Abstract
Aberrant or prolonged immune responses often affect bone metabolism. The investigation on bone destruction observed in autoimmune arthritis contributed to the development of research area on effect of the immune system on bone. A number of reports on bone phenotypes of immunocompromised mice indicate that the immune and skeletal systems share various molecules, including transcription factors, signaling molecules, and membrane receptors, suggesting the interplay between the two systems. Furthermore, much attention has been paid to the modulation of immune cells, including hematopoietic progenitor cells, by bone cells in the bone marrow. Thus, osteoimmunology which deals with the crosstalk and shared mechanisms of the bone and immune systems became the conceptual framework fundamental to a proper understanding of both systems and the development of new therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The musculoskeletal system is made up of bones, cartilage, tendons, ligaments, muscles, and other connective tissues. The major functions of bone are to support the body, to enable the motion and to maintain the calcium storage system but also serves as the crucial site for hematopoiesis. Therefore, bone is a unique organ, which belongs to both musculoskeletal and immune systems [1, 2]. Bone marrow is a primary lymphoid organ, harboring hematopoietic stem cells as well as lymphoid and myeloid progenitors [3]. These immune cell progenitors share the microenvironment with bone cells. However, as the bone biology and immunology had been investigated separately, the close relationship between the bone and immune systems had been overlooked for a long time.
Rheumatoid arthritis (RA) is an autoimmune disease, but the bone destruction in the joints is a major clinical problem [4]. Therefore, to understand the mechanism of bone destruction in arthritis is to explore the molecular basis for the effect of immune system on bone. Receptor activator of NF-κB ligand (RANKL), the osteoclast differentiation factor aberrantly expressed in RA synovium, was found to connect the activated immune response to enhanced osteoclastic bone resorption [5, 6]. Thus, RANKL emerged as one of the key molecules linking the bone and immune systems. Studies on RANKL signal transduction showed that most of the downstream molecules play a role in the immune system [7]. In addition, genetically modified mice deficient in immunomodulatory molecules were identified to exhibit various bone phenotypes, providing evidence for the shared molecular regulatory units [1].
However, immune cells infiltrate into any organs and communicate with resident cells, which often results in the tissue damage, raising a question of whether the relationship between bone and immune system is not something special. Then, it is very important to know the role of bone cells in the regulation of immune system and hematopoiesis. It was proposed that osteoblasts comprise the hematopoietic stem cell niche, but the detailed studies revealed that osteoblasts are not equal to HSC niche cells but instrumental in HSC regulation under certain conditions [8, 9]. Thus, the bone is not merely a target organ of the immune system. From the evolutionally point of view, the development of the immune system was coincident with the emergence of terrestrial vertebrate with strong bony skeleton [10]. All the cells in the bone marrow share the same microenvironments, making it vital to understand the differentiation and function of all of these cells in the context of osteoimmune system. Here, we summarize the historically important topics as well as recent progress in the field of osteoimmunology.
Multiple RANKL Functions in Bone and the Immune System
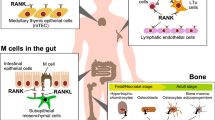
RANKL was initially identified as an activator of dendritic cells expressed by activated T cells [11, 12]. Later, RANKL was found to bind to RANK on the surface of osteoclast precursors and induce osteoclast differentiation [13, 14]. RANKL function is inhibited by osteoprotegerin (OPG), which binds to RANKL and prevents its binding to RANK [15]. Both Rankl−/− and Rank−/− mice exhibit a severe osteopetrotic phenotype and have a defect in tooth eruption due to a lack of osteoclasts. In contrast, mice lacking Opg exhibit severe osteoporosis, resulting from both an increased number and enhanced activity of osteoclasts.
Interestingly, both Rankl−/− and Rank−/− mice lack the lymph nodes (LNs) and display a defect in Peyer’s patches (PPs) and cryptopatches (CPs), demonstrating the critical role for RANK activation during the early stages of development of lymphoid tissue inducer cells in the peripheral lymphoid organs [16, 17]. A recent study shows that RANKL on mesenchymal cells induces M cell differentiation in the gut, resulting in the induction of IgA production and gut microbiota diversity [18]. Severe immunodeficiency was not observed in RANKL-deficient mice, possibly because the loss of RANKL was compensated by CD40L in Rankl−/− mice, but the pathogenic effect of RANKL expressed on CD4+ T cells was reported in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis [19, 20]. RANKL also induces the development of autoimmune regulator (Aire)-expressing medullary thymic epithelial cells, playing a role in the establishment of central tolerance in the thymus [21, 22]. Thus, RANKL is an essential regulator of both bone and immune systems.
RANKL Signals in Osteoclastogenesis
Signals driven by RANKL and macrophage-colony-stimulating factor (M-CSF) are indispensable for osteoclastogenesis. RANKL binding to RANK induces the trimerization of RANK and TRAF6, which leads to the activation of NF-κB and the mitogen-activated kinases (MAPKs), including Jun N-terminal kinase (JNK) and p38. The key role of NF-κB and activator protein 1 (AP-1) transcription factor complex in osteoclast differentiation has been demonstrated genetically [1]. Mice lacking c-fos, a component of AP-1, exhibit an osteopetrotic phenotype and have no tooth eruption [23]. In addition, it was reported that c-fos is induced not only by the NF-kB pathway but also by the calcium/calmodulin-dependent protein kinase (CaMKIV) with cyclic AMP-responsive element-binding protein (CREB) pathway [24]. Since the NF-κB p50 and p52 double-deficient mice develop severe osteopetrosis due to the defective osteoclast precursor differentiation, both the canonical (p65/p50) and non-canonical (p52) NF-κB pathways activated by the RANKL are essential for osteoclastogenesis [25].
Activation of NF-κB and AP-1/c-Fos by RANKL induces nuclear factor of activated T cells cytoplasmic 1 (NFATc1), the master regulator of osteoclast differentiation [26]. The Nfatc1 promoter contains NFAT binding sites and NFATc1 specifically autoregulates its own promoter during osteoclastogenesis, resulting in a robust induction of NFATc1 [27]. NFATc1 regulates a number of osteoclast-specific genes, such as osteoclast-associated receptor (OSCAR), dendritic cell-specific transmembrane protein (DC-STAMP), and β3-integrin in cooperation with other transcription factors such as AP-1, PU.1, and MITF [28]. It is notable that NFATc1 has an exclusive role in osteoclasts but NFATc1 and NFATc2 have a redundant role in T cells.
RANK signaling also stimulates the NFATc1 activation through Ca2+-dependent phosphatase calcineurin, which is regulated by phospholipase Cγ (PLCγ) that mediates Ca2+ release from intracellular stores [29]. The activation of PLCγ by RANK requires the protein tyrosine kinases Btk/Tec and Syk, along with immunoreceptor tyrosine-based activation motif (ITAM)-bearing molecules, such as DNAX-activating protein (DAP12) and the Fc receptor common gamma chain (FcRγ) [29, 30]. Tec tyrosine kinases are predominantly expressed in immune cells such as lymphoid and myeloid cells, and have critical roles in their development and function by regulating multiple pathways. Btk and Tec, which belong to Tec family kinases, have already known to play a key role in proximal BCR signaling. In osteoclastogenesis, Btk and Tec are associated with SH2-containing leukocyte protein (SLP) family proteins, B cell linker protein (BLNK), and lymphocyte cytosolic protein 2 (Lcp2; also known as SH2-containing leukocyte protein of 76 kDa, SLP76) together with PLCγ, which is dependent on the activation of RANK and ITAM signals. The osteopetrotic phenotype in Tec and Btk double-deficient mice revealed that these two kinases have a vital role in linking RANK and ITAM signals in the regulation of osteoclastogenesis [30]. Studies on RANKL signaling revealed that there is a huge overlap of the molecules used by the bone and immune systems. Therefore, we should consider the interactions between the two systems as well as adverse effects in the therapeutic targeting of osteoimmune molecules.
T Cell Regulation of Osteoclastogenesis in Arthritis
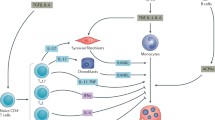
Osteoclast-like giant cells at the interface between the synovium and bone were characterized as authentic osteoclasts by bone resorbing activity as well as the expression of tartrate-resistant acid phosphatase (TRAP) and the calcitonin receptor [31, 32]. This finding prompted the rheumatologists to investigate the role of osteoclasts in the bone destruction in the synovium of RA patients. Inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-17, and tumor necrosis factor (TNF)-α, highly detected in RA synovium, have the potential to induce RANKL on synovial fibroblasts, which causes enhanced osteoclastogenesis in joints [33]. Although RANKL is also found to be expressed by T cells in the inflamed synovium, the major RANKL-expressing cell subset in arthritic joints was shown to be synovial fibroblasts using Tnfsf11flox/ΔCol6a1-cre mice [34].
How T cells induce osteoclastogenesis in arthritis? Th1 and Th2 cells produce interferon (IFN)-γ and IL-4, both of which inhibit osteoclastogenesis [1, 35]. In RA synovium, autoreactive CD4+ T cells differentiate into pathological Th17 cells that are induced by the pro-inflammatory cytokines including IL-6 and IL-23. Th17 cells accumulate in the synovial joints and produce IL-17 that upregulates RANKL on synovial fibroblasts [36, 37]. The elevated expression of RANKL increased osteoclastogenesis, resulting in severe bone destruction in the joint. IL-17 derived from Th17 further induces inflammatory cytokine including IL-6, TNF-α, and IL-1β by innate immune cells, which exacerbate the inflammation as well as RANKL induction and stimulation of the osteoclast precursor cells. In addition, the inflammatory cytokine stimulates synovial cells to produce matrix-damaging enzymes associated with cartilage destruction.
The regulatory T cells (Tregs) modulate the immune response and are responsible for maintaining tolerance to self-antigens and preventing autoimmune diseases. The development and function of Tregs require the Foxp3 transcription factor. Using Foxp3-Cre-GFP ROSA-YFP mice, in which Foxp3 in Treg cells can be monitored by the expression of GFP and YFP, CD25loFoxp3+ T cells frequently lost Foxp3 expression under arthritic conditions. Interestingly, the CD25loFoxp3+ T cells converted into IL-17-producing T cells, which are termed exFoxp3Th17 cells, whereas CD25hiFoxp3+ T cells maintained Foxp3 expression and their regulatory activity. This conversion was promoted by arthritic synovial fibroblasts [38]. The exFoxp3Th17 cells have a powerful potential to induce osteoclastogenesis and promote local inflammation.
Cooperation Between Immune and Bone Cells During Fracture Healing
As described above, the understanding of the interaction between bone and immune cells in RA reminds us of the importance of immune cell involvement in other inflammatory bone disorders and bone regeneration. Fracture repair requires multiple processes: blood clot formation at the site of injury, an inflammatory phase, callus generation, primary bone formation, and secondary bone remodeling [39]. Some reports suggest the local inflammation initiates bone regeneration by stimulating the migration of mesenchymal stem cells, fibroblast, and immune cells such as macrophages, neutrophils, and lymphocytes. The immune cells are detected in fracture hematoma. Removal of the hematoma, which contains macrophages and neutrophils, causes delayed fracture healing, indicating the importance of the hematoma during fracture healing [40]. Not only myeloid cells but also lymphoid cells infiltrate in the fracture site. A recent study suggested that IL-17A accelerates osteogenesis upon fracture healing. Further analysis revealed that IL-17-producing Vγ6+ γδ T cells promotes bone formation by stimulating the mesenchymal progenitor cells, which express IL-17 receptor A at the injury site [41]. These findings provide evidence for the role of γδ T cells in fracture repair (Fig. 1).
Contribution of Bone Cells to the Regulation of Immune Cells
Although the osteoimmunological researches first centered on the understanding that immunological responses affect bone metabolism, recent studies show that bone cells also have a role in the regulation of immune cells. Since mature blood cells are short lived, HSCs replenish multilineage progenitors throughout life. Therefore, the function of HSCs should affect host defense and immune system. The importance of the bone marrow microenvironment is evident from the fact that prolonged culturing functional HSCs outside of the animal body is difficult. Bone marrow consists of several types of cells including bone cells, hematopoietic cells, endothelial cells, and neural cells [42, 43].
Osteoblasts create new bone that provides a platform to maintain HSCs. The endosteal surface in the bone marrow was initially proposed to be a key component for supporting HSCs [44,45,46]. These reports attracted the attention of researchers on the role of osteolineage cells in regulating the HSC functions. Osteoblasts were first reported to contribute to the regulation of the HSC number, but it was shown that osteoblasts are less relevant to the maintenance of HSCs [8, 9, 47, 48]. SCF derived from perivascular cells and CXCL12-expressing mesenchymal stem cells were both vital for HSC maintenance, while SCF derived from osteoblasts did not have much function in HSC regulation [49,50,51]. Osteoblast-specific deletion of CXCL12 reduced B-lymphoid progenitors in the bone marrow but not HSC number [51]. Furthermore, DLL4 expressed by osteoblasts supported T-lineage competent cells [52].
An acute inflammatory condition leads to a lymphopenia caused by the reduction of the numbers of peripheral lymphocytes and common lymphoid progenitors (CLPs) in the bone marrow, which associated with a decrease in the osteoblast number [53]. A study of osteoblast-specific deletion of IL-7 revealed that IL-7 derived from osteoblasts supported CLPs in the bone marrow. Furthermore, virus infection was shown to induce a rapid loss of osteoblasts associated with elevated levels of circulating inflammatory cytokines [54]. Osteoblasts may also have a role in maintaining the bone marrow microenvironments to control HSC malignancy. Disruption in murine osteoblasts of Dicer, which is an RNase III endonuclease essential for microRNA synthesis and processing, resulted in HSC malignancy with concomitant osteoporosis [55].
As mice lacking osteoclasts do not have enough space to support immune cell differentiation, it seems that bone marrow cavity created by osteoclasts are also essential for maintaining immune homeostasis. Extramedullary hematopoiesis observed in osteopetrotic mice might influence the differentiation and function of the immune cells [17, 56]. Osteopetrotic patients develop anemia and severe infection due to abnormal hematopoiesis [57,58,59]. Pharmacological ablation of osteoclasts by injections of zoledronic acid led to a reduction in the expression of IL-7 and CXCL12 by stromal cells [60]. When osteoclasts degrade the bone matrix, certain factors stored in the bone matrix are released. It is possible that some of them are involved in maintaining HSCs. During bone resorption, TGF-β is released and activated by proteolytic enzymes secreted from osteoclasts. TGF-β plays an important role in regulating HSC quiescence and self-renewal. Furthermore, there is a report showing that osteoclasts enhance HSC mobilization from bone marrow to peripheral blood [61]. Although G-CSF-induced mobilization of HSC was detected in osteoclast-deficient mice, such as op/op, c-fos, and Rankl−/− mice, it has been reported that RANKL injection induced HSC mobilization by secreting protease, which is able to degrade niche components [61, 62]. These studies indicated that osteoclasts contribute to HSC support by regulating bone marrow microenvironment.
Compared to the understanding of the roles of osteoblasts and osteoclasts in the regulation of immune cells in the bone marrow, a role of osteocytes in the immune cell differentiation remains unclear. Osteocyte-ablated mice have a defect in the bone marrow, thymus, and spleen, resulting in severe lymphopenia [63]. Sclerostin is a potent inhibitor of bone formation produced by osteocytes. Sclerostin-deficient mice exhibits osteosclerosis and a reduction of mature B cells due to a decrease in the CXCL12 in bone marrow, suggesting that bone marrow microenvironment supported by osteocytes is required for the normal B hematopoiesis [64]. Thus, the bone cells regulate the differentiation and function of immune cells under resting and certain pathological conditions (Fig. 2).
The bone marrow microenvironment. Bone cells provide the bone marrow microenvironment to support the self-renewal ability, multipotency, and quiescence of HSCs. Further, osteoblasts produce IL-7, DLL4, and CXCL12 to support common lymphoid progenitors, T-lineage competent cells, and B cells, respectively. Lepr+ perivascular cells maintain HSCs by producing Stem cell factor (SCF). CAR cells, Nestin+ cells, and mesenchymal stem cells, which can differentiate into osteoblasts and adipocytes, express SCF and CXCL12 to support HSCs. Non-myelinating Schwann cells contribute to the quiescence of HSCs. The impairment of B cell differentiation in sclerostin-deficient mice suggested that osteocytes are involved in the B cell differentiation in the bone marrow. The function of osteoclasts in the mobilization of HSCs is still controversial
Cellular Communication in the Bone Marrow
Cells in the bone marrow communicate with each other to maintain various bone function, but the molecules mediating the cells in the bone marrow are not fully characterized. Osteoclasts can produce bone cell communicating factors including ephrinB2, S1P, BMP6, and platelet-derived growth factor (PDGF)-BB [65,66,67,68]. Bidirectional signaling ephrinB2 on osteoclasts and EphB4 on osteoblasts enhances both osteoblast differentiation and osteoclast suppression [65]. Blocking of BMP6 and S1P in osteoclasts inhibits mineralization based on an in vitro study [67]. Osteoclast precursors also express S1P receptor, and the concentration of S1P in blood is higher than in tissues. It has been reported that S1P-mediated chemotaxis of osteoclast precursors would contribute to their recirculation from bone to systemic blood flow, regulating osteoclastogenesis [66]. PDGF-BB derived from preosteoclasts promotes vessel formation needed for the subsequent bone resorption and new bone formation. Osteoclasts can also suppress bone formation by expressing semaphorin (Sema) 4D. The binding of Sema4D to Plexin-B1 on osteoblasts inhibits osteoblastic bone formation through RhoA activation. Sema4D guards the resorption area from bone formation [69].
The source of RANKL in bone remodeling had not been clear, until osteocyte-specific deletion of Rankl had shown that osteocytes are the critical source of Rankl in the adult mice. Osteoblast lineage cells also produce osteoprotegerin (OPG) which suppresses osteoclast activation by masking RANKL. A study of Opg-deficient mice indicated that another anti-osteoclastogenic factor, Sema3a, is expressed by osteoblasts and inhibits osteoclast differentiation. Sema3a binds to a receptor complex of the ligand-binding subunit Neuropilin-1 and one of the class A plexins. PlexinA1 promotes osteoclast differentiation by activating ITAM signal through the formation of the PlexinA1-TREM2-DAP12 complex in response to ligands such as Sema6D. The binding of Sema3a to Neuropilin-1 inhibits osteoclast differentiation by sequestering PlexinA1 from TREM2. Furthermore, Sema3a stimulate osteoblast differentiation through the activation of the canonical Wnt pathway. Sema3a can be considered an osteoprotective molecule derived from osteoblasts [70] (Fig. 3). Differentiating osteoblasts also express Wnt14. Although low Wnt14 expression promotes chondrocyte maturation and enhances endochondral bone formation, high levels of the protein blocked endochondral bone formation [71].
The communication in the bone marrow. Bone cells secrete the modulating factors of bone resorption and formation. Osteoclastogenesis is stimulated by RANKL which is secreted from osteoblast lineage cells including osteocytes. Osteoclasts inhibit bone formation through the expression of Sema4D. TGF-β and IGF are stored in the bone matrix and released as soluble factors by bone resorption. EphrinB2-EphB4 bidirectional signaling links suppression of osteoclast differentiation to stimulation of bone formation. PDGF-BB secreted by preosteoclasts stimulates angiogenesis, further supporting osteogenesis. S1P receptor expressed on osteoclast precursors contributes to control the migratory behavior, leading to regulate the bone homeostasis. Sema3a not only suppresses osteoclast differentiation but also promotes osteoblastogenesis. OPG is a soluble decoy receptor for RANKL and functions as an inhibitor of RANK activation. Osteocytes produce sclerostin, an inhibitor of bone formation
Coupling factors were thought to regulate the bone remodeling processes in the bone marrow, but studies on bone cell communication factors suggested that bone remodeling is regulated by many more factors functioning in a different manner from the classical coupling factors. Identification of communication factors mediating interplay between bone and immune cells will be an important issue in the future.
Conclusion
Studies on arthritic bone destruction shed light on the effect of the immune system on bone and the close interplay between the immune and bone systems. Research on the maintenance of HSCs by various cell types including osteoclasts and osteoblasts has shown the clear effect of the bone on the immunity, proving the concept of mutual dependency of bone and immune systems. Recent studies have indicated the role of bone cells in the immune cell malignancy or the control of immunodeficiency as well as the role of unrecognized immune cells in the regulation of bone metabolism, expanding the horizon of the osteoimmunology. These findings may provide novel molecular basis for the treatment of immune diseases by modulating the bone cells in addition to the immunotherapy for bone diseases. Further studies are needed to get the overall picture of the interaction of bone and immune systems.
References
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Takayanagi H (2012) New developments in osteoimmunology. Nat Rev Rheumatol 8:684–689
Mercier FE, Ragu C, Scadden DT (2012) The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol 12:49–60
Komatsu N, Takayanagi H (2012) Inflammation and bone destruction in arthritis: synergistic activity of immune and mesenchymal cells in joints. Front Immunol 3:77
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S (2000) Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43:259–269
Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43:250–258
Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T, Takayanagi H (2017) Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev 97:1295–1349
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841
Vivier E, van de Pavert SA, Cooper MD, Belz GT (2016) The evolution of innate lymphoid cells. Nat Immunol 17:790–794
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179
Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, Lee SY, Choi Y (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272:25190–25194
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, Nakashima T, Takayanagi H (2017) Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol 18:675–682
Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y (1999) TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med 189:1025–1031
Guerrini MM, Okamoto K, Komatsu N, Sawa S, Danks L, Penninger JM, Nakashima T, Takayanagi H (2015) Inhibition of the TNF family cytokine RANKL prevents autoimmune inflammation in the central nervous system. Immunity 43:1174–1185
Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2012) Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol 189:5519–5526
Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G (2007) RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204:1267–1272
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480
Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H (2006) Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med 12:1410–1416
Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med 3:1285–1289
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Nakashima T, Hayashi M, Takayanagi H (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab 23:582–590
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763
Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, Kitamura D, Kurosaki T, Ellmeier W, Takayanagi H (2008) Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132:794–806
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29:403–440
Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152:943–951
Takayanagi H (2009) Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol 5:667–676
Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H (2016) RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 75:1187–1195
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408:600–605
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H (2013) Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20:62–68
Dimitriou R, Tsiridis E, Giannoudis PV (2005) Current concepts of molecular aspects of bone healing. Injury 36:1392–1404
Park SH, Silva M, Bahk WJ, McKellop H, Lieberman JR (2002) Effect of repeated irrigation and debridement on fracture healing in an animal model. J Orthop Res 20:1197–1204
Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H (2016) IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 7:10928
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H (2011) Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147:1146–1158
Gong JK (1978) Endosteal marrow: a rich source of hematopoietic stem cells. Science 199:1443–1445
Lord BI, Testa NG, Hendry JH (1975) The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood 46:65–72
Taichman RS, Emerson SG (1994) Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med 179:1677–1682
Kiel MJ, Radice GL, Morrison SJ (2007) Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1:204–217
Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F (2008) Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood 111:1173–1181
Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462
Ding L, Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495:231–235
Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495:227–230
Yu VWC, Saez B, Cook C, Lotinun S, Pardo-Saganta A, Wang YH, Lymperi S, Ferraro F, Raaijmakers M, Wu JY, Zhou L, Rajagopal J, Kronenberg HM, Baron R, Scadden DT (2015) Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J Exp Med 212:759–774
Terashima A, Okamoto K, Nakashima T, Akira S, Ikuta K, Takayanagi H (2016) Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 44:1434–1443
Maltby S, Lochrin AJ, Bartlett B, Tay HL, Weaver J, Poulton IJ, Plank MW, Rosenberg HF, Sims NA, Foster PS (2018) Osteoblasts are rapidly ablated by virus-induced systemic inflammation following lymphocytic choriomeningitis virus or pneumonia virus of mice infection in mice. J Immunol 200:632–642
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464:852–857
Lowell CA, Niwa M, Soriano P, Varmus HE (1996) Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood 87:1780–1792
Gerritsen EJ, Vossen JM, van Loo IH, Hermans J, Helfrich MH, Griscelli C, Fischer A (1994) Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 93:247–253
Reeves JD, August CS, Humbert JR, Weston WL (1979) Host defense in infantile osteopetrosis. Pediatrics 64:202–206
Sreehari S, Naik DR, Eapen M (2011) Osteopetrosis: a rare cause of anemia. Hematol Rep 3:e1
Mansour A, Anginot A, Mancini SJ, Schiff C, Carle GF, Wakkach A, Blin-Wakkach C (2011) Osteoclast activity modulates B-cell development in the bone marrow. Cell Res 21:1102–1115
Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T (2006) Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12:657–664
Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, Miyamoto H, Hao W, Morioka H, Chiba K, Yasuda H, Penninger JM, Toyama Y, Suda T, Miyamoto T (2011) Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med 208:2175–2181
Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H, Sada A, Ikeda K, Matsui T, Katayama Y (2013) Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab 18:749–758
Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO (2012) Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res 27:1451–1461
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K (2006) Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4:111–121
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458:524–528
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105:20764–20769
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, Li C, Xie L, Crane J, Wan M, Zhen G, Bian Q, Yu B, Chang W, Qiu T, Pickarski M, Duong LT, Windle JJ, Luo X, Liao E, Cao X (2014) PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20:1270–1278
Negishi-Koga T, Takayanagi H (2012) Bone cell communication factors and Semaphorins. Bonekey Rep 1:183
Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H (2012) Osteoprotection by semaphorin 3A. Nature 485:69–74
Day TF, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750
Funding
This study was funded by Grants-in-Aid for Specially Promoted Research (15H05703), Chugai Pharmaceutical Co., LTD., AYUMI Pharmaceutical Corporation, Noevir Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terashima, A., Takayanagi, H. Overview of Osteoimmunology. Calcif Tissue Int 102, 503–511 (2018). https://doi.org/10.1007/s00223-018-0417-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0417-1