Abstract
Purpose
Postmenopausal osteoporosis (PMO) is usually managed by conventional drug treatment. However, prolonged use of these drugs cause side effects. Gut microbiota may be a potential target for treatment of PMO. This work was a three-month intervention trial aiming to evaluate the added effect of probiotics as adjunctive treatment for PMO.
Methods
Forty patients with PMO were randomized into probiotic (n = 20; received Bifidobacterium animalis subsp. lactis Probio-M8 [Probio-M8], calcium, calcitriol) and placebo (n = 20; received placebo material, calcium, calcitriol) groups. The bone mineral density of patients was measured at month 0 (0 M; baseline) and month 3 (3 M; after three-month intervention). Blood and fecal samples were collected 0 M and 3 M. Only 15 and 12 patients from Probio-M8 and placebo groups, respectively, provided complete fecal samples for gut microbiota analysis.
Results
No significant change was observed in the bone mineral density of patients at 3 M. Co-administering Probio-M8 improved the bone metabolism, reflected by an increased vitamin D3 level and decreased PTH and procalcitonin levels in serum at 3 M. Fecal metagenomic analysis revealed modest changes in the gut microbiome in both groups at 3 M. Interestingly, Probio-M8 co-administration affected the gut microbial interactive correlation network, particularly the short-chain fatty acid-producing bacteria. Probio-M8 co-administration significantly increased genes encoding some carbohydrate metabolism pathways (including ABC transporters, the phosphotransferase system, and fructose and mannose metabolism) and a choline-phosphate cytidylyltransferase.
Conclusions
Co-administering Probio-M8 with conventional drugs/supplements was more efficacious than conventional drugs/supplements alone in managing PMO. Our study shed insights into the beneficial mechanism of probiotic adjunctive treatment.
Registration number of clinical trial
Chinese Clinical Trial Registry (identifier number: ChiCTR1800019268).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common aging-related diseases, which is characterized by bone loss and bone tissue structural degradation [1]; it affects over 200 million individuals worldwide, causing 8.9 million fractures annually [2]. Moreover, over 30% of postmenopausal women aged over 50 years suffer from osteoporosis [3]. Many factors influence the occurrence and development of postmenopausal osteoporosis (PMO), e.g., genetic predisposition, lifestyle, and diet; however, menopause and aging-associated estrogen deficiency play the most determining role [4]. Bone tissues are continuously remodeled by concerted actions of bone resorption and formation, and the dynamics of bone metabolism are regulated together by estrogen, parathyroid hormone (PTH), and vitamin D [5]. Estrogen deficiency, PTH suppression, and reduction in the final renal activation of vitamin D in postmenopausal women may lead to calcitriol deficiency in the circulation and at target tissues, consequently affecting intestinal absorption of calcium (Ca) and phosphate. This also causes dysregulation in mineralization homeostasis and bone absorption [6]. Considering the etiology of PMO, vitamin D analogs (e.g., calcitriol), Ca complex, and anti-bone resorption drugs (e.g., alendronate and estrogen) are used in clinical treatment of PMO. An early, large controlled clinical study showed that 3-year treatment with calcitriol resulted in a threefold reduction in the rate of new vertebral fractures in postmenopausal women compared with those receiving only 1 g of elemental Ca per day over the same period [7]. Nowadays, calcitriol has been introduced to the European market, particularly for PMO and renal bone disease [8]. However, the treatment of PMO is a long-term process, and prolonged intake of drugs for PMO treatment may cause side effects, e.g., constipation and hypercalcemia [9]. Therefore, it would be of interest to explore alternative complementary treatments that ease side effects of currently available regimens.
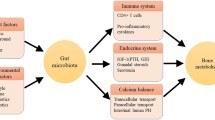
The gut microbiome is the microbial communities inhabiting in the gastrointestinal tract, comprising up to 10 trillion microorganisms. The gut microbiome and its metabolites are closely related to human health, including bone metabolism [3, 10, 11]. The reduced bone mineral density (BMD) in senile osteoporosis is most probably linked with gut dysbiosis. For example, more Escherichia/Shigella and Veillonella have been found in individuals with osteopenia than osteoporosis, while more Actinomyces, Eggerthella, Clostridium Cluster XlVa, and Lactobacillus have been detected in individuals with osteoporosis than those with normal BMD [12]. Thus, the gut microbiota may be a potential target for treating PMO. Probiotics are live microorganisms that exert beneficial effects on the host when adequate amounts are administered [13], and probiotic administration has been shown to confer favorable effects on bone metabolism [14]. Thus, Montazeri-Najafabady et al. (2019) compared the beneficial effects of five probiotic strains on bone health and demonstrated strain-specific probiotic effects in rats suffering from ovariectomy-induced bone loss. Among the five investigated strains, Lactobacillus (L.) acidophilus and L. casei showed the highest efficacy in improving BMD, bone marrow concentration, bone area, and biochemical parameters, including Ca and alkaline phosphatase (ALP) [15]. The intake of Ca, vitamin D, and complex probiotics (Bifidobacterium longum, L. acidophilus, L. rhamnosus) significantly decreased the levels of bone-specific ALP and collagen type 1 cross-linked C-telopeptide [16]. Bacillus subtilis C-3102 intake decreased the serum urinary type I collagen cross-linked N-telopeptide, accompanied by changes in colonic abundances of Bifidobacterium and Fusobacterium [17]. On the other hand, there have been conflicting results as to whether probiotics could improve bone metabolism. For example, ingesting a probiotic mix of three Lactobacillus paracasei strains or a L. reuteri-based product had little effects on bone formation, bone resorption, and inflammatory markers [18, 19]. The inconsistent clinical efficacy could be related to probiotic strain-specific effects and/or inter-individual variations [15, 20]. Moreover, the role of probiotic-driven gut microbiome-/metabolome-modulation effects in improving bone metabolism has not been elucidated in current literature.
Bifidobacterium animalis subsp. lactis Probio-M8 (Probio-M8) is a novel probiotic isolated from the breast milk of a healthy woman [21]. Probio-M8 could alleviate neurodegenerative diseases [22] and asthma [23] via increasing the abundance of health-promoting gut microbes. Thus, this work served as a pilot study, aiming to investigate the added beneficial effect and mechanism of co-administering Probio-M8 in adjunct to conventional treatment (Ca and calcitriol) in improving the bone metabolism of patients with PMO.
Methods
Trial design and subject recruitment
As this work was a pilot study to evaluate the effect of Bifidobacterium animalis subsp. lactis Probio-M8 in management of PMO, power calculation was not employed. However, the number of patients in present study was referenced to similar reports, in which 20–50 postmenopausal women participated [16, 24].
Fifty-three patients firstly diagnosed with PMO were recruited at the Affiliated Hospital of Inner Mongolia Medical University for this three-month trial during 2018.09 to 2019.06. The inclusion criteria were: (1) − 2.5 > T value > − 1.0, measured by dual-energy X-ray absorptiometry (DXA), and high fracture risk assessed by the fracture risk assessment tool, FRAX® (threshold of 7%); or T value > − 2.5 measured by DXA; (2) 50 to 80 years old postmenopausal female; (3) agreed to participate in this study.
Thirteen volunteers were excluded based on clinical presentation and willingness to participate: (1) secondary osteoporosis (i.e., patients with comorbid illnesses, e.g., endocrine and metabolic diseases, connective tissue diseases, renal bone malnutrition caused by multiple chronic kidney diseases, gastrointestinal and nutritional diseases, blood system diseases, neuromuscular system diseases, long-term braking or space travel, organ transplantation, drugs and poisons; n = 3); (2) active stage of peptic ulcer or with gastrointestinal malignant tumor (n = 1); (3) past intestinal diseases (e.g., irritable bowel syndrome, inflammatory bowel disease, habitual diarrhea; n = 2); (4) infectious diseases requiring antibiotic treatment (n = 1); (5) history of allergy to lactic acid bacteria and their products (n = 1); (6) declined to participate (n = 5).
After the screening, a final cohort of forty individuals were enrolled and randomized into Probio-M8 (receiving [Bifidobacterium animalis subsp. lactis Probio-M8, Probio-M8, mix with maltodextrin, 1.5 × 1010 CFU/day] and conventional drugs; n = 20) and placebo (receiving placebo [maltodextrin, 2 g] and conventional drugs; n = 20) groups, respectively (Fig. 1, Table S1). The conventional treatment used in this study was Ca tablet (D-Cal®, 600 mg/day) and calcitriol (FEADWAY®, 0.25 μg/day). Although Ca and calcitriol are not considered as first-line treatment option in some countries, they are commonly prescribed to patients with postmenopausal or senile osteoporosis in China due to fewer side effects compared with other first-line drugs and a lower cost. The Probio-M8 and placebo materials were prepared as powder of identical appearance and taste, packed in individually sealed plastic sachets, and stored at 4℃ (JinHua YinHe Biological Technology Co., Ltd., Zhejiang, China; prepared under ISO9001 and HALAL standards). During the trial, patients were asked not to take fermented dairy products, and none of them took any antibiotics during the study.
BMD measurement, serum and fecal sample collection
This trial lasted three months. The BMD of three anatomical compartments of patients, i.e., lumbar spine (L1-L4), left femoral neck, and left hip joint, was measured by DXA (DEXA; MEDIX90, Medlink, France) at month 0 (0 M; baseline) and month 3 (3 M; after intervention). Obtained data were converted into T values. The smallest T value was used as the BMD score for a diagnosis. At 0 M, BMD was measured for all patients. However, at 3 M, three and four patients from the Probio-M8 and placebo groups, respectively, failed to participate in BMD measurement.
Blood samples were collected at 0 M and 3 M during clinic visit. All blood samples were immediately centrifuged for 5 min (3000 × g, at 4℃) to collect the sera. Serum bone metabolites and inflammatory factors, including PTH, vitamin D3 (VD3), Ca, phosphorus (P), ALP, osteocalcin (OC), total procollagen 1 N-Terminal propeptide (tP1NP), and β-C-terminal cross-linked telopeptides of type I collagen (β-CTX), and procalcitonin (PCT), were detected by an automated analyzer (DXA5000, Beckman Coulter, Inc, USA).
Fecal samples were provided by the subjects at 0 M and 3 M for gut microbiome sequencing. Collected fecal samples were stored in a -80℃ freezer before sequencing. Only 15 and 12 patients from Probio-M8 and placebo groups, respectively, provided complete fecal samples for gut microbiota analysis.
Extraction of DNA and metagenomic sequencing
Metagenomic DNA was extracted from patients’ stool samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instruction. The quality/integrity/purity/concentration of extracted metagenomic DNA were assessed by 1% agarose gel electrophoresis, a Nanodrop spectrophotometer, and the Qubit® dsDNA Assay Kit with a Qubit® 2.0 fluorometer (Life Technologies, CA, USA). Qualified samples (DNA concentration > 20 ng/μL; optical density (260 nm to 280 nm) ratio between 1.8 and 2.0) were used for sequencing. Sequencing libraries were generated by NEBNext® Ultra™ DNA Library Prep Kit for Illumina (New England Biolabs, Inc., USA). Samples were indexed by PCR with random sequences of 16 bases, under the cycling conditions: 95 °C for 3 min; 12 cycles of 98 °C for 20 s, 58 °C for 30 s, and 72 °C for 30 s with a final extension at 72 °C for 5 min. The DNA library preparations were sequenced on an Illumina NovaSeq platform to generate paired-end reads (Tianjin Novogene Technology Co., Ltd., Tianjin, China).
Quality control of reads
Fifty-four samples were sequenced (n = 15 and 12, in Probio-M8 and placebo groups, collected at 0 M and 3 M, respectively), generating 0.40 Tbp of high-quality paired-end reads (7.33 ± 1.39 Gbp raw metagenomic reads per sample). KneadData (http://huttenhower.sph.harvard.edu/kneaddata; v0.7.5) was used to filter low-quality reads (length of reads < 60 nt) by Trimmomatic (a flexible trimmer for Illumina datasets [25]) and removing human contaminating reads by Bowtie2 (v2.3.5.1) [26]. Finally, 0.39 Tbp of clean data (7.23 ± 1.37 Gbp clean metagenomic reads per sample, Table S2) remained for downstream analysis.
Metagenomic assembly, contig binning, genome dereplication
In metagenomics, binning is the process of grouping and assigning reads/contigs to individual genomes. Thus, to generate the taxonomic profile of samples, species-level genome bins (SGBs) were assembled from the metagenomic dataset after quality control. First, MEGAHIT was used to assemble reads of each sample into contigs (an average N50 length of 7.73 Kbp, Table S2). Second, contigs > 2000 bp were selected for binning to obtain metagenome-assembled genomes (MAGs) by MetaBAT2 using the default options [27]. Third, reads were mapped back to the corresponding contigs using BWA-MEM2 [28, 29], and Samtools and the jgi_summarize_bam_contig_depths function in MetaBAT2 were used to calculate the contig depth [30]. Then, VAMB software was used to obtain MAGs, which was classed by CheckM based on completeness and contamination (partial-quality: completeness ≥ 50%, contamination ≤ 5%; medium-quality: completeness ≥ 70%, contamination ≤ 10%; high-quality: completeness ≥ 80%, contamination ≤ 5%)[23, 31]. Only high-quality MAGs were further analyzed. Finally, dRep (v3.0.1) was used to cluster and obtain SGBs with the parameters -pa 0.95 and -sa 0.95[32]. This study yielded a total of 259 high-quality genomes.
Taxonomic annotation, abundance, functional prediction of SGBs
Kraken2 tool and NCBI nonredundant Nucleotide Sequence Database were used to annotate the MAGs with default settings [33]. Prodigal was used to predict putative genes in the contigs [34], followed by annotation by UniProt Knowledgebase using the blastp function of DIAMON. CoverM (https://github.com/wwood/CoverM) calculated the abundance of each SGB (parameters: "–min-read-percent-identity 0.95 –min-covered-fraction 0.4"). Gene abundance was expressed in reads per kilobase million (RPKM) to calculate SGB diversity by two R packages (vegan and optparse). Proteins in SGBs were predicted by Prodigal and functionally annotated by the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthologies (KOs) database. The best hit of each gene was selected for calculating the gene abundance profile for each sample.
Statistical analyses
All statistical analyses were performed using the R software (v.4.0.3). Data were expressed as mean ± SD. The Shannon index and principal coordinates analysis (PCoA) were used to assess post-intervention changes in the fecal microbiota diversity and structure using two R packages, vegan and ggpubr. Wilcoxon test and t-test were used to evaluate differences in the fecal microbiome inter-group and intra-group. Correlation and strength of correlation between bacteria were assessed with the Spearman’s rank correlation coefficient, calculated by R packages (psych, gplots, and dplyr). Here, significantly associated taxa (defined as r > 0.5 or r < − 0.5, and P < 0.05) were presented as correlation networks constructed by Cytoscape (v3.5.1). Pathway enrichment was analyzed by STAMP (v.2.1.3). All graphics were generated under R and Adobe Illustrator environment.
Results
Co-administrating Probio-M8 improved bone metabolism and osteoblast activity
At 0 M, there was no significant difference in all monitored parameters between Probio-M8 and placebo groups (Table 1). At 3 M, significant differences were shown in serum concentrations of PCT, PTH, VD3, and Ca between two groups (Table 1). The level of PCT, an indicator of systemic inflammation [35], decreased significantly in Probio-M8 group compared with placebo group at 3 M (Probio-M8 group: 0.03 ± 0.01 ng/mL, placebo group: 0.04 ± 0.01 ng/mL; P < 0.01). Both PTH and VD3 are indicators of bone metabolism and Ca homeostasis [36]. The levels of PTH (Probio-M8 group: 36.49 ± 18.24 pg/L, placebo group: 45.05 ± 19.39 pg/L; P = 0.039) and Ca (Probio-M8 group: 2.27 ± 0.12 mmol/L, placebo group: 2.38 ± 0.12 mmol/L; P = 0.013) were significantly lower in Probio-M8 group compared with placebo group, and an opposite trend was seen in VD3 level (Probio-M8 group: 26.22 ± 7.90 ng/L, placebo group: 20.52 ± 7.07 ng/L; P = 0.032).
Longitudinal comparison revealed significant decreases in ALP levels in both groups at 3 M (Probio-M8 group: 78.45 ± 18.34 U/L at baseline decreased to 66.00 ± 10.25 U/L at 3 M, P < 0.01; and placebo group: 75.75 ± 12.17 U/L at baseline decreased to 67.65 ± 17.90 U/L at 3 M, P = 0.22; Table 1). Significant reduction in OC was observed only in Probio-M8 group (Probio-M8 group: 20.25 ± 5.80 ng/mL at baseline decreased to 16.82 ± 6.57 ng/mL at 3 M, P = 0.012; placebo group: 19.25 ± 5.65 at baseline to 18.38 ± 5.81, P = 0.29; Table 1). No significant difference was observed in the P, tP1NP and β-CTX levels between groups or time points (Table 1).
These results together suggested that co-administering Probio-M8 with Ca and calcitriol offers better beneficial effects to bone metabolism and protection against bone loss than just taking Ca and calcitriol.
Co-administering Probio-M8 did not modify subjects’ gut microbiota diversity drastically
No significant intra-group or inter-group differences were observed in the alpha diversity of subjects’ gut microbiota (reflected by the Shannon–Wiener diversity index; Fig. 2a). Similarly, the PCoA (Bray–Curtis distance) score plot did not show any group- or time-based clustering patterns (Fig. 2b). Such results indicated that probiotic intake did not cause drastic changes in subjects’ gut microbiota diversity and structure.
Microbial diversity and differentially abundant species-level genome bins (SGBs) between groups. a Shannon diversity index of fecal microbiome at baseline (0 M) and after three-month intervention (3 M) in Probio-M8 (Pro) and placebo (Pla) groups, respectively. Violin plots represent the data distribution of each subgroup, and the horizontal line inside the box represents the median. b Principal coordinates analysis (PCoA) score plots of two groups at different time points. Symbols of each subgroup were represented by a different color. c Comparison of amounts (in reads per kilobase million, RPKM) of differentially abundant SGBs. All the shown SGBs were not significantly different between groups at baseline, but only become significantly differential after the intervention (* P < 0.05)
However, finer taxonomic analysis revealed four intervention responsive SGBs that showed no significant difference in abundance between Probio-M8 and placebo groups initially but only became differentially abundant after the intervention (Fig. 2c; Table S3). Significantly more Eubacterium ventriosum (SGB101) was found in Probio-M8 group than in placebo group (P < 0.05), and Coprococcus sp. (SGB40), Alistipes putredinis (SGB10), and Lachnospiraceae sp. (SGB203) showed an opposite trend (P < 0.05 in all cases, Wilcoxon test).
Co-administering Probio-M8 enhanced the gut microbiota interactions in patients with POM
Genus-level interactive gut microbial networks were construced (Fig. 3). The correlation strength was defined as: very strong, |r|≥ 0.8; strong, 0.6 ≤|r|< 0.8; moderate, 0.5 ≤|r|< 0.6). At baseline, a total of 31 detected correlations (eight strongly positive, 16 moderately positive, seven moderately negative). Although the microbial interconnectedness of both groups increased at 3 M compared with 0 M, it was obviously stronger in Probio-M8 group compared with placebo group at 3 M, characterized by a higher number of significant correlation (76 detected correlations; one very strong positive, 31 strongly positive, 15 moderately positive, 12 strongly negative, 17 moderately negative; including some well-recognized butyrate-producers, e.g., Ruminococcus, Butyricicoccus, and Eubacterium). In contrast, the correlation network of placebo group was less interconnected, with only 39 detected correlations (three very strongly positive, 18 strongly positive, four moderately positive, one very strong negative, 12 strongly negative, one moderately negative). Importantly, Bifidobacterium correlated positively with Eubacterium, Blautia and Ruminococcus, and the correlations strengthened further at 3 M only in Probio-M8 group but not placebo group. Our results suggested that Probio-M8 co-administration enhanced the strength of interconnectedness of patients’ gut microbiota.
Co-administering Probio-M8 modulated bone metabolism-related function encoded in the gut microbiome
The pathways and function encoded in subjects’ gut microbiota were annotated by KEGG database (Fig. 4, Table S4). A total of 6095 KOs were annotated across all samples. Twenty-two responsive KOs were identified, which were not significantly different between groups at baseline, but only became significantly differential abundant at 3 M. Four responsive KOs were significantly more abundant in placebo group than Probio-M8 group (K00338, K03617, K18377, K03346, involved in Energy metabolism and Protein families: genetic information processing), while the remaining 18 KOs were significantly more abundant in Probio-M8 group than placebo group (mainly related to Carbohydrate metabolism [K00847, K02770, K02781, K03332; these four KOs encoded different pathways of Fructose and mannose metabolism], Cell motility [K02389, K02397], Metabolism of other amino acids [K00968], Membrane transport [K02806, K18891], Protein families: genetic information processing [K19339], Poorly characterized pathways [K02426, K06877, K06921, K07075, K07128, K09976], and Unclassified pathways [K01447, K19336]). Finally, pathways of Phosphonate and phosphonate metabolism significantly increased after intervention with Probio-M8.
Discussion
Osteoporosis is a metabolic bone disease, which relates with the dynamic balance between bone resorption mediated by osteoclasts and bone formation mediated by osteoblasts, and it is commonly managed by administrating conventional drugs or dietary supplements like Ca and calcitriol. There is a strong link between the gut microbiota and osteoporosis [5]. Provided the ability of probiotics in restoring the host gut microbiota from disease-associated dysbiotic states, this work investigated the added benefit of co-administering Probio-M8 with Ca and calcitriol in bone metabolism of patients with PMO. We found that co-administering Probio-M8 with Ca and calcitriol for three months could improve patients’ bone metabolism.
Our results showed that co-administrating Probio-M8 significantly improved Ca and P metabolism, meanwhile improving the anti-inflammatory index in patients with osteoporosis. The two most important regulators for blood Ca are PTH and VD3. A high PTH concentration increases Ca release from bone, causing bone resorption [16]. Several previous studies showed that oral intake of Ca and multispecies probiotics could decrease the blood PTH level, mitigating bone resorption [16, 37, 38], which is consistent with our results. We also found the serum Ca concentration significantly increased only in placebo but not Probio-M8 group at 3 M, which might be resulted from the decrease in PTH, implicating an enhanced retention of bone minerals and reduction in bone resorption. Vitamin D plays a crucial role in the overall health by sustaining bone Ca homeostasis, maintaining BMD, and preventing from bone resorption [39]. A range of below 30 ng/mL of serum 25-hydroxy vitamin D is regarded as vitamin D deficiency [40]. Co-administering Probio-M8 improved subjects’ overall serum VD3 level. A higher serum vitamin D level is indicative of improvement in Ca absorption [41]. Thus, the increase in VD3 and decrease in Ca in serum of subjects in Probio-M8 group might be indicative of an increased bone formation activity. Additionally, the ratio of subjects in Probio-M8 group to placebo group that achieved a normal serum VD3 level (> 30 ng/mL) was 8:1 after the trial, implicating the effectiveness of probiotic administration in increasing serum VD3 and preventing bone loss. The serum PCT level is a biomarker of systemic inflammation and autoimmune diseases [35], which was significantly lowered after Probio-M8 consumption, suggesting that Probio-M8 could alleviate systemic inflammation in patients with osteoporosis. Apart from being an inflammatory biomarker, it is the precursor of calcitonin, mainly produced by the parafollicular C cells in the thyroid. Some studies consistently found that applying calcitonin at supraphysiologic doses promptly inhibited osteoclast function [42, 43]. In contrast, the biological function of PCT is still not entirely clear. Some studies found a positive correlation between PCT and the severity of osteoporosis, while other studies found an inverse association between PCT and OC [44, 45]. Thus, the exact role and mechanism of drop in serum PCT subsequent to probiotic intake need further elucidation.
Co-administering Probio-M8 significantly decreased the ALP and OC levels, which are biomarkers of bone formation [46], and ALP has also been proposed as a marker of bone turnover [16]. Few studies have reported the impact of probiotic intake on these two biomarkers in human. Britton et al. (2014) showed in a menopausal ovariectomized mouse model that L. reuteri intake could decrease the level of bone-specific ALP, indicating a diminished bone turnover and bone loss [47], which is in line with the current study. Our study observed a significant decrease in the serum OC level in Probio-M8 group but not placebo group at 3 M, contrasting to the observation of an increased serum OC level in ovariectomized rats after 16-week intervention with B. longum [48]. During bone formation, OC can be released directly into blood after osteoblastic synthesis; however, it can also enter the circulation from osteoclastic bone matrix degradation during bone resorption [49]. On the other hand, a meta-analysis found that serum OC might not be a good indicator for bone formation but rather an indicator of bone turnover due to its heterogeneity in the circulation influenced by glucose metabolism [50]. Therefore, the decrease in serum OC in Probio-M8 group seemed to reflect a lower bone turnover status at 3 M compared with baseline.
The human gut microbiota has been described as “a key mediator of osteoporosis and osteogenesis” [5], and the gut microbiome of women with osteoporosis and osteopenia are altered[51]. As the gut microbiota is likely playing an active role in the pathogenesis of PMO, it may also serve as a therapeutic target for managing PMO. Probiotics can modulate the gut microbiota and improve clinical symptoms in a variety of medical conditions, e.g., coronary artery disease [52], asthma [53] and gastrointestinal disease [54]. Moreover, some previous clinical studies reported that applying probiotics could improve bone health, but only few studies have focused on analyzing probiotic-driven gut microbiota responses in association with symptom improvement. Thus, here we focused on analyzing changes in subjects’ gut microbiota after probiotic intake. No significant change was observed in both the alpha diversity (represented by the Shannon index) and structure of subjects’ gut microbiota after the three-month intervention regardless of Probio-M8 co-administration. In contrast, Takimoto et al. (2018) observed significant increases in both gut microbiota richness and diversity after 24-week intervention with Bacillus subtilis C-3102 in healthy postmenopausal Japanese women [17]. The inconsistency could be a result of different intervention duration and probiotic strains between studies. Although it is generally thought that a high diversity is indicative of a “healthy microbiota”, such view point has been questioned [55]. Our observations suggested that ingesting Probio-M8 was only accompanied by moderate changes in the host gut microbiota diversity and structure. Interestingly, the interconnectedness between the interactive gut microbial correlation network of Probio-M8 and placebo groups was different at 3 M. Interactive network-based analysis facilitates the decipherment of complex microbial interaction patterns, reflecting relationships among microbes and their ecological interconnectedness [56]. Provided the close link between gut microbiota and host health, gut microbial interactive network analysis reflects improvement in health state and/or symptom alleviation in relationship with the dynamic changes in the gut microbiota [57]. It has been shown that probiotic intake could enriched indigenous bacterial correlation, inhibiting potential gut pathogens; and strengthened networking of differentially abundant-disease associated gut microbes in pancreatic cancer patients could reflect poor prognostic factors [58, 59]. Such approach has also been used to identify link between diet-induced changes in human gut microbiota and metabolic health [60]. We found an enhanced gut microbiota interactive correlation network in Probio-M8 group compared with placebo group at 3 M. Particularly, the generally considered beneficial genus, Bifidobacterium, showed positive correlation with some major short-chain fatty acids-producing taxa, e.g., Eubacterium, Blautia, and Ruminococcus; and Ruminococcus has been reported to be inversely associated with the presence of osteoporosis [61]. Therefore, it is reasonable to speculate that Probio-M8 intake enhanced the interconnection among specific beneficial bacteria in the gut ecological niche, promoting the health of patients with PMO. However, it is important to note that interactive gut microbiota analysis as such does not provide solid proof of causal relationship, which needs to be further verified.
Gut microbiota modulation will naturally affect the functional potential of the gut metagenome; thus, changes in the metagenomic functional features after probiotic intervention were analyzed. In Probio-M8 group, significant increases were observed in genes coding ABC transporters, the phosphotransferase system, and fructose and mannose metabolism pathways. ABC transporters are frequently co-located and co-regulated with glycoside hydrolase-encoding genes. The phosphotransferase system is a common mechanism of high affinity carbohydrate uptake in microbial species [62]. Increases in these genes are suggestive of enhanced carbohydrate uptake and utilization [63]. It was previously shown that feeding fructose to rats could induce insulin resistance and accelerated osteoporosis [64], suggesting that fructose intake might worsen the pathogenesis of osteoporosis. The observation of an increased abundance in genes coding pathways of fructose and mannose metabolism after Probio-M8 intake suggested that probiotic co-administration could accelerate fructose utilization in patients’ gut, reducing PMO symptoms.
In addition, the gene abundance of a choline-phosphate cytidylyltransferase (K00968) increased after Probio-M8 treatment. Choline-phosphate cytidylyltransferases are major rate-determining enzymes in phosphatidylcholine biosynthesis in mammalian cells. The serum levels of phosphatidylcholines have been shown to associate with BMD in Chinese subjects; however, the correlation direction is dependent on their chemical structure and fatty acid combination. For example, two phosphatidylcholines (16:0/18:3 and O-18:0/22:6, respectively) were associated with BMD in opposite direction [65]. Similarly, Farina et al. (2012) found differential association between blood phosphatidylcholine concentrations and hip BMD/hip fracture in older adults [66]. More serum phosphatidylcholines were found in osteoporotic patients than osteopenic patients [67]. The increase in choline-phosphate cytidylyltransferase-encoding genes associated with Probio-M8 intake seems to be beneficial to the current cohort of subjects, but further confirmation would be required.
In conclusion, this study demonstrated that probiotics could act together with conventional drugs (Ca and calcitriol) to improve bone metabolism in patients with PMO. This work has provided insights into potential mechanisms of probiotic intake in clinical improvement in patients with PMO. However, a larger sample size would be desirable in future studies to further validate our results.
Data availability
The sequence dataset was deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession number PRJNA773596).
References
Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM (2015) The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging 10:583
Noh J-Y, Yang Y, Jung H (2020) Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci 21(20):7623
He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, Fu J, Lin X, Zheng G, Yu G (2020) Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY) 12(9):8583
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. The Lancet 377(9773):1276–1287
Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE (2021) The Human gut microbiota: a key mediator of osteoporosis and osteogenesis. Int J Mol Sci 22(17):9452
Ringe JD (2020) Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteoporos 15(1):182. https://doi.org/10.1007/s11657-020-00842-0
Tilyard MW, Spears GFS, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326(6):357–362. https://doi.org/10.1056/nejm199202063260601
Janoušek J, Pilařová V, Macáková K, Nomura A, Veiga-Matos J, Silva DDD, Remião F, Saso L, Malá-Ládová K, Malý J, Nováková L, Mladěnka P (2022) Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Criti rev clin lab sci. https://doi.org/10.1080/10408363.2022.2070595
Rizzoli R, Biver E (2020) Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep 18(3):273–284
Ling C-w, Miao Z, Xiao M-l, Zhou H, Jiang Z, Fu Y, Xiong F, L-s-y Z, Liu Y-p, Wu Y-y (2021) The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab 106(10):e3852–e3864
Shen Q, Zhang C, Qin X, Zhang H, Zhang Z, Richel A (2021) Modulation of gut microbiota by chondroitin sulfate calcium complex during alleviation of osteoporosis in ovariectomized rats. Carbohyd Polym 266:118099
Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, Molloy C, O’Toole PW, Shanahan F, Molloy MG (2019) Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 58(12):2295–2304
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Jia L, Tu Y, Jia X, Du Q, Zheng X, Yuan Q, Zheng L, Zhou X, Xu X (2021) Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif 54(7):e13075
Montazeri-Najafabady N, Ghasemi Y, Dabbaghmanesh MH, Talezadeh P, Koohpeyma F, Gholami A (2019) Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics antimicrob proteins 11(4):1145–1154
Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA (2017) Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr 36(7):497–506
Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, Morita H, Nakamura T (2018) Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized placebo-controlled double-blind clinical trial. Bioscience of Microbiota Food and Health. https://doi.org/10.12938/bmfh.18-006
Jansson P-A, Curiac D, Ahrén IL, Hansson F, Niskanen TM, Sjögren K, Ohlsson C (2019) Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol 1(3):e154–e162
Nilsson A, Sundh D, Bäckhed F, Lorentzon M (2018) Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med 284(3):307–317
Billington EO, Mahajan A, Benham JL, Raman M (2021) Effects of probiotics on bone mineral density and bone turnover: a systematic review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1998760
Zhang W, Wang Y, Li K, Kwok L-Y, Liu W, Zhang H (2020) Modulation of fatty acid metabolism improves oxygen tolerance of Bifidobacterium animalis ssp lactis Probio-M8. J Dairy Sci 103(10):8791–8795
Sun H, Zhao F, Liu Y, Ma T, Jin H, Quan K, Leng B, Zhao J, Yuan X, Li Z, Li F, Kwok L-Y, Zhang S, Sun Z, Zhang J, Zhang H (2022) Probiotics synergized with conventional regimen in managing Parkinson’s disease. npj Parkinson’s Disease. https://doi.org/10.1038/s41531-022-00327-6
Liu A, Ma T, Xu N, Jin H, Zhao F, Kwok L-Y, Zhang H, Zhang S, Sun Z (2021) Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbiol spectr 9(2):e00859-e1821
Narva M, Nevala R, Poussa T, Korpela R (2004) The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr 43(2):61–68. https://doi.org/10.1007/s00394-004-0441-y
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiat 74(10):720–726
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359
Kang DD, Li F, Kirton E, Thomas A, Egan R, An H, Wang Z (2019) MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7:e7359
Lee J, Jang JY, Kwon MS, Lim SK, Kim N, Lee J, Park HK, Yun M, Shin MY, Jo HE, Oh YJ, Ryu BH, Ko MY, Joo W, Choi HJ (2018) Mixture of two lactobacillus plantarum strains modulates the gut microbiota structure and regulatory t cell response in diet-induced obese mice. Mol Nutr Food Res 62(24):11. https://doi.org/10.1002/mnfr.201800329
Vasimuddin M, Misra S, Li H, Aluru S (2019) Efficient architecture-aware acceleration of BWA-MEM for multicore systems. 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). Piscataway, IEEE, pp 314–324
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25(7):1043–1055
Olm MR, Brown CT, Brooks B, Banfield JF (2017) dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J 11(12):2864–2868. https://doi.org/10.1038/ismej.2017.126
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20(1):1–13
Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11(1):1–11
Becker KL, Snider R, Nylen ES (2010) Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol 159(2):253–264. https://doi.org/10.1111/j.1476-5381.2009.00433.x
Yerlikaya FH, Onmaz DE (2022) Inflammation and Bone turnover markers in adult obesity. J Clin Densitom. https://doi.org/10.1016/j.jocd.2022.08.002
Kärkkäinen MU, Lamberg-Allardt CJ, Ahonen S, Välimäki M (2001) Does it make a difference how and when you take your calcium? The acute effects of calcium on calcium and bone metabolism. Am J Clin Nutr 74(3):335–342
Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, Korpela R (2004) Effects of long-term intervention with Lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab 48(4):228–234
Bell TD, Demay MB, Burnett-Bowie SAM (2010) The biology and pathology of vitamin D control in bone. J Cell Biochem 111(1):7–13
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22(2):142–146
Zaidi M, Moonga BS, Abe E (2002) Calcitonin and bone formation: a knockout full of surprises. J Clin Investig 110(12):1769–1771
Davey RA, Findlay DM (2013) Calcitonin: physiology or fantasy? J Bone Miner Res 28(5):973–979
Li J, Qiao H, LIN T, (2021) Diagnostic value of serum procalcitonin level combined with quantitative CT in elderly women with painful osteoporosis and its correlation with disease severity. Chin J Endocr Surg 6:189–192
MacDonald IJ, Tsai H-C, Chang A-C, Huang C-C, Yang S-F, Tang C-H (2021) Melatonin Inhibits osteoclastogenesis and osteolytic bone metastasis: implications for osteoporosis. Int J Mol Sci 22(17):9435
Kuo T-R, Chen C-H (2017) Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark res 5(1):1–9
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J cell physiol 229(11):1822–1830
Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:10. https://doi.org/10.1155/2015/897639
Singer FR, Eyre DR (2008) Using biochemical markers of bone turnover in clinical practice. Clevel Clin J Med 75(10):739–750
Liu ZY, Chen RQ, Jiang YT, Yang Y, He L, Luo CX, Dong JW, Rong LM (2019) A meta-analysis of serum osteocalcin level in postmenopausal osteoporotic women compared to controls. BMC Musculoskelet Disord 20(1):7. https://doi.org/10.1186/s12891-019-2863-y
Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC (2021) The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR plus 5(3):e10452. https://doi.org/10.1002/jbm4.10452
Sun B, Ma T, Li Y, Yang N, Li B, Zhou X, Guo S, Zhang S, Kwok L-Y, Sun Z, Zhang H (2022) Bifidobacterium lactis probio-M8 adjuvant treatment confers added benefits to patients with coronary artery disease via target modulation of the gut-heart/-brain axes. Msystems. https://doi.org/10.1128/msystems.00100-22
Liu A, Ma T, Xu N, Jin H, Zhao F, Kwok L-Y, Zhang H, Zhang S, Sun Z (2021) Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbio spectr. https://doi.org/10.1128/Spectrum.00859-21
Xu HY, Ma C, Zhao FY, Chen P, Liu YH, Sun ZH, Cui LH, Kwok LY, Zhang HP (2021) Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur J Nutr 60(5):2553–2565. https://doi.org/10.1007/s00394-020-02437-4
Shanahan F, Hill C (2019) Language, numeracy and logic in microbiome science. Nat Rev Gastroenterol Hepatol 16(7):387–388. https://doi.org/10.1038/s41575-019-0163-5
Matchado MS, Lauber M, Reitmeier S, Kacprowski T, Baumbach J, Haller D, List M (2021) Network analysis methods for studying microbial communities: a mini review. Comput Struct Biotechnol J 19:2687–2698
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, Chan FKL, Sung JJY, Yu J (2019) Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 68(4):654–662
Xu H, Zhao F, Hou Q, Huang W, Liu Y, Zhang H, Sun Z (2019) Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct 10(5):2618–2629
Kelder T, Stroeve JHM, Bijlsma S, Radonjic M, Roeselers G (2014) Correlation network analysis reveals relationships between diet-induced changes in human gut microbiota and metabolic health. Nutr Diabetes 4(6):e122–e122. https://doi.org/10.1038/nutd.2014.18
Ling CW, Miao ZL, Xiao ML, Zhou HW, Jiang ZL, Fu YQ, Xiong F, Zuo LSY, Liu YP, Wu YY, Jing LP, Dong HL, Chen GD, Ding D, Wang C, Zeng FF, Zhu HL, He Y, Zheng JS, Chen YM (2021) The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab 106(10):E3852–E3864. https://doi.org/10.1210/clinem/dgab492
Hayes CA, Dalia TN, Dalia AB (2017) Systematic genetic dissection of PTS in Vibrio cholerae uncovers a novel glucose transporter and a limited role for PTS during infection of a mammalian host. Mol Microbiol 104(4):568–579
Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10(5):323–335
Morishita, (2009) Fluvastatin improves osteoporosis in fructose-fed insulin resistant model rats through blockade of the classical mevalonate pathway and antioxidant action. Int J Mol Med. https://doi.org/10.3892/ijmm_00000167
Mei ZD, Dong X, Qian Y, Hong D, Xie ZA, Yao GF, Qin A, Gao SY, Hu JY, Liang LM, Zheng Y, Su JC (2020) Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 62:10. https://doi.org/10.1016/j.ebiom.2020.103111
Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL (2012) Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The framingham osteoporosis study. J Bone Miner Res 27(5):1222–1230. https://doi.org/10.1002/jbmr.1581
Aleidi SM, Alnehmi EA, Alshaker M, Masood A, Benabdelkamel H, Al-Ansari MM, Rahman AMA (2021) A distinctive human metabolomics alteration associated with osteopenic and osteoporotic patients. Metabolites 11(9):14. https://doi.org/10.3390/metabo11090628
Funding
This research was supported by Inner Mongolia Science and Technology Major Projects (2021ZD0014) and the Earmarked Fund for CARS-36.
Author information
Authors and Affiliations
Contributions
JZ and HZ conceived and designed the trial. ZS supervised all bench work. ZG recruited patients, collected and summarized data. FZ, ZZ, KW, and YL analyzed the metagenomic sequencing data. FZ wrote the manuscript. LY Kwok reviewed and revised the manuscript critically. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This intervention trial was approved by Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University (project number KY 2018010) and registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/; identifier number: ChiCTR1800019268). An informed consent was signed by all subjects prior to the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, F., Guo, Z., Kwok, LY. et al. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr 62, 965–976 (2023). https://doi.org/10.1007/s00394-022-03042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03042-3