accelerated functional recovery, with treatment outcomes of patients receiving probiotic at month 4 at comparable levels with those of patients receiving placebo at month 6, suggesting that probiotic may accelerate the fracture healing process [49].
Additional studies are required to confirm these data and to optimize the choice of probiotic strains, usually based for the clinical interventions reported above on preclinical works in animal models, and the dose of these strains to maximize the benefit on bone. It is likely that the benefit on bone loss prevention may depend of time since menopause and bone loss rate, as suggested in the largest intervention study showing that the protective effect of Lactobacillus treatment was significant for participants below, but not above the median time since menopause [46]. A major limitation is certainly the amount of bacteria ingested. For instance, in adult monozygotic tweens, 2 servings per day of fermented milk products containing 5 different species of bacteria did not modify the large intestine GM composition. In contrast, when the same fermented milk products were given to gnotobiotic mice by gavage, there was a rapid change (within 24 h) in microbiome-encoded enzymes affecting carbohydrate metabolism [50].
Fermented Dairy Products
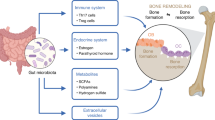
In humans, fermented dairy products are the primary source of probiotics [51•]. However, the specific effects of probiotics on bone as compared with calcium, protein, phosphorus, or zinc, as well as prebiotics as also provided by dairy products, are difficult to specifically identify (Fig. 2). Furthermore, the problem remains as to whether a sufficient amount of bacteria is capable of reaching the distal part of the gastrointestinal tract. Some data suggest that when yogurt is consumed on a regular basis, it influences the composition and metabolism of the human intestinal microbiota. Indeed, yogurt consumers have lower level of Enterobacteriaceae and higher beta-galactosidase activity in their GM. Beta-galactosidase activity (and Bifidobacterium population) is positively correlated to the quantity of fermented products ingested [52].
Effects of fermented dairy products on bone mass and metabolism. Adapted from Rizzoli and Biver [51•], with permission from Springer Nature
Some observational studies have examined the associations between bone traits and fermented milk products. In an Irish cohort of 4310 community dwelling older adults (> 60 years), each unit increase in yogurt intake was associated with a 39% lower risk in women, and 52% lower risk of osteoporosis in men [53]. The associations between yogurt and bone were of higher magnitude than with milk intake. In a cross-sectional and longitudinal study in older women (65 years of age) from the Geneva Retirees cohort, yogurt consumers had larger bone size at the distal tibia and radius. Compared with non-consumers, cortical bone loss at the radius was attenuated in these subjects, not in milk or ripened cheeses consumers, independently of total calcium, protein, and energy intakes [54]. In a 12-year follow-up of the Framingham Offspring Study, dairy product intake, including yogurts, was associated with lower trochanter BMD loss, with a weak protective trend for hip fracture, while there was no significant association with other dairy groups [55]. In a long-term follow-up of Swedish postmenopausal women, mortality and fracture rate were lower in the women with a high compared with low intake of cheese or fermented milk products. For each serving, the rates of mortality and of hip fracture were lower by 10–15% (P < 0.001) [56].
Intervention studies using fermented dairy products, fortified or not, to promote bone health have been reviewed previously [51•]. They increase IGF-I and are effective to promote bone mineral accretion during growth, whereas in adults, a dairy improves calcium balance and prevents secondary hyperparathyroidism, and age-related increases in bone resorption and bone loss.
Mechanisms of Interaction Between Probiotics and Bone Health
GM Metabolites and Intestinal Wall Permeability
GM produces various metabolites, either from endogenous compounds that are generated by the microorganisms themselves and their hosts or from the fermentation of undigested dietary components that reach the colon. They are key regulators of the integrity of the gut epithelium. These metabolites can also translocate from the gut across a disrupted intestinal barrier to modulate multiple inflammatory or metabolic processes [57]. A compromised gut permeability may contribute to multiple chronic diseases including osteoporosis by promoting the absorption of toxins and pathogens and decreasing nutrient bioavailability. In sex hormone deficiency, intestinal wall permeability is increased in relation with a reduction in gap junction protein transcripts [58]. The administration of probiotics prevents this increase in gut permeability and lowers the production of osteoclastogenic cytokines [29]. In addition, treatment with a non-absorbable mucus supplement that enhances intestinal barrier function prevents glucocorticoid-mediated osteoblast and osteocyte apoptosis in mice or Salmonella-induced bone loss in broiler chickens [26, 59].
Interaction with Diet and Prebiotics
Prebiotics are non-digestible fiber saccharides that pass undigested the upper part of the gastro-intestinal tract. By acting as substrate for GM, they stimulate the growth and/or metabolism of bacteria of the large bowel [60]. Various prebiotics such as galactooligosaccharides, fructooligosaccharides, fiber dextrin, inulin, and agarve fructans, at doses up to 20 g/day in human, increase the number of bifidobacteria and lactobacilli, and decrease that of coliforms. Prebiotics may also have direct effects on the immune system without being metabolized [61]. The fiber content of the diet markedly influences GM composition and metabolism [62], as does the ingestion of probiotics [63], and may interfere in the pathogenesis of several chronic diseases. For instance, an animal-based diet increases fat and protein intakes and leads to inter-individual differences in GM composition and microbial gene expression which may support the link between dietary fat, bile acids, and the outgrowth of microorganisms capable of triggering inflammatory bowel disease [10]. A Mediterranean diet, which is rich in fiber, fermented dairy products, and polyphenols, is associated with changes in the GM and various health benefits, including a lower hip fracture risk [64,65,66].
Both experimental animal models and intervention studies in human suggest that prebiotics influence calcium absorption and retention, and bone mineral density (for review see [60]). The evidence on BMD in human is however limited with few studies of relatively low quality (low number of participants and heterogeneity of the populations, not at risk of low calcium diet/absorption which may have minimize the effects) [67,68,69,70,71].
Fermentation of prebiotic fibers by saccharolytic microbes within the large intestine leads to a reduction in intestinal pH and to the synthesis of short-chain fatty acids (SCFAs), including acetate, propionate, valerate, isovalerate, butyrate, and isobutyrate [60, 62, 72, 73]. These microbial metabolites have emerged as key bone regulatory factors produced by the GM and diffusing into the circulation [74]. In addition to their influence on limiting intestinal wall permeability [75], SCFAs may indeed not only inhibit osteoclast number and activity, and thereby bone resorption, but also stimulate bone formation by promoting Wnt10b signaling in bone marrow stromal cells, leading to their proliferation and differentiation into osteoblasts. Both mechanisms involve interactions of SCFAs with Treg cells. These data indicate that probiotics, prebiotics, and diet may influence bone remodeling. This is supported by studies showing that SCFAs have direct effects on bone metabolism and bone mass. For instance, when mice are given SCFAs (acetate, propionate, or butyrate) in the drinking water, there are an increase in trabecular bone volume, and a reduction in osteoclast number and biochemical markers of bone resorption [76]. Furthermore, propionate or butyrate prevents OVX-induced as well as inflammation-dependent bone loss by inhibiting osteoclast differentiation and bone resorption [76]. Butyrate increases osteoblast differentiation [77] and bone formation, and is associated with higher bone sialoprotein and osteoprotegerin production [78].
Interaction with the Immune System and Inflammation
There is a close interplay between the immune and bone systems, and it is well established that chronic inflammatory conditions are associated with osteoporosis [79]. GM can modulate the immune system development, since in GF animals, hence lacking GM, mucosal and spleen immune systems are immature [80]. The beneficial effect of probiotics on BMD involves, as reported above for SCFAs, the contribution of the immune system. In male mice, lymphocytes are critical for the beneficial effects of L. reuteri on BMD and experiments using L. reuteri supernatants demonstrated that the regulation of T-lymphocytes is mediated, at least partially, by factors secreted by the probiotic strain [81]. OVX-induced bone loss is not observed in OVX mice depleted of T cells or lacking the T cell costimulatory molecule CD40 ligand [82]. In OVX germ-free mice, there is no increase of TNFα+ T cells in the bone marrow, contrary to what is observed in control OVX mice [83]. Since TNFalpha is a central cytokine involved in bone loss induced by estrogen deficiency, GM may thus be necessary to present the antigens stimulating TNFα production by T cells [84]. In addition, aging, which is one of the main risk factor of osteoporosis, is associated with modifications of the GM characterized by the increase of the proportion of opportunistic pro-inflammatory bacteria, a reduction in genes involved in pathways responsible for the production of SCFAs, and an increase in bacterial genes involved in tryptophan metabolism pathways [85]. The amount of gut pro-inflammatory bacteria is correlated with plasma levels of cytokines such as IL-6 and IL-8, and therefore with systemic low-grade inflammation [86]. Multiple additional factors (place of residence, frailty, comorbidities, drugs, markers of inflammation, and nutritional status…), including well-established risk factors of bone fragility, contribute to GM composition and its greater inter-individual variations in older people compared with younger adults [7, 8].
Interaction with Estrogens
The interaction of sex steroids with GM and its impact on bone metabolism have been shown in hypogonadal mice. Sex hormone deficiency (ovariectomy, OVX) is associated with attenuated cortical and trabecular bone loss in germ-free animals compared with controls, in relation with a lower bone resorption [29]. In these models, estrogen deficiency is associated with increased intestinal permeability, possibly due to the reduction in gap junction protein transcripts [29, 58]. In addition, OVX increases GM diversity and number of Bacteroidetes phylum, and reduces short-chain fatty acid production [87]. Interestingly, probiotics supplementation prevents sex steroid deficiency–associated bone loss in these mice [29]. These data suggest that GM composition and its metabolites may modulate postmenopausal bone loss. It remains unknown how it may interfere with menopausal hormone therapy or antiresorptive drugs used in the treatment of postmenopausal osteoporosis.
Interaction with Vitamin D
Preclinical studies demonstrated that vitamin D receptor plays a critical role in mucosal barrier homeostasis by preserving the integrity of junction complexes of the colonic epithelium [88]. In addition, lack of VDR induces dysbiosis since cecal content and stools of VDR knock-out animals are depleted in lactobacillus and enriched in clostridium and bacteroides [89]. In human, GM composition and circulating levels of lipopolysaccharide, an endotoxin from the outer membrane of most Gram-negative bacteria known to promote low-grade inflammation, vary according to vitamin D intake or circulating calcifediol levels [90,91,92]. These data suggest that vitamin D deficiency may compromise the mucosal barrier, leading to increased intestinal permeability and potentially chronic low-grade inflammation. The modulation of gut microbiome with vitamin D3 supplementation seems to predominate in the upper gastrointestinal tract [93]. In addition, probiotic strains such as Lactobacillus rhamnosus and Lactobacillus plantarum increase VDR expression in both mouse and human intestinal epithelial cells [94]. A protection of Salmonella-induced colitis by these probiotics is observed in VDR+/+, but not in VDR−/− mice, indicating that vitamin D pathways are required for probiotic protection in colitis [94].
Interaction with Calcium
GM is associated with the digestion and availability for absorption of various ingested nutrients including dietary carbohydrates, proteins, plant polyphenols, bile acids, and vitamins, and is therefore a key factor in shaping the biochemical profile of the diet [95]. This interaction between GM and prebiotics promotes calcium absorption via various mechanisms: first, the reduction in bowel content pH increases calcium bioavailability [73, 96]. Second, calcium surface absorption is increased, since cellular uptake of SCFAs increases intestinal cell proliferation resulting in increased intestinal crypt depth and greater cell density and blood flow in the villi [73, 97]. Last, SCFAs may signal for greater gene expression of the intracellular calcium transporters [60]. Higher calcium absorption decreases parathyroid hormone (PTH) production and may thereby lower bone resorption [98].
In addition, studies in animals suggest that calcium from the diet or supplements might interfere with gut microbiota, and partly explains the beneficial effects of calcium on body weight/fat loss [99]. Calcium supplementation in dietary obese animals has a prebiotic-like effect which modulates GM composition in favor of potentially beneficial bacteria in the gut, and in turn may modulate systemic low-grade inflammation, as demonstrated by the lower plasma endotoxin LPS in host animals receiving calcium supplements compared with controls [100].
Interaction with Other Bone Regulatory Pathways
Interactions between GM and several bone regulatory pathways, including PTH, IGF-I, or serotonin, have also been reported. Bone loss associated with primary hyperparathyroidism involves microbial-dependent expansion of intestinal TNFα+ T cells and Th17 cells [101]. In addition, butyrate production by gut luminal microbiota is required for the bone anabolic activity of PTH [102]. Serum IGF-I levels in mice are increased in response to microbial colonization, while it decreases after antibiotic treatment. Supplementation of antibiotic-treated mice with SCFAs restores IGF-I concentrations and bone mass to levels observed in control mice [17]. In GF animals, serotonin, known to reduce bone formation secretion, is decreased in relation to lower tryptophan hydroxylase-1 expression in the large intestine [103].
Conclusion
There is compelling evidence supporting that probiotics may improve bone health. In animal models, probiotics prevent bone loss associated with estrogen deficiency, diabetes or glucocorticoid treatments, by modulating both bone resorption by osteoclasts and bone formation by osteoblast in relation with GM composition and metabolism. In humans, they interfere with 25-hydroxyvitamin D levels, and calcium intake and absorption, and slightly decrease bone loss in elderly postmenopausal women, in a quite similar magnitude as observed with calcium and vitamin D supplements. A dietary source of probiotics is fermented dairy products which benefits calcium balance and prevents secondary hyperparathyroidism, age-related increase of bone resorption, and age-related bone loss. However, some important issues remain to be elucidated. In some models, the response to probiotics is sex specific and seems more readily detectable in subjects with high bone turnover like children or adolescents or early postmenopausal women [23, 46]. The types and doses of probiotics, in terms of efficacy and tolerance, the time and duration of administration, and the offset of the effects upon probiotics discontinuation need still to be defined. Finally, there is currently no data demonstrating whether probiotics may reduce fracture risk. With this respect, genetic background, sex, immune status, age, diet, living conditions, geography, and drugs are likely important confounding factors in evaluating the effects of probiotics on bone health. Additional studies are required to determine whether probiotics or any other interventions targeting GM and its metabolites such as prebiotics may be adjuvant treatment to calcium and vitamin D supplements, anti-osteoporotic drugs and also to promotion of a balance diet and regular physical activity in the general management of patients with bone fragility.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Harvey NC, Biver E, Kaufman JM, Bauer J, Branco J, Brandi ML, et al. The role of calcium supplementation in healthy musculoskeletal ageing : an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int. 2017;28(2):447–62. https://doi.org/10.1007/s00198-016-3773-6.
• Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. https://doi.org/10.1007/s00198-018-4704-5An updated summary of the management of patients with osteoporosis.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. Jama. 2010;303(18):1815–22. https://doi.org/10.1001/jama.2010.594.
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–83. https://doi.org/10.1001/jamainternmed.2015.7148.
Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA Jr, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5(6):438–47. https://doi.org/10.1016/s2213-8587(17)30103-1.
•• McCabe LR, Parameswaran N. Advances in probiotic regulation of bone and mineral metabolism. Calcif Tissue Int. 2018;102(4):480–8. https://doi.org/10.1007/s00223-018-0403-7An extensive and updated review on the role of probiotics in bone metabolism.
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–91. https://doi.org/10.1073/pnas.1000097107.
Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. https://doi.org/10.1038/nature11319.
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. https://doi.org/10.1371/journal.pone.0010667.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. https://doi.org/10.1038/nature12820.
Beaumont M, Portune KJ, Steuer N, Lan A, Cerrudo V, Audebert M, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am J Clin Nutr. 2017;106(4):1005–19. https://doi.org/10.3945/ajcn.117.158816.
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. https://doi.org/10.1126/science.1237439.
Devkota S. MICROBIOME. Prescription drugs obscure microbiome analyses. Science. 2016;351(6272):452–3. https://doi.org/10.1126/science.aaf1353.
Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–8. https://doi.org/10.1038/nature25979.
Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67. https://doi.org/10.1002/jbmr.1588.
Schwarzer M, Strigini M, Leulier F. Gut microbiota and host juvenile growth. Calcif Tissue Int. 2017;102:387–405. https://doi.org/10.1007/s00223-017-0368-y.
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554–e63. https://doi.org/10.1073/pnas.1607235113.
Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. https://doi.org/10.1038/nature11400.
Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13(1):7–27. https://doi.org/10.1081/abio-120005767.
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21. https://doi.org/10.1016/j.cell.2014.05.052.
Pytlik M, Folwarczna J, Janiec W. Effects of doxycycline on mechanical properties of bones in rats with ovariectomy-induced osteopenia. Calcif Tissue Int. 2004;75(3):225–30. https://doi.org/10.1007/s00223-004-0097-x.
Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, et al. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19(6):637–44.
McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228(8):1793–8. https://doi.org/10.1002/jcp.24340.
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–30. https://doi.org/10.1002/jcp.24636.
Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156(9):3169–82. https://doi.org/10.1210/en.2015-1308.
Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2019. https://doi.org/10.1002/jbmr.3947.
Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus -fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011;59(14):7734–42. https://doi.org/10.1021/jf2013716.
Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J, Mizumachi K. Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr. 2007;98(6):1178–86. https://doi.org/10.1017/s0007114507787469.
Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–63. https://doi.org/10.1172/jci86062.
Narva M, Rissanen J, Halleen J, Vapaatalo H, Vaananen K, Korpela R. Effects of bioactive peptide, valyl-prolyl-proline (VPP), and lactobacillus helveticus fermented milk containing VPP on bone loss in ovariectomized rats. Ann Nutr Metab. 2007;51(1):65–74. https://doi.org/10.1159/000100823.
Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. https://doi.org/10.1371/journal.pone.0092368.
Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, et al. Probiotics (Bifidobacterium longum) increase bone mass density and Upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. 2015;2015:897639. https://doi.org/10.1155/2015/897639.
Rodrigues FC, Castro AS, Rodrigues VC, Fernandes SA, Fontes EA, de Oliveira TT, et al. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food. 2012;15(7):664–70. https://doi.org/10.1089/jmf.2011.0296.
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J Clin Immunol. 2011;31(2):147–54. https://doi.org/10.1007/s10875-010-9457-7.
Foureaux Rde C, Messora MR, de Oliveira LF, Napimoga MH, Pereira AN, Ferreira MS, et al. Effects of probiotic therapy on metabolic and inflammatory parameters of rats with ligature-induced periodontitis associated with restraint stress. J Periodontol. 2014;85(7):975–83. https://doi.org/10.1902/jop.2013.130356.
Garcia VG, Knoll LR, Longo M, Novaes VC, Assem NZ, Ervolino E, et al. Effect of the probiotic Saccharomyces cerevisiae on ligature-induced periodontitis in rats. J Periodontal Res. 2016;51(1):26–37. https://doi.org/10.1111/jre.12274.
Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(7):2944–51. https://doi.org/10.1210/jc.2012-4262.
Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(5):2098–105. https://doi.org/10.1016/j.clnu.2018.10.028.
Karbaschian Z, Mokhtari Z, Pazouki A, Kabir A, Hedayati M, Moghadam SS, et al. Probiotic supplementation in morbid obese patients undergoing one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB) surgery: a randomized, double-blind, placebo-controlled. Clin Trial Obes Surg. 2018;28(9):2874–85. https://doi.org/10.1007/s11695-018-3280-2.
Mokhtari Z, Karbaschian Z, Pazouki A, Kabir A, Hedayati M, Mirmiran P, et al. The effects of probiotic supplements on blood markers of endotoxin and lipid peroxidation in patients undergoing gastric bypass surgery; a randomized, double-blind, placebo-controlled, clinical trial with 13 months follow-up. Obes Surg. 2019;29(4):1248–58. https://doi.org/10.1007/s11695-018-03667-6.
Agustina R, Bovee-Oudenhoven IM, Lukito W, Fahmida U, van de Rest O, Zimmermann MB, et al. Probiotics Lactobacillus reuteri DSM 17938 and Lactobacillus casei CRL 431 modestly increase growth, but not iron and zinc status, among Indonesian children aged 1-6 years. J Nutr. 2013;143(7):1184–93. https://doi.org/10.3945/jn.112.166397.
Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind. Controlled Trial J Am Coll Nutr. 2017;36(7):497–506. https://doi.org/10.1080/07315724.2017.1318724.
Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, et al. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017;106(3):909–20. https://doi.org/10.3945/ajcn.117.153353.
Nilsson AG, Sundh D, Backhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018. https://doi.org/10.1111/joim.12805.
Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, et al. s Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health. 2018;37(4):87–96. https://doi.org/10.12938/bmfh.18-006.
Jansson P-A, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1(3):e154–e62. https://doi.org/10.1016/S2665-9913(19)30068-2.
Ohlsson C, Curiac D, Sjogren K, Jansson PA. Probiotic treatment using a mix of three Lactobacillus strains protects againts lumbar spine bone loss in healthy early postmenopausal women. Journal of Bone and Mienral Research. 2018;33:S24.
Hauselmann HJ, Rizzoli R. A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int. 2003;14(1):2–12. https://doi.org/10.1007/s00198-002-1301-3.
Lei M, Hua LM, Wang DW. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benef Microbes. 2016;7(5):631–7. https://doi.org/10.3920/bm2016.0067.
McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3(106):106ra. https://doi.org/10.1126/scitranslmed.3002701.
• Rizzoli R, Biver E. Effects of fermented milk products on bone. Calcif Tissue Int. 2018;102(4):489–500. https://doi.org/10.1007/s00223-017-0317-9Fermented dairy products are providing probiotics, protein, mineral and various micronutrients, and their effects on bone represent an example of the role of food matrix.
Alvaro E, Andrieux C, Rochet V, Rigottier-Gois L, Lepercq P, Sutren M, et al. Composition and metabolism of the intestinal microbiota in consumers and non-consumers of yogurt. Br J Nutr. 2007;97(1):126–33. https://doi.org/10.1017/s0007114507243065.
Laird E, Molloy AM, McNulty H, Ward M, McCarroll K, Hoey L, et al. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos Int. 2017;28(8):2409–19. https://doi.org/10.1007/s00198-017-4049-5.
Biver E, Durosier-Izart C, Merminod F, Chevalley T, van Rietbergen B, Ferrari SL, et al. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos Int. 2018;29(8):1771–82. https://doi.org/10.1007/s00198-018-4535-4.
Sahni S, Tucker KL, Kiel DP, Quach L, Casey VA, Hannan MT. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: the Framingham Offspring Study. Arch Osteoporos. 2013;8:119. https://doi.org/10.1007/s11657-013-0119-2.
Michaëlsson K, Wolk A, Langenskiöld S, Basu S, Warensjö Lemming E, Melhus H, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015.
Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40–54. https://doi.org/10.1038/s41577-019-0198-4.
Collins FL, Schepper JD, Rios-Arce ND, Steury MD, Kang HJ, Mallin H, et al. Immunology of gut-bone signaling. Adv Exp Med Biol. 2017;1033:59–94. https://doi.org/10.1007/978-3-319-66653-2_5.
Raehtz S, Hargis BM, Kuttappan VA, Pamukcu R, Bielke LR, McCabe LR. High molecular weight polymer promotes bone health and prevents bone loss under Salmonella challenge in broiler chickens. Front Physiol. 2018;9:384. https://doi.org/10.3389/fphys.2018.00384.
Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcif Tissue Int. 2018;102(4):443–79. https://doi.org/10.1007/s00223-017-0339-3.
Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–10. https://doi.org/10.1038/nrgastro.2015.47.
Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–30. https://doi.org/10.1007/s11914-015-0257-0.
Druart C, Alligier M, Salazar N, Neyrinck AM, Delzenne NM. Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv Nutr. 2014, 5(5):624s–33s. https://doi.org/10.3945/an.114.005835.
Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117(12):1645–55. https://doi.org/10.1017/s0007114517001593.
De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21. https://doi.org/10.1136/gutjnl-2015-309957.
Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. 2018;73(3):318–26. https://doi.org/10.1093/gerona/glx227.
Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82(2):471–6. https://doi.org/10.1093/ajcn.82.2.471.
Abrams SA, Griffin IJ, Hawthorne KM. Young adolescents who respond to an inulin-type fructan substantially increase total absorbed calcium and daily calcium accretion to the skeleton. J Nutr. 2007;137(11 Suppl):2524s–6s. https://doi.org/10.1093/jn/137.11.2524S.
Kim Y-Y, Jang K-H, Lee E-Y, Cho Y-H, Kang S-A, Ha W-K, et al. The effect of chicory fructan fiber on calcium absorption and bone metabolism in Korean postmenopausal women. Nutritional sciences. 2004;7(3):151–7.
Slevin MM, Allsopp PJ, Magee PJ, Bonham MP, Naughton VR, Strain JJ, et al. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J Nutr. 2014;144(3):297–304. https://doi.org/10.3945/jn.113.188144.
Tu MY, Chen HL, Tung YT, Kao CC, Hu FC, Chen CM. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One. 2015;10(12):e0144231. https://doi.org/10.1371/journal.pone.0144231.
Garcia-Vieyra MI, Del Real A, Lopez MG. Agave fructans: their effect on mineral absorption and bone mineral content. J Med Food. 2014;17(11):1247–55. https://doi.org/10.1089/jmf.2013.0137.
Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem. 2011;59(12):6501–10. https://doi.org/10.1021/jf2009777.
Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129(8):3018–28. https://doi.org/10.1172/jci128521.
Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26. https://doi.org/10.1016/j.cmet.2011.02.018.
Lucas S, Omata Y, Hofmann J, Bottcher M, Iljazovic A, Sarter K, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. https://doi.org/10.1038/s41467-017-02490-4.
Lee HW, Suh JH, Kim AY, Lee YS, Park SY, Kim JB. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol. 2006;20(10):2432–43. https://doi.org/10.1210/me.2006-0061.
Katono T, Kawato T, Tanabe N, Suzuki N, Iida T, Morozumi A, et al. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch Oral Biol. 2008;53(10):903–9. https://doi.org/10.1016/j.archoralbio.2008.02.016.
Terashima A, Takayanagi H. Overview of osteoimmunology. Calcif Tissue Int. 2018;102(5):503–11. https://doi.org/10.1007/s00223-018-0417-1.
Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–85. https://doi.org/10.1038/nri1373.
Collins FL, Rios-Arce ND, Schepper JD, Jones AD, Schaefer L, Britton RA, et al. Beneficial effects of Lactobacillus reuteri 6475 on bone density in male mice is dependent on lymphocytes. Sci Rep. 2019;9(1):14708. https://doi.org/10.1038/s41598-019-51293-8.
Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108(2):768–73. https://doi.org/10.1073/pnas.1013492108.
Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest. 1997;99(7):1699–703. https://doi.org/10.1172/jci119333.
Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12(6):935–41. https://doi.org/10.1359/jbmr.1997.12.6.935.
Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and 'Garb-aging'. Trends Endocrinol Metab. 2017;28(3):199–212. https://doi.org/10.1016/j.tem.2016.09.005.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90. https://doi.org/10.1038/s41574-018-0059-4.
Cox-York KA, Sheflin AM, Foster MT, Gentile CL, Kahl A, Koch LG, et al. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol Rep. 2015, 3(8). https://doi.org/10.14814/phy2.12488.
Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208–16. https://doi.org/10.1152/ajpgi.00398.2007.
Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. 2015, 37(5):996–1009.e7. https://doi.org/10.1016/j.clinthera.2015.04.004.
Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LG, Ferreira SR. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism. 2017;69:76–86. https://doi.org/10.1016/j.metabol.2017.01.007.
Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551–6. https://doi.org/10.21873/anticanres.13984.
Kanhere M, He J, Chassaing B, Ziegler TR, Alvarez JA, Ivie EA, et al. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2018;103(2):564–74. https://doi.org/10.1210/jc.2017-01983.
Bashir M, Prietl B, Tauschmann M, Mautner SI, Kump PK, Treiber G, et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55(4):1479–89. https://doi.org/10.1007/s00394-015-0966-2.
Wu S, Yoon S, Zhang YG, Lu R, Xia Y, Wan J, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G341–9. https://doi.org/10.1152/ajpgi.00105.2015.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. https://doi.org/10.1007/s00394-017-1445-8.
Ammann P, Rizzoli R, Fleisch H. Influence of the disaccharide lactitol on intestinal absorption and body retention of calcium in rats. J Nutr. 1988;118(6):793–5.
Mineo H, Amano M, Minaminida K, Chiji H, Shigematsu N, Tomita F, et al. Two-week feeding of difructose anhydride III enhances calcium absorptive activity with epithelial cell proliferation in isolated rat cecal mucosa. Nutrition. 2006;22(3):312–20. https://doi.org/10.1016/j.nut.2005.06.015.
Rizzoli RB, Bonjour JP. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Physiology of calcium and phosphate homeostasis. Dynamics of bone and cartilage metabolism: principles and clinical applications. San Diego: Academic Press; 2006. p. 345–60.
Zhang F, Ye J, Zhu X, Wang L, Gao P, Shu G, et al. Anti-obesity effects of dietary calcium: the evidence and possible mechanisms. Int J Mol Sci. 2019;20(12). https://doi.org/10.3390/ijms20123072.
Chaplin A, Parra P, Laraichi S, Serra F, Palou A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol Nutr Food Res. 2016;60(2):468–80. https://doi.org/10.1002/mnfr.201500480.
Yu M, Malik Tyagi A, Li JY, Adams J, Denning TL, Weitzmann MN, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat Commun. 2020;11(1):468. https://doi.org/10.1038/s41467-019-14148-4.
Li JY, Yu M, Pal S, Tyagi AM, Dar H, Adams J, et al. Microbiota dependent production of butyrate is required for the bone anabolic activity of PTH. J Clin Invest. 2020. https://doi.org/10.1172/jci133473.
Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–37. https://doi.org/10.1016/j.cell.2008.09.059.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Rizzoli reports personal fees from Abiogen, Amgen, EuropeanMilkForum, Danone, Echolight, Mylan, Radius Health, Nestlé, Rejuvenate, Sandoz, and Theramex, outside the submitted work.
Dr. Biver reports fees from Nestle paid to the institution.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition, Exercise and Lifestyle in Osteoporosis
Rights and permissions
About this article
Cite this article
Rizzoli, R., Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health?. Curr Osteoporos Rep 18, 273–284 (2020). https://doi.org/10.1007/s11914-020-00591-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00591-6