Abstract
Purpose of Review
Mechanical loading is an essential stimulus for skeletal tissues. Osteocytes are primarily responsible for sensing mechanical stimuli in bone and for orchestrating subsequent responses. This is critical for maintaining homeostasis, and responding to injury/disease. The osteocyte mechanotransduction pathway, and the downstream effects it mediates, is highly complex. In vivo models have proved invaluable in understanding this process. This review summarizes the commonly used models, as well as more recently developed ones, and describes how they are used to address emerging questions in the field.
Recent Findings
Minimally invasive animal models can be used to determine mechanisms of osteocyte mechanotransduction, at the cell and molecular level, while simultaneously reducing potentially confounding responses such as inflammation/wound-healing.
Summary
The details of osteocyte mechanotransduction in bone are gradually becoming clearer. In vivo model systems are a key tool in pursing this question. Advances in this field are explored and discussed in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical loading is an essential stimulus for musculoskeletal tissues. In bone, it is generally accepted that osteocytes are primarily responsible for sensing mechanical stimuli and for orchestrating the subsequent tissue responses in the local microenvironment [1, 2•]. This mechanosensory ability is critical during development, and throughout life, in maintaining homeostasis and responding to injury/disease. The osteocyte mechanotransduction pathway, and the downstream effects it mediates, is a highly complex process. However, the details are gradually becoming clearer thanks to advances made by the bone research community. Some of the recent advances in osteocyte mechanotransduction, particularly those that rely on in vivo model systems, are summarized and discussed here.
Osteocytes are terminally differentiated osteoblasts, which arise from mesenchymal stem cells, and are ubiquitous throughout healthy bone matrix. Approximately 20% of osteoblasts become osteocytes, with the remaining population undergoing either apoptosis, transformation to bone lining cells or potentially even trans-differentiation [3,4,5], although the latter remains controversial. In situ osteocytes comprise approximately 90% of resident cells in mature bone tissue (with the remainder being made up of osteoblasts, osteoclasts, and bone lining cells). They are identifiable by their unique embedded position in the lacunar-canalicular system. At a cellular level, osteocytes are characterized morphologically by the multiple dendritic processes (canaliculi) that project outwards to meet other canaliculi, nearby blood vessels, and lining cells at the bone surface. Osteocytes also have a unique molecular signature including expression of SOST, DMP1, PHEX, and MEPE which are expressed to varying degrees during the lifetime of the cell [6]. These cells were once thought to exist merely as “placeholders” in mineralized matrix [7•], without any significant function, while osteoblasts and osteoclasts were considered more central to bone physiology. It is now clear that this is not the case, and that osteocytes play a critical role in regulating bone adaptation and remodeling processes. Indeed, osteocytes have important functions even beyond the skeleton [1, 2•] such as in the regulation of kidney function [7•, 8].
Since the mineralized microenvironment is so central to the function and nature of osteocytes, removing them for study under standard in vitro conditions was traditionally a challenge. Important advances were made in this area of osteocyte biology by Bonewald et al. [7•, 9, 10•] and others who created the first osteocyte immortalized cell line. This led to the development of a rich new area of osteocyte research (reviewed in [10]). In parallel with this, much of the early work on the osteocyte, and its mechanosensory role, was carried out using in vivo animal models. Most of the early studies examined the effects of extrinsic mechanical loading on osteocytes in situ, using surgical methods [11, 12]. The details of these models are discussed below, but in general, they proved useful in establishing some of the basic underlying principles of bone mechanotransduction. However, it also became clear that the surgical approach was somewhat hindered by injury/inflammatory effects on surrounding tissues, making mechanistic interpretation of the localized bone response challenging. To address these difficulties, non-surgical/non-invasive methods were developed, which centered on the application of controlled extrinsic loads using standardized laboratory-based mechanical systems. These methods allow forces to be applied and modulated in a controlled way, and thus can titrate the extent of injury/inflammation induced. This ability to carefully control loading conditions means that most non-invasive models can be successfully used without generating any appreciable inflammation, if required. These methods proved very successful and quickly gained widespread popularity in the field. In comparison to surgical methods, non-invasive loading seemed better able to replicate the mechanical signals generated during physiological (and pathological) loading of the skeleton. Importantly these methods also avoided complications of wound-healing/infection [7•, 8, 13, 14]. Furthermore, these models were cost-effective, reproducible, and relatively easy to conduct using the sensitive control systems that are available on most commercial mechanical testing systems [6, 15].
This review will briefly cover the background of in vivo mechanical loading as an experimental approach in the field of osteocyte biology, from its initial applications to the more recent developments and will largely focus on advances made during the past 5 years.
Early Models
Surgical Models
Mechanical loading models to study the bone response to external forces were first examined in the mid-1800s by Charles Sedillot [16]. Later, Jiri Hert [17, 18] developed a more controlled system where an external loading device, acting through transfixing pins, was used to alter the mechanical loads across rabbit tibiae. In those studies, adaptive bone remodeling in the region around the pins was observed. However, it was not possible to define the connection between externally applied loads and localized tissue level stress/strain fields [19]. To address this, Lanyon et al. [20, 21•] used strain gauges at the bone surface in the area of interest, thus bringing an extra level of control. This was a particularly important refinement and led to a greater understanding of how extrinsic loads in one place, can change the localized strain field at another. A particular advantage of these models was that the section of loaded bone was functionally isolated after surgery, preventing any confounding strain stimulus from normal activity. Unfortunately, however, the surgically placed pins caused local periosteal woven bone responses, which complicated subsequent analyses. This model was also somewhat limited by the time required for post-operative healing, which was necessary to allow full integration of the pins [20, 21•]. The development of a surgical vertebra-loading model in the rat overcame some of these challenges [22, 23]. As before, surgically placed pins were used to transmit extrinsically controlled loads. However, in this case, pins were placed through the vertebrae in the tail cranial and caudal to the vertebra of interest. This approach has an advantage in that the injury-induced woven bone response occurs only in the pinned vertebra, i.e., adjacent to the vertebra under study, therefore avoiding some of the difficulties discussed above. However, a time lag between surgery and load application remained in this model. A subsequent approach, which did not require placement of surgical pins, was the ulnar osteotomy model. In that model, the concept was to take advantage of the natural load-sharing relationship between the ulna and radius in the animal forelimb. The surgical removal of a central segment of the ulna increases the loading on the radius, under regular bodyweight. This provides the opportunity to examine the response of the tissue in the radius to increased loading, without the need for surgical pins [11, 12, 24]. Nonetheless, inflammatory and wound-healing responses still occurred, and in order to overcome some of these limitations, non-invasive mechanical methods were developed.
Non-invasive Models
Some of the earliest non-invasive extrinsic mechanical loading models were carried out in order to interrogate the mechanosensory role of osteocytes, and to understand the underlying mechanism—without the complicating factors of surgery, wound-healing, and inflammation [25, 26•, 27]. One of the initial concepts used a 4-point bending system (a common technique used to characterize structural properties in mechanical engineering), to apply external forces at the surfaces of long bones, through the soft tissues, and generate a bending moment (Fig. 1a). This proved very useful for studying endocortical adaptation, but the periosteal reaction at the point of application of load, again involving inflammation and woven bone formation, was difficult to interpret [11, 24]. In an attempt to overcome these issues, a cantilever model was developed whereby the knee joint was secured and the ankle was used as the point of application of load, to induce mediolateral bending in the longitudinal plane of the tibia, without direct force applied to the periosteum [28]. This model was successful in eliminating the woven bone formation response in the periosteum—but was slightly more challenging to carry out experimentally [29, 30].
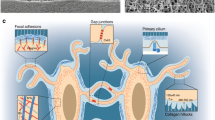
Schematic illustrations of non-invasive mechanical loading models. (a) Four-point bending model of rat tibia where a bending moment is generated in the medio-lateral direction (adapted from [28, 82]). (b) Rat ulnar loading model where forelimb is fixed and a force is applied along the diaphysis (adapted from [33, 34]). (c) Rat knee injury model shows the hind limb positioned and fixed such that torsional and valgus forces are induced across the knee joint (adapted from [54])
The next model that was developed has become possibly the most widely used in vivo bone loading system in the field. The premise of the ulnar axial loading system [31,32,33] is that the flexed wrist and elbow of an animal (usually a rodent) are secured into a testing machine (Fig. 1b). Compressive loads are then applied and transmitted along the length of the forelimb. Its natural curved shape allows the linear force to be translated into a bending moment. Importantly, this is achieved without direct force application to the periosteum. One of the important early discoveries using this approach was that loads, above a certain magnitude, must be applied cyclically (rather than monotonically) to achieve an osteogenic, or bone forming, response. This model has been widely used by many researchers to advance our understanding of how osteocytes orchestrate an osteogenic response to mechanical stimulus [13, 34,35,36,37]. A modified version of this system has also become the gold standard for the controlled introduction of microdamage to diaphyseal cortical bone and to determine the role of osteocytes in microdamage repair. Once again, cyclical loading at a specific magnitude was found to be necessary to initiate and propagate linear microcracks. Some of the principles of this model were also derived initially from the engineering/material science fields, in particular, fracture mechanics. Using this approach, it became possible to introduce controlled levels of microdamage to a defined area of bone tissue. Within that defined area the resident osteocyte response to damage could be characterized at a cellular and molecular level [2, 6, 38,39,40,41,42,43]. This method was recently used to determine that osteocyte apoptosis, in response to microdamage generated by ulnar loading, was an obligate step in the initiation of the targeted remodeling response [38]. In this scenario, one population of osteocytes undergo apoptosis, while a neighboring population upregulate osteoclastogenic factors such as RANKL, and thus orchestrate the reparative targeted remodeling response to linear microcracks.
The concept of in vivo axial loading of rodent long bones was then adapted for use in the lower limb [44•, 45, 46,47,48,49]. In this system, controlled forces are applied to the distal femur of the flexed knee, and the calcaneus of the dorsiflexed ankle—thus creating compressive forces in the tibia. Like the ulnar loading model, the curved bone shape allows for the transformation of linear compressive forces into a bending moment. However, in contrast to the ulnar model, forces are not applied directly to the bone under study—which has advantages and disadvantages. It is advantageous in terms of understanding the resultant stress-state in the tibia. However, there are limitations in how much force can be passed through a mobile joint (in particular the knee) without causing damage/dislocation to that joint. Another particularly useful attribute of this model is that it can target the trabecular bone compartment, specifically in the proximal tibia, whereas the ulnar loading model largely targets mid-diaphyseal cortical bone.
Recent developments of each of these systems have expanded their utility and broadened their application base. The ulnar damage induction protocol described above was modified to create a different type of microdamage. While it was known that cyclical loading generated linear microcracks on the order of 10–100 μm—it was also apparent that clusters of smaller < 1 mm cracks were generated simultaneously [39, 50]. These clusters are known as areas of “diffuse damage,” but little was known about their functional significance. By applying a constant load (instead of cyclical) to the ulna, Seref-Ferlengez et al. [24] were able to reproducibly generate diffuse damage, without introducing linear microdamage. By studying this in isolation, they determined that this damage occurs at physiological load levels and that it also is repaired over time. However, intriguingly, this seemed to occur in an osteocyte independent manner. This suggests that there may be an entirely different bone repair mechanism, distinct from osteonal remodeling, which has remained undiscovered until now. Whether this is an entirely cell-independent process (i.e., purely physico-chemical) is not known.
The tibial loading model has also been adapted recently to address questions relating to joint injury and disease. In most cases, the concept is to increase/alter the loading mechanism to introduce some amount of controlled damage to the knee joint, and then to observe the pathophysiological response. These models ultimately focus on osteoarthritis and are thus outside the remit of this review. Nonetheless, in most cases, the subchondral bone compartment plays a role, to a greater or lesser extent, and so are worth mentioning briefly. Various different models have been described to study different aspects of joint injury and disease. Olsen et al. described an intra-articular tibial plateau fracture model in mice. This model captures the aspect of a high-impact, high-energy joint injury which induces degenerative joint changes over an expected time period post-injury [51]. Christiansen et al. [52] used single compressive overload to rupture the anterior cruciate ligament in a model of post-traumatic osteoarthritis (PTOA). This model also nicely replicates human injury and subsequent OA develops due to a known etiology—and also evaluates bony responses [29, 46, 52, 53]. Ramme et al. also described a PTOA model, this time using the rat, whereby anterior cruciate ligament (ACL) rupture and subchondral bone microdamage was generated (Fig. 1c). Subchondral bone microdamage was quantified as was its relationship with subchondral bone remodeling response. This model also results in cartilage degradation over the expected timeframe for these models [54]. Single tibial compression models have also been described [55], whereby a single bout of loading was shown to damage articular cartilage, but not induce progressive joint changes. Whereas proteoglycan loss and cartilage lesions were observed after 2 weeks of cyclical loading. A similar model has also been used with a range of different load magnitudes to study a spectrum of joint responses including chondrocyte apoptosis, matrix degradation, synovitis as well and pathological bony responses [56,57,58]. Most recently, a novel model for trans-cortical induction of subchondral microdamage showed that such subchondral microdamage overlaps spatially with bone marrow lesions (BMLs) [59]. BMLs are a common feature of clinical joint injury that may be involved in joint changes via some form of bone-cartilage crosstalk.
Lewis et al. [60••] recently developed an entirely new in vivo extrinsic bone loading system for real-time visualization of the osteocyte response to mechanical stimulus in mouse metatarsals. Using a miniature 3-point bending system, in conjunction with multiphoton microscopy, their work evaluated the Ca2+ response in osteocytes to physiological strains between 250 and 3000 με and frequencies from 0.5 to 2 Hz. This study showed that, at all frequencies examined, the size of the responding osteocyte population increased strongly with applied strain, while Ca2+ intensity remained unchanged [60••]. This suggests that osteocytes respond to this stimulus in a binary manner, either “on” or “off” and with increased load magnitude, the number of respond cells changes rather than response/activity level of any given cell. This Ca2+ response profile is common in other cell types and systems including glia and cardiac muscle [61]. The high correlation between applied strain and size of the Ca2+-responding osteocyte population suggests this cell network is remarkably sensitive in how it encodes the mechanical frequency input. This work fits well with a computational model that was recently reported to predict the response of mouse bone to anabolic loading using the tibial loading model described above [62]. The model was based on established osteocytes characteristics and predicted that the commonly observed load-induced bone adaptation could only result from osteocytes responding in a binary manner. Furthermore, the model predicted that osteocyte recruitment number would vary with load. Taken together, these studies are consistent with the “set-point” hypothesis that was originally described by Frost [63].
All of the models described here are used to examine the effect of extrinsic loading on the skeleton and the role of osteocytes in the subsequent response. However, it is also clear that underloading, or lack of mechanical stimulus, has a significant effect on the skeleton. This is traditionally discussed in terms of spaceflight or microgravity, which is a well-known cause of bone loss in astronauts—but is also important for long-term bed rest and spinal cord injury patients. Due to the obvious difficulties in mimicking a microgravity environment in a laboratory setting, the rodent hind limb suspension (HLS) model has been used to study the effects of unloading on the skeleton [64, 65] In the HLS model, body weight is removed from the hind limbs by suspending the animal by its tail from an overhead wire system. This stimulus generates a robust osteoclast-mediated bone loss response. Until recently, the role of osteocytes in this process was unknown. Osteocyte apoptosis is known to be essential in activating bone remodeling in response to a mechanical stimulus, such as cyclical fatigue loading (as discussed above). Although it had been spatially linked to bone resorption activity following disuse, whether osteocyte apoptosis played an active controlling role was unclear. Studies using the HLS model showed that osteocyte apoptosis was increased significantly in both cortical and trabecular bone, after just 5 days [66]. Increases in osteocyte apoptosis and RANKL production were found to precede bone resorption. Furthermore, pharmacological inhibition of apoptosis completely abrogated the remodeling response.
In summary, it is clear that the use of extrinsic mechanical models that alter skeletal loading have contributed greatly to understanding the way in which osteocytes respond to changes in their mechanical/physical environment. It is still unclear exactly how this is achieved—defining the mechanosensor in osteocytes remains one of the most exciting challenges in the field.
Mechanotransduction
The process of translating mechanical forces into cellular biochemical signals is complex. The real-time load-induced osteocyte Ca2+ signaling discussed above clearly demonstrates that the bone response to loading occurs very rapidly after the stimulus is applied. Cell culture studies have shown that adenosine triphosphate (ATP) is also released on a similarly short timescale from stimulated bone cells [67]. Subsequent to this, second messengers prostaglandin E2 (PGE2) and nitric oxide (NO), are also released quickly from mechanically activated cells [68, 69].
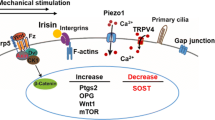
The extremely short timescale over which these events occur is partly why identification of the primary mechanosensor has been so challenging [70••]. There are several general signaling pathways that seem to play a role in the process, but none among them has yet been identified as the mechanosensor. Primary cilia, ion channels, integrin/cytoskeletal complexes, and G protein-coupled receptors are found in many cell types and seem to contribute, at least to some extent, to osteocyte mechanotransduction [71].
Primary cilia are rigid multifunctional organelles that extend out from the cell body and have the ability to contribute to mechanotransduction via direct deflection under fluid flow and/or pressure [71]. These structures are extremely important in growth and development and seem to be involved in osteocytes pericellular mechanics as well as in mechanotransduction in other tissues including liver and kidney [72, 73]. Ion channels are pore-forming structures at the cell membrane that permit passage of charged ions in and out of the cell through the otherwise impermeable lipid bilayer cell membrane. Ions flow passively through channels towards equilibrium in response to an electrochemical stimulus or to a physical stimulus (such as stretch-activated channels). A particular type of channel protein that is highly expressed in bone cells is connexin-43 [74]. These gap-junction proteins are normally found in opposition to an identical structure in adjacent cells and thus facilitate direct cell-cell communication. However, they can also exist unopposed in one cell membrane, and in this scenario, are called “hemi-channels.” Such channels allow egress of PGE2 from osteocytes via physical perturbation of their associated integrin network that serves to anchor the cells to their substrate. The integrin/cytoskeletal complex likely plays a central role in mechanotransduction in various cell types. In osteocytes, the cell processes are physically linked to the lacunar-canalicular system by tethering structures including αvβ3 integrins and the glycocalyx. In vivo, fluid movement through the lacunar-canalicular system across these structures results in strain amplification at the cell body, which ultimately mediates the osteocyte response. Intriguingly, given the discussion above, in osteocytes, these sites lack the typical integrin transduction mechanism but are known to respond by altering Ca2+ signaling [66, 75]. G protein-coupled receptors (GPCR) are the largest family of plasma membrane receptors at the cell surface. GPCRs can be activated by a variety of factors including hormones, peptides, and amino acids. Thus, perhaps unsurprisingly, these proteins are involved in the process of mechanotransduction. However, and again unsurprisingly, their role is not straightforward. Purified G proteins in empty phospholipid vesicles can be activated (GTP hydrolysis) through application of fluid shear alone [76], independent of their receptor (GPCR). Their behavior also appears to be modulated by membrane or substrate stiffness. GPCRs themselves are also sensitive to ligand-independent activation, in response to mechanical stimulation. Conformation-sensitive fluorescence resonance energy transfer (FRET) imaging has been used to demonstrate shear stress-induced changes in parathyroid hormone/parathyroid hormone-related peptide receptor in MC3T3 osteoblast-like cells [77]. The same group reported that similar changes occur in the B2 bradykinin receptor on endothelial cells [78]. This suggests that the mechanosensitive role of GPCRs might be highly conserved among many cell types [70••]. However, this still does not confirm that this, or the other broad categories discussed above, is the ultimate mechanosensory mechanism in osteocytes.
As with many highly conserved and ubiquitous signaling pathways (such as those discussed above), using genetic methods to modify their function can be challenging. However, using a genetic approach to modulate very specific pathways, related to a system/process of interest, can be extremely useful. For example, the Wnt signaling pathway is central to the process of bone formation and is also implicated in mechanotransduction. The canonical Wnt signaling cascade is initiated when Wnt proteins form receptor complexes with co-receptors called “Frizzled,” and either low-density lipoprotein-related receptor-5 (Lrp5) or 6 (Lrp6). Successful activation of this receptor complex leads to accumulation of β-catenin in the cytoplasm and subsequent transcriptional activity. Heterozygous deletion of β-catenin in osteocytes reduces load-induced bone formation [79]. Lrp5 and Lrp6 are closely related, but also have distinct functional properties. The full extent of their downstream effects is yet to be fully characterized; the current state-of-the-art is comprehensively reviewed in [80]. These studies demonstrate the impact that genetic manipulations in pre-clinical models can have in parsing out cell/molecular mechanisms, even in complex systems such as mechanotransduction in bone and bone formation.
In summary, the use of in vivo animal models in osteocyte mechanotransduction has undoubtedly been crucial to progress in the area. It is also likely that their use will be central to continued progress in the future. This may come via use of existing, or newly developed, non-invasive mechanical models and/or through generation of novel transgenic model systems to probe important signaling pathways. Indeed, it is likely that some combination of both will provide key future insights, due to the inherent multi-disciplinary nature of mechanobiological research.
Conclusion
Our understanding of how osteocytes respond to mechanical loading in vivo has continued to grow in recent years, due to the development of new techniques and methodologies. These advances have opened up new areas of research and can provide a better understanding of how osteocyte mechanotransduction is integrated into broader skeletal health. This work is complemented greatly by continuing advances that are being made on the basic functionality and physiology of osteocytes [81••]. While there is now a suite of in vivo extrinsic mechanical models available to interrogate different aspects of bone mechanotransduction, such as the ulnar/tibial axial loading models discussed in this review, it will also be important to continue innovating and developing new systems [60••]. This will allow us to address limitations of existing techniques and to answer newer, more challenging, questions as they arise.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700.
• Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24 Provides a detailed review of experimental and theoretical approaches used in osteocyte mechanobiology.
Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–43.
Lerner UH. Osteoblasts, osteoclasts, and osteocytes: unveiling their intimate-associated responses to applied orthodontic forces. Remodeling and Modeling of Bone Tissue YSODO. 2012;18:237–48.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–90.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10:118–25.
• Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38 Provides an excellent review of osteocyte biology and function.
Budyn E, et al. How the morphology of osteocytes contributes to their mechanotransduction near microdamage. MRS Proc. 2015;1724:mrsf14–1724–h12–24.
Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 2010;12:2014–23.
• Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17:61–5 Describes the establishment of the first osteocyte cell line.
Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. An experimental study. J Bone Jt Surgery. 1979;61:539–46.
Lee TC, Staines A, Taylor D. Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat. 2002;201:437–46.
Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci. 2010;1192:422–8.
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. https://doi.org/10.1038/382448a0.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Current Osteoporosis Reports. 2015;13:180–5.
Sedillot, C. Des moyens d’assurer la réussite des amputations des membres Résultats statistiques des amputations pratiquées par moi pendant la dernière année scolaire. C R Acad Sci. 1852;34
Hert J, Lisková M, Landa J. Reaction of bone to mechanical stimuli. 1. Continuous and intermittent loading of tibia in rabbit. Praha. 1971;19:290–300.
Hert J, Sklenská A, Lisková M. Reaction of bone to mechanical stimuli. 5. Effect of intermittent stress on the rabbit tibia after resection of the peripheral nerves. Praha. 1971;19:378–87.
Meakin LB, Price JS, Lanyon LE. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front Endocrinol. 2014;5.
Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905.
• Lanyon LE. Analysis of surface bone strain in the calcaneus of sheep during normal locomotion Strain analysis of the calcaneus. J Biomech. 1973;6:41–9 Describes one of the earliest methods of measuring surface strain of bone in vivo.
Stokes IA, Mente PL, Iatridis JC, Farnum CE, Aronsson DD. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Jt Surg - Ser A. 2002;84:1842–8.
Lambers FM, Koch K, Kuhn G, Ruffoni D, Weigt C, Schulte FA, et al. Trabecular bone adapts to long-term cyclic loading by increasing stiffness and normalization of dynamic morphometric rates. Bone. 2013;55:325–34.
Seref-Ferlengez Z, Basta-Pljakic J, Kennedy OD, Philemon CJ, Schaffler MB. Structural and mechanical repair of diffuse damage in cortical bone in vivo. J Bone Miner Res. 2014;29:2537–44.
Skerry TM, Lanyon LE, Bitensky L, Chayen J. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–8.
• Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone. 1991;12:73–9 Describes of the early noninvasive bone adaptation models.
Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63:442–9.
Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17:493–501.
Connelly JT, Fritton JC & Van Der Meulen MC Simulation of in vivo loading in the tibia of the C57BL/6 mouse. 49th Annu Meet Orthop Res Soc (2003).
Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2012;20:773–82.
Torrance AG, Mosley JR, Suswillo RFL, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54:241–7.
Lee KCL, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–12.
Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–13.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75.
Lara-Castillo N, et al. In vivo mechanical loading rapidly activates β–catenin signaling in osteocytes through a prostaglandin mediated mechanism HHS public access. Bone. 2015;76:58–66.
Guo D, Bonewald LF. Advancing our understanding of osteocyte cell biology. Therapeutic Advances in Musculoskeletal Disease. 2009;1:87–96.
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28:865–74.
Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–7.
Vashishth D. Hierarchy of bone microdamage at multiple length scales. Int J Fatigue. 2007;29:1024–33.
Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54:250–7.
Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–7.
Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, et al. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–83.
Cardoso L, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J BONE Miner Res J Bone Min Res. 2009;2424:597–605.
• Fritton JC, Myers ER, Wright TM, Van Der Meulen MCH. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8 Describes the first tibial loading model to study osteocyte mechanotransduction in trabecular bone.
Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, et al. Mechanical load increases in bone formation via a Sclerostin-independent pathway. J Bone Miner Res. 2014;29:2456–67. https://doi.org/10.1002/jbmr.2278.
De Souza RL, et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8.
Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–15.
Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91.
Zaman G, Jessop HL, Muzylak M, de Souza RL, Pitsillides AA, Price JS, et al. Osteocytes use estrogen receptor α to respond to strain but their ERα content is regulated by estrogen. J Bone Miner Res. 2006;21:1297–306.
Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, et al. In vivo diffuse damage in human vertebral trabecular bone. Bone. 2000;26:147–52.
Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–92.
Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23:1627–38.
Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32:79–88.
Ramme AJ, Lendhey M, Raya JG, Kirsch T, Kennedy OD. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthr Cartil. 2016;24:1776–85.
Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–47.
Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets. Arthritis Rheum. 2014;66:1256–65.
Yan H, et al. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci. 2017;114:–E3871.
Rai, M. F. et al. Post-traumatic osteoarthritis in mice following mechanical injury to the synovial joint. Sci Rep 2017;7.
Matheny JB, Goff MG, Pownder SL, Koff MF, Hayashi K, Yang X, et al. An in vivo model of a mechanically-induced bone marrow lesion. J Biomech. 2017;64:258–61.
•• Lewis KJ, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci. 2017;114:11775–80 Describes a new method of noninvasive mechanical loading to study osteocyte response.
Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–7.
Pereira AF, Javaheri B, Pitsillides AA, Shefelbine SJ. Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface. 2015;12:20150590.
Frost H. A determinant of bone architecture. The minimum effective strain. Send to Clin Orthop Relat Res. 1983;175:286–92.
Morey ER, Sabelman EE, Turner RT, Baylink DJ. A new rat model simulating some aspects of space flight. Physiologist. 1979;22:S23–4.
Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–2.
Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hind limb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–65.
Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–9.
Forwood MR, Kelly WL, Worth NF. Localization of prostaglandin endoperoxide H synthase (PGHS)-1 and PGHS- 2 in bone following mechanical loading in vivo. Anat Rec. 1998;252:580–6.
Rawlinson SCF, et al. Loading-related increases in prostaglandin production in cores of adult canine cancellous bone in vitro: a role for prostacyclin in adaptive bone remodeling? JBMR. 1991;6:1345–51.
•• Robling, A. G. The interaction of biological factors with mechanical signals in bone adaptation: recent. Reviews recent developments in osteocyte mechanotransduction.
Nguyen AM, Young Y-N, Jacobs CR. The primary cilium is a self-adaptable, integrating nexus for mechanical stimuli and cellular signaling. Biol Open. 2015;4:1733–8.
Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrime signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun Biochem Biophys Res Commun August. 2011;19:182–7.
Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400.
Plotkin LI, Speacht TL, Donahue HJ. Cx43 and mechanotransduction in bone. Current Osteoporosis Reports. 2015;13:67–72.
Cabahug-Zuckerman P, Stout RF Jr, Majeska RJ, Thi MM, Spray DC, Weinbaum S, et al. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J Orthop Res. 2018;36:642–52.
Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A. 1998;95:2515–9.
Zhang Y-L, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am J Phys Cell Phys. 2009;296:C1391–9.
Chachisvilis M, Zhang Y-L, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–8.
Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, et al. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29:705–15.
Kang KS, Robling AG. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front Endocrinol. 2015;6:246.
•• Bonewald LF. The role of the osteocyte in bone and nonbone disease. Endocrinol Metab Clin N Am. 2017;46:1–18 Describes an updated review of role of osteocytes in multiple pathological states.
Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1:249–62.
Acknowledgements
The authors gratefully acknowledge the contribution of Jack Roberts on the illustrative work in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paige V Hinton, Susan M. Rackard, and Oran D. Kennedy declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Hinton, P.V., Rackard, S.M. & Kennedy, O.D. In Vivo Osteocyte Mechanotransduction: Recent Developments and Future Directions. Curr Osteoporos Rep 16, 746–753 (2018). https://doi.org/10.1007/s11914-018-0485-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0485-1