Abstract
This is a review on second primary tumors in patients with head and neck cancer. These patients have a high risk of developing other cancers simultaneously or subsequently. The incidence of multiple primary tumors in this population can be as high as 27%. Recurrences are the most common cause of treatment failure within the first 2 years of follow-up. After the third year the diagnosis of a second primary tumor becomes the most important cause of morbimortality in head and neck cancer patients, especially in those treated for cancers early diagnosed. Most second primary tumors occur in the upper aerodigestive tract (40%–59%), lung (31%–37.5%), and esophagus (9%–44%). Patients who develop second primary tumor have a significant reduction of survival expectancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinomas represent approximately 3% of all human malignancies and most occur in the upper aerodigestive tract (UADT) [1]. In the United States, the estimated number of newly diagnosed oral cavity, pharynx, and larynx cancers is 49,260 cases and 11,480 deaths in 2010 [1]. The treatment of these patients depends on several factors, including the institutional experience, primary tumor site and clinical stage, and the patients’ medical conditions and acceptance [2]. Most recurrences are diagnosed within the first 2 or 3 years after initial treatment, and the majority are local and regional [3, 4]. After the third year, the diagnosis of a second primary tumor (SPT) becomes an important cause of morbimortality [5–8].
Patients with UADT squamous cell carcinomas have a high risk of developing other cancers simultaneously or subsequently. The incidence of multiple primary tumors in these patients can be as high as 27%. Most of these tumors are located in the oral cavity, pharynx, larynx, lungs, or esophagus [9–14, 15•, 16–18]. The main reason for this topographical specificity is the exposure of the squamous epithelium of these organs to the same carcinogenic agents, especially tobacco and alcohol [14, 17, 19, 20].
During the last 20 years, multiple primary tumors in patients with UADT cancer have been considered an important problem because a second cancer has become the major cause of death in patients treated for cancers early diagnosed [7, 8, 21].
Historical Aspects and Basic Considerations
The first description of simultaneous tumors that occurred in one patient was presented by Billroth in 1860, cited by Warren and Gates [22]. Warren and Gates [22] published in 1932 a comprehensive review of several case series of multiple primary tumors and also reported 1078 autopsies among which 40 new cases (3.7%) of multiple tumors were reported. In this study, the authors proposed and used the following criteria for the diagnosis of multiple primary tumors: confirmation of malignancy in both tumors, each tumor must be distinct, and it is necessary to exclude the possibility that one tumor is a metastasis of the other.
Slaughter et al. [20] in 1953 proposed the “condemned mucosa” theory to explain the high incidence of SPT in patients with carcinomas induced by environmental factors and introduced the concept of “field cancerization” to explain the occurrence of multicentric squamous cell carcinomas in the oral cavity. They showed that the epithelium around the tumor had histologic changes, possibly related to the same carcinogen exposure and then more susceptible to malignant transformation. Other authors have confirmed the existence of multicentric dysplasia foci and in situ carcinoma in patients with squamous cell carcinomas of the larynx [23], pharynx [24], and trachea or bronchi [25]. The existence of premalignant epithelial changes in macroscopic normal epithelium in patients with upper aerodigestive tract carcinomas was also demonstrated [26].
Califano et al. [27] proposed a model of head and neck carcinoma carcinogenesis. Cytogenetic analysis and polymerase chain reaction (PCR) used for the evaluation of microsatellite instability allowed the identification of sequential chromosome losses and possible sites of tumor suppressor genes. These changes are detected cumulatively during tumor progression, while all the tumor genetic alterations identified in situ are present in invasive carcinomas, to which are added progressive additional ones. Different genetic alterations involving the same chromosomes distributed irregularly in the mucous epithelium support the thesis of “field cancerization” and the polyclonal origin of multiple squamous cell carcinomas.
The first tumor diagnosed is arbitrarily defined as the primary or index tumor. Considering the time of diagnosis, multiple tumors can be classified into 1) synchronous (diagnosed simultaneously or within 6 months after the diagnosis of the index tumor); or 2) metachronous (diagnosed after a time interval of 6 months).
Incidence and Localization of Second Primary Tumors
The incidence of SPT varies across several studies, depending mainly on the follow-up time and systematic screening of the cases [9–14, 15•,[16–18]. In three large series of cases, Chuang et al. [15•] with 99,257 patients, Haughey et al. [11] with 40,287, and Panosetti et al. [9] with 9089, the SPT incidence was 10.9%, 14.2%, and 9.4%, respectively.
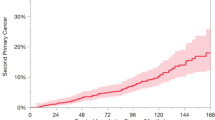
In patients with UADT primary tumors, the SPT diagnosis occurs at a rate of approximately 3.7% to 6% per year during the follow-up period [5, 16, 17]. The risk of SPT remains high even after 10 years of follow-up and the cumulative risk at 20 years is around 36% [15•]. Most SPT occurs in the UADT (40%–59%), lung (31%–37.5%), and esophagus (9%–44%) [5, 12, 16–18].
Risk Factors and Prevention
Several studies have attempted to identify predictive factors of SPT in patients treated for UADT tumors [10, 12, 14, 15•, 17, 19]. Day and Blot [10] collected data from nine population-based cancer registries in the United States and assessed the risk of SPT in 21,371 patients diagnosed with oral and pharyngeal cancer. They found a relative risk (RR) of SPT development ranging from 4.2 to 23 times (esophagus [RR 23.0], mouth and pharynx [RR 20.0], larynx [RR 6.8], nasal cavity and paranasal sinus [RR 4.9], and lung [RR 4.2]). The elevated risk persisted for over 5 years after the primary tumor diagnosis and was higher in patients aged 60 years or less.
In a case-control study with 85 cases of SPT and 170 controls matched by tumor location, risk factors related to the occurrence of metachronous SPT were smoking (RR 4.9), metal industry work (RR 6.2), clinical stage III and IV tumors (RR 0.1), and follow-up between 6 and 24 months (RR 4.4) or greater than 24 months (RR 11.5) [19].
A study with 1257 patients with oral cavity and larynx primary tumors identified as independent predictors of SPT only tobacco and alcohol consumption (risk five and two times greater, respectively). Five-year disease-free survival after SPT was better in those patients who were nonsmokers or nondrinkers than in the tobacco or alcohol addicts (98% and 90% respectively, P = 0.01) [14].
In another study, the risk factors to the diagnosis of SPT were male sex, age less than 60 years, and early primary tumors (T1 and T2) without nodal metastases (N0) located in the larynx or oral cavity. In multivariate analysis only T and N stages were related to the diagnosis of SPT [12]. A positive correlation between SPT and index tumor at clinical stages I and II, age less than 66 years. Early-stage tumors of the larynx or oral cavity were also identified as risk factors by other authors [16].
Leon et al. [17] conducted a case-control study to evaluate the influence of the persistence of tobacco and alcohol use on risk of SPT in patients treated for UADT squamous cell carcinomas. The risk for the development of SPT in patients who continued smoking was 2.9 and for those who continued to consume alcohol was 5.2. The authors identified a strong association between continuity of tobacco and alcohol consumption with the development of SPT after treatment of primary tumor, being the habit responsible for development of at least 33% of the SPT.
SPT chemoprevention in patients with primary head and neck cancer treated was evaluated in various randomized clinical trials [28–32]. Hong et al. [28] randomized patients to receive high dose of isotretinoin (13-cis-retinoic acid; 50–100 mg per square meter of body-surface area per day) or placebo, to be taken daily for 12 months. After a median follow-up of 32 months, the incidence of second primary tumors was lower in the isotretinoin than in the placebo group (P = 0.005). But 33% of the patients in the isotretinoin group did not complete de treatment because of drug toxicity. In another study, when low-dose isotretinoin was used, it was not demonstrated effective in reducing the rate of second primary tumors [29].
Other substances (alpha-tocopherol [30], retinyl palmitate and/or N-acetylcysteine [31], beta-carotene [32]) were studied, but reduction of SPT incidence was not significant.
Screening and Diagnosis of Second Primary Tumors
A close follow-up, using routine triple endoscopy (laryngoscopy, endoscopy, and bronchoscopy), has been recommended aiming to diagnose precursor lesions and early-stage asymptomatic invasive tumors [33–36]. However, most reports only describe the frequency of diagnoses and not the long-term results of treatment of these patients (Table 1) [11, 33–41]. The efficacy of triple endoscopy was considered better in the earlier reports than in the most recent ones.
On the other hand, there are some doubts on the cost-effectiveness of triple endoscopy used routinely. A study that followed-up 140 patients with UADT primary tumor for 1 to 4 years reported that 18 SPT were diagnosed, and the authors´ conclusion was that in the absence of symptoms endoscopy and bronchoscopy have high cost and minimal benefit [42].
The use of Lugol chromoendoscopy allows identifying areas of suspected mucosal lesions or with premalignant transformation, and can orient the best site for biopsy [3, 34]. More recently, narrow-band imaging combined with magnifying endoscopy have been demonstrated to improve the detection accuracy of a larger number of asymptomatic mucosal premalignant lesions and early cancers of the UADT [43••]. New techniques, like fluorescence spectroscopy and microendoscopy in addition to modern flexible endoscopic techniques, may have an important role in SPT screening in the near future [44].
Positron emission tomography (18F-FDG-PET/CT) seems to be a promising test for early diagnosis of SPT in patients with primary UADT cancer. Haerle et al. [45••] demonstrated that 18F-FDG-PET/CT diagnosed more SPT than triple endoscopy (6.1% and 4.5%, respectively), but with a higher number of false positives. In a series of 589 patients with UADT squamous cell carcinoma submitted to 18F-FDG-PET/CT, Strobel et al. [46] diagnosed 56 SPT in 44 patients, 55% of them at early clinical stage.
Several molecular biology techniques have been used aiming to identify genetic abnormalities associated with the tumor progression and are possible predictors of SPT. Among the genetic alterations, p53 expression [47], and polymorphisms of p21 [48], p73 [49], and glutathione S-transferases [50], were related to the SPT risk in head and neck cancer patients.
Survival and Prognoses
Panosetti et al. [9] identified a better survival in metachronous SPT than in synchronous (55% and 18% in 5 years, respectively). In 49.4% of the synchronic tumors the treatment of the primary tumor had to be modified. The prognosis was worse when a change was needed in the initial treatment of the primary tumor. When the first treatment was performed according to the standard guidelines the 5-year survival was 28%, and it was only 8% when treatment planning was modified.
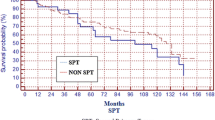
Two other studies also showed better survival rates for metachronous tumors. In the study by Di Martino et al. [13], the 5-year post-SPT survival was also significantly higher in the metachronous tumors (26.0%) than in synchronous (11.9%; P < 0.001). In the study by Lin et al. [14], the 5-year overall survival for synchronous tumors was 45% and for metachronous it was 70% (P = 0.003).
For patients with early glottic tumors (Tis, T1, and T2) treated with radiotherapy alone, Lee et al. [8] showed that the main cause of death in this group was the SPT. In the group of patients with SPT, the 5-year overall survival was 68%, and it was 88% for those without SPT
Franchin et al. [7] also studied patients with early larynx tumors treated with radiotherapy alone and concluded that the development of SPT was the first cause of death in this group, especially in those patients who continued to smoke after primary tumor diagnosis. The 10-year overall survival was 32% for patients who developed SPT and 77% for those who did not.
Rennemo et al. [16] evaluated the impact of SPT on survival rates of patients with head and neck primary cancer. They identified that in patients who developed SPT the overall median survival was 6 years and it was 3 years in those who did not have SPT (P < 0.05). In the first 6 years of follow-up, the cancer-specific survival was better in the group who developed SPT (70%) than in the group without SPT (50%). However, after 6 years of follow-up, the group with SPT has worse survival (5-year survival after SPT diagnosis was 16% and 90% of cancer-related deaths were due to SPT).
Jones et al. [12] reported 5-year overall survival was similar in patients with and without SPT (around 49%); however, the overall survival after 15 years of follow-up was 20% in the group that developed SPT and 44% in patients without SPT (P = 0.029). In this study, five-year survival after SPT diagnosis was 26% (31% for UADT SPT and 8% for other locations). In Cox regression multivariate analysis the variables related to worse survival were age (older than 60 years), primary tumor T and N stage (T3 and T4 and N positive), and presence of SPT (outside of UADT).
Lin et al. [14] also found better survival in patients with SPT located in UADT than in the lung (66% and 19%, respectively; P < 0.001). In the study by Rennemo et al. [16], no patient with lung SPT had survived more than 5 years. Of the 13 patients with esophageal SPT, the highest survival was 14 months. The best survival rates (20% in 5 years) were found in patients with UADT SPT and other locations.
Conclusions
The patients who develop an SPT have a significantly worse prognosis. Thus, the best strategies are prevention and early diagnosis (especially premalignant lesions). It is essential to provide the orientation and psychology support for tobacco and alcohol cessation.
After treatment of the primary tumor, it is necessary to maintain close follow-up of patients and always properly investigate their complaints and any suspicious lesion. The routine use of triple endoscopy (laryngoscopy, bronchoscopy, and endoscopy) allows, in selected cases, the diagnosis of premalignant lesions and invasive tumors. More recently introduced in clinical practice, 18F-FDG-PET/CT is a promising examination in SPT early detection.
Most SPT develops in the UADT mucosa accessible to routine head and neck clinical examination. Patients with metachronous SPT located in UADT have a better prognosis than those with synchronous or located in other sites. The best treatment, offering higher survival expectancy, is the classic set for each tumor site and clinical stage, always respecting the patient’s general condition and their choice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cancer Facts & Figures 2010 [online]. Atlanta: American Cancer Society; 2010 [cited 2010 Oct 3]. Available http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010.
Mendenhall WM, Amdur RJ, Stringer SP, Villaret DB, Cassisi NJ. Radiation therapy for squamous cell carcinome of the tonsillar region: a preferred alternative to surgery? J Clin Oncol. 2000;18:2219–25.
Cooney TR, Poulsen MG. Is routine follow-up useful after combined-modality therapy for advanced head and neck cancer? Arch Otolaryngol Head Neck Surg. 1999;125:379–82.
Haas I, Hauser U, Ganzer U. The dilemma of follow-up in head and neck cancer patients. Eur Arch Otorhinolaryngol. 2001;258:177–83.
Vikram B, Strong EW, Shah JP, Spiro R. Second malignant neoplasms in patients successfully treated with multimodality treatment for advanced head and neck cancer. Head Neck Surg. 1984;6:734–7.
Sturgis EM, Miller RH. Second primary malignancies in the head and neck cancer patient. Ann Otol Rhinol Laryngol. 1995;104:946–54.
Franchin G, Minatel E, Gobitti C, Talamini R, Vaccher E, Sartor G, Politi D, Trovo MG, Barzan L. Radiotherapy for patients with early-stage glottic carcinoma: univariate and multivariate analyses in a group of consecutive, unselected patients. Cancer. 2003;98:765–72.
Lee JH, Machtay M, McKenna MG, Weinstein GS, Markiewicz DA, Krisch RE, Kligerman MM. Radiotherapy with 6-megavolt photons for early glottic carcinoma: potential impact of extension to the posterior vocal cord. Am J Otolaryngol. 2001;22:43–54.
Panosetti E, Luboinski B, Mamelle G, Richard JM. Multiple synchronous and metachronous cancers of the upper aerodigestive tract: a nine-year study. Laryngoscope. 1989;99:1267–73.
Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14–9.
Haughey BH, Gates GA, Arfken CL, Harvey J. Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol. 1992;101:105–12.
Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343–53.
Di Martino E, Sellhaus B, Hausmann R, Minkenberg R, Lohmann M, Esthofen MW. Survival in second primary malignancies of patients with head and neck cancer. J Laryngol Otol. 2002;116:831–8
Lin K, Patel SG, Chu PY, Matsuo JM, Singh B, Wong RJ, Kraus DH, Shaha AR, Shah JP, Boyle JO. Second primary malignancy of the aerodigestive tract in patients treated for cancer of the oral cavity and larynx. Head Neck. 2005;27:1042–8.
• Chuang SC, Scelo G, Tonita JM, Tamaro S, Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E, Tracey E, Friis S, Pompe-Kirn V, Brewster DH, Martos C, Chia KS, Boffetta P, Brennan P, Hashibe M. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer. 2008;123:2390–6. This is an important review on risk factors of second primary tumors, using population-based cancer registry data.
Rennemo E, Zätterström U, Boysen M. Impact of second primary tumors on survival in head and neck cancer: an analysis of 2,063 cases. Laryngoscope. 2008;118:1350–6.
León X, del PradoVenegas M, Orús C, López M, García J, Quer M. Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with a head and neck cancer. A case-control study. Cancer Causes Control. 2009;20:645–52
Priante AVM, Carvalho AL, Kowalski LP. Second primary tumor in patients with upper aerodigestive tract cancer. Braz J Otorhinolaryngol. 2010;76:251–6.
Franco EL, Kowalski LP, Kanda JL. Risk factors for second cancers of the upper respiratory and digestive systems: a case-control study. J Clin Epidemiol. 1991;44:615–25.
Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Cancer. 1953;6:963–8.
Kowalski LP, Batista MB, Santos CR, Scopel AA, Salvajoli JV, Novaes PE, Trippe N. Prognostic factors in glottic carcinoma clinical stage I and II treated by surgery and radiotherapy. Am J Otolaryngol. 1993;14:122–7.
Warren S; Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–403
Sirtori C, Leonardelli GB, Parolari P. Plurifocalitá del cancro laringeo e significato di recidiva: studio istopatologico su cento casi. Arch Ital Oto’ Rinol Laringol. 1963;74:483–98.
Ballantyne AJ. Principles of surgical management of cancer of the pharyngeal walls. Cancer. 1967;20:663–7.
Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lunng cancer. New Engl J Med. 1961;265:253–67.
Incze J, Vaughan CW Jr, Lui P, Strong MS, Kulapaditharom B. Premalignant changes in normal appearing epithelium in patients with squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1982;144:401–5.
Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–92.
Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801.
Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, Winn R, Pajak TF, Williams B, Shenouda G, Hodson I, Fu K, Shin DM, Vokes EE, Feng L, Goepfert H, Hong WK. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–50.
Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, Mercier JP, Têtu B, Harel F, Mâsse B, Vigneault E, Vass S, del Vecchio P, Roy J. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–8.
van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–86.
Mayne ST, Cartmel B, Baum M, Shor-Posner G, Fallon BG, Briskin K, Bean J, Zheng T, Cooper D, Friedman C, Goodwin WJ Jr. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001;61:1457–63
Tincani AJ, Brandalise N, Altemani A, Scanavini RC, Valério JB, Lage HT, Molina G, Martins AS. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck. 2000;22:170–4.
Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–82.
Kesting MR, Schurr C, Robitzky L, Steinstraesser L, Nieberler M, Baurecht H, Wolff KD, Loeffelbein DJ, Mücke T. Results of esophagogastroduodenoscopy in patients with oral squamous cell carcinoma--value of endoscopic screening: 10-year experience. J Oral Maxillofac Surg. 2009;67:1649–55.
Kesting MR, Robitzky L, Al-Benna S, Steinstraesser L, Baurecht H, Wolff KD, Hölzle F, Nieberler M, Mücke T, Loeffelbein DJ. Bronchoscopy screening in primary oral squamous cell carcinoma: a 10-year experience. Br J Oral Maxillofac Surg. 2009;47:279–83.
McGuirt WF, Matthews B, Koufman JA. Multiple simultaneous tumors in patients with head and neck cancer: a prospective, sequential panendoscopy study. Cancer. 1982;50:1195–9.
Leipzig B, Zellmer JE, Klug D. The role of endoscopy in evaluating patients with head and neck cancer. A multi-institutional prospective study. Arch Otolaryngol. 1985;111:589–94.
Davidson J, Gilbert R, Irish J, Witterick I, Brown D, Birt D, Freeman J, Gullane P. The role of panendoscopy in the management of mucosal head and neck malignancy - a prospective evaluation. Head Neck. 2000;22:449–55.
Kerawala CJ, Bisase B, Lee J. Panendoscopy and simultaneous primary tumours in patients presenting with early carcinoma of the mobile tongue. Br J Oral Maxillofac Surg. 2009;47:363–5.
Guardiola E, Pivot X, Dassonville O, Poissonnet G, Marcy PY, Otto J, Poudenx M, Francois E, Bensadoun RJ, Thyss A, Demard F, Schneider M. Is routine triple endoscopy for head and neck carcinoma patients necessary in light of a negative chest computed tomography scan? Cancer. 2004;101:2028–33.
Shaha A, Hoover E, Marti J, Krespi Y. Is routine triple endoscopy cost-effective in head and neck cancer? Am J Surg. 1988;155:750–3.
•• Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M, Muto M. Endoscopy. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010 ;42:185–90. This study describes the most promising new method for early diagnosis of second primary tumors in the esophagus.
Upile T, Jerjes W, Sterenborg HJ, El-Naggar AK, Sandison A, Witjes MJ, Biel MA, Bigio I, Wong BJ, Gillenwater A, MacRobert AJ, Robinson DJ, Betz CS, Stepp H, Bolotine L, McKenzie G, Mosse CA, Barr H, Chen Z, Berg K, D’Cruz AK, Stone N, Kendall C, Fisher S, Leunig A, Olivo M, Richards-Kortum R, Soo KC, Bagnato V, Choo-Smith LP, Svanberg K, Tan IB, Wilson BC, Wolfsen H, Yodh AG, Hopper C. Head & neck optical diagnostics: vision of the future of surgery. Head Neck Oncol. 2009 Jul 13;1:25.
•• Haerle SK, Strobel K, Hany TF, Sidler D, Stoeckli SJ. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck. 32(3):319–25, 2010. This study describes the impact of PET-CT on the diagnosis of second primary tumors, showing that it has more sensitivity but less specificity than panendoscopy.
Strobel K, Haerle SK, Stoeckli SJ, Schrank M, Soyka JD, Veit-Haibach P, Hany TF. Head and neck squamous cell carcinoma (HNSCC)-detection of synchronous primaries with (18)F-FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:919–27.
Homann N, Nees M, Conradt C, Dietz A, Weidauer H, Maier H, Bosch FX. Overexpression of p53 in tumor-distant epithelia of head and neck cancer patients is associated with an increased incidence of second primary carcinoma. Clin Cancer Res. 2001;7:290–6.
Lei D, Sturgis EM, Liu Z, Zafereo ME, Wei Q, Li G. Genetic polymorphisms of p21 and risk of second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Carcinogenesis. 2010;31:222–7.
Li F, Sturgis EM, Zafereo ME, Liu Z, Wang LE, Wei Q, Li G. p73 G4C14-to-A4T14 polymorphism and risk of second primary malignancy after index squamous cell carcinoma of head and neck. Int J Cancer. 2009;125:2660–5.
Zafereo ME, Sturgis EM, Aleem S, Chaung K, Wei Q, Li G. Glutathione S-transferase polymorphisms and risk of second primary malignancy after index squamous cell carcinoma of the head and neck. Cancer Prev Res (Phila). 2009;2:432–9.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priante, A.V.M., Castilho, E.C. & Kowalski, L.P. Second Primary Tumors in Patients with Head and Neck Cancer. Curr Oncol Rep 13, 132–137 (2011). https://doi.org/10.1007/s11912-010-0147-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-010-0147-7