Abstract
Purpose of Review
In this article, we review recent updates to the epidemiology, diagnostic testing, genetics, pathophysiology, and management of hemiplegic migraine.
Recent Findings
While three genes have been historically associated with hemiplegic migraine, recent studies suggest two additional genes may also be implicated including PPRT2 and SLC1A3.
Summary
Hemiplegic migraine is a severe subset of migraine with aura with symptoms including reversible hemiparesis in addition to other aura symptoms such as visual, sensory, or speech. The exact pathophysiology of hemiplegic migraine is not clear, but it is thought that this phenomenon is due to neuronal and glial depolarization causing cortical spreading depression. Due to the severity of presentation as well as the numerous mimickers, it is important to know a comprehensive differential and work-up. Given the low prevalence of the disease, most studies regarding treatment are limited to case studies. There is still an important need for further and larger studies regarding management of these cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemiplegic migraine (HM) is a rare subtype of migraine with aura. Per International Classification of Headache Disorders, 3rd edition (ICHD-3) criteria, patients experience migraine with aura with symptoms of both fully reversible motor weakness and fully reversible visual, sensory, and/or speech symptoms [1]. They can further be classified as sporadic hemiplegic migraine (SHM) or familial hemiplegic migraine (FHM). Patients with FHM have at least one first- or second-degree relative with symptoms of HM. If no family members are identified, they are diagnosed with SHM. Given the severity of symptoms, these individuals often undergo a thorough diagnostic work-up prior to making a diagnosis.
In this review, we discuss updates and recent studies in HM including epidemiology, genetics, pathophysiology, diagnostic testing, and treatment.
Case
A 39-year-old female with a history of migraine with visual aura presents to headache clinic for follow-up. She describes a new symptom associated with her most recent migraine. Prior to the onset of the headache, she noticed that her right arm and leg were significantly weaker than her left side. She did not appreciate any facial symptoms at the time. She took aspirin and laid down. Her hemiparesis resolved after 30 min, but she went on to develop a severe headache, typical of her usual migraine. In hindsight, she recalls a similar event at the age of 15 when she also developed right-sided weakness. At that time, she was admitted to the hospital and underwent a complete work-up including lab work and imaging studies, which were normal.
Epidemiology
Historically, very few studies have looked at the epidemiology of HM. In 2002, a Danish study found the overall estimated national prevalence of HM to be approximately 0.01% (n = 291) based on three recruitment methods including a national patient registrar search, screening neurologists’ case records, and advertisements. Of those individuals, 147 subjects had FHM, 105 had SHM, and 39 were unclassifiable. The identified sex ratio was 1:3.3 (male:female) [2]. Onset of symptoms has been found to be between the ages of 12 and 17 [2].
A more recent retrospective US military cohort study was studied from 1990 to 2017. Interestingly, no cases of HM were reported prior to 2008. Incidence rates in the US military were between 0.04 and 0.05%. There were more women observed with HM than expected (p < 0.01, observed n = 240, expected n = 90). Additionally, there were more black and white service members than expected (p < 0.01, observed n = 390, expected n = 130). It should be noted that this population is biased given that upon entry into the military, these individuals tend to be healthier but then during their active service may be more prone to headaches due to physical stressors, dehydration, and sleep disruptions [3].
Presentation
The type of headache associated with HM can vary, from unilateral to bilateral and can be ipsilateral or contralateral to the aura symptoms [4•]. Symptoms in HM can be classified by aura symptoms, which occur during attacks, and chronic symptoms which can occur interictally. Aura symptoms always include motor weakness, and in addition can include sensory, visual, and language deficits. Visual deficits that may be noted include scintillating scotomas and hemianopia. Other aura symptoms that may be noted with these migraine attacks include seizure, fever, bilateral visual deficits, and posterior circulation-related deficits (described in more detail below). Chronic symptoms are usually based on the gene involved. For instance, familial hemiplegic migraine type 1 (FHM1) is associated with cerebellar involvement such as gaze-evoked nystagmus and progressive ataxia. CACNA1A and ATP1A2 have a known association with cognitive impairment after severe attacks. Children with a pathogenic CACNA1A mutation have been noted to have paroxysmal events such as vertigo, torticollis, or tonic upgaze. Epilepsy is commonly associated with HM as well, though seizures are not typically seen with migraine attacks [4•].
Duration of motor symptoms typically lasts less than 72 h, though in some patients, it may persist for weeks [1]. More commonly, the duration of aura is 20–60 min. There have been reported cases of irreversible brain injury including infarcts, cerebral atrophy, and death due to severe HM attacks in those with CACNA1A mutations. Common triggers for HM include stress (physical and emotional), head trauma, and viral infections [4•]. Notably, patients can have unilateral hyperreflexia and sensorimotor deficits affecting mostly the upper extremities if examined during a migraine attack [5].
As noted previously, it has been well established that there is an association of FHM with cerebellar symptoms, previously known as “brainstem auras” [6]. These are more commonly seen in patients with FHM1 (rarely in type 2) and include gaze-evoked nystagmus, ataxia, vertigo, tinnitus, dysarthria, impaired consciousness, and even sometimes coma [4•]. Although it is not entirely clear if these symptoms are due to cerebellum or brainstem involvement, cerebellar atrophy noted in this subgroup suggests that the cerebellum likely plays a role. These symptoms are typically transient; however, about 20% of patients show some level of permanent cerebellar deficits. Of note, besides the focal neurologic deficits seen with FHM, this phenotype can also be associated with seizure, prolonged coma, cognitive dysfunction, and hyperthermia [6].
A recent case report from 2019 describes a 24-year-old male with history of right-sided HM who presented with an episode of hemiparesis in addition to concomitant expressive and receptive aphasia and disorientation. Given left frontal, central, and temporal slowing seen on electroencephalogram (EEG) during this hospitalization, this was repeated 2 months later with persistent slowing. Given this finding on EEG had not resolved, neuropsychological testing was completed with evidence of low-average scores in semantic fluency and processing speed. The authors speculate whether there may be some association with cerebellar cognitive affective syndrome (CCAS) given overlap in areas of low performance, although less pronounced in this case report. The conclusions from this case are limited given this was a single neuropsychological evaluation. Further studies should consider if screening with the CCAS/Schmahmann syndrome scale should be completed in patients with FHM given its association with cognitive dysfunction [7].
Genetics
FHM1
CACNA1A is the gene mutation associated with FHM1. It is located on chromosome 19p13 and encodes for a pore-forming alpha-1 subunit on the Cav2.1 calcium channel [4•], [8]. This gene is also implicated in episodic ataxia type 2 and spinocerebellar ataxia type 6 [4•]. A gain of function mutation allows for an increase in calcium influx, which in turn leads to cortical spreading depression (CSD) [4•], [8]. Twenty-one unique mutations have been identified. One of the most recent missense mutations affecting c.622 isoform 1 G>A; p.Gly208Arg was recently discovered. Nine family members met criteria for FHM by the ICHD-3 criteria, and no mutations were identified in the other two FHM genes. Seven family members had at least one attack affecting both sides of the body, which were often more severe and longer lasting episodes. These HM attacks were often associated with ipsilateral paresthesia and aphasia. Most were women (n = 8) and onset was generally in childhood [8]. With a small sample size, it is difficult to draw conclusions but this variant may represent a more severe phenotype involving diplegia.
FHM2
In familial hemiplegic migraine type 2 (FHM2), there is a mutation in the ATP1A2 gene, which encodes for the alpha-2 subunit of glial Na+/K+ ATPase pump [4•]. Mutations result in loss of function in the reuptake of potassium and glutamate from the synaptic cleft, which plays a role in CSD. Recently, some new variants within the gene have been identified [9].
An Italian family with a newly identified substitution mutation, L425H, had milder symptoms of sensory and aphasia aura symptoms with the proband being the only one with associated hemiparesis. No family members were treated with prophylactic medications [9]. Another study identified a different nucleotide mutation in a Chinese family, G762S. Using whole exome sequencing, 10 out of 16 family members were affected with 100% penetrance across four generations. This mutation is thought to disrupt the three-dimensional structure of the alpha-2 subunit leading to a loss of function mutation. All family members had triggers of physical or emotional stress with aura symptoms lasting from 0.5 to 2 days. Family members experienced a frequency of up to 6 events per year with no family members on prophylactic medications [10].
In an effort to identify more variants of HM, patients with HM who had tested negative for CACNA1A, ATP1A2, and SCN1A mutations after undergoing routine diagnostic screening service underwent next generation sequencing (NGS) multigene panel screening. Of the variants identified, 11 out of 29 were deemed “likely pathogenic” or “pathogenic.” Most variants (22 out of 44 cases) were within the ATP1A2 gene, unlike in many other studies where CACNA1A mutation is typically of highest proportion. Seven of the ATP1A2 variants were in individuals with concussion-like symptoms after head trauma, which is similar to the patient population of CACNA1A p.Ser218Leu mutation [11]. In the future, this may lead to increased use of NGS if patients test negative on the first round of standard genetic screening. This type of testing may be difficult to obtain in standard clinical practice, but would be helpful in identifying genetic variations that may be the underlying cause of disease.
FHM3
FHM3 is characterized by the gene mutation, SCN1A, affecting the neuronal voltage gated sodium channel on GABAergic interneurons, Nav 1.1 [4•]. Previously, it has been challenging to sequence and study this mutation given the plasmid easily rearranged during propagation and had low yield. In a recent study, Bertelli et al. were able to create an optimized plasmid to evaluate specific mutations within the gene, L1670W and F1774S. Their results helped to confirm a gain of function mechanism and a useful tool for further evaluations of these neuronal voltage gated sodium channels [12].
Other Mutations
Through further genetic studies, other genes have recently been discovered and may also be implicated in HM, including PPRT2 and SLC1A3. PPRT2 encodes for a presynaptic transmembrane protein involved in regulation of voltage gated calcium channels in glutaminergic neurons and synaptic vesicle fusion [4•]. It also plays an important role in brain development and is associated with paroxysmal kinesigenic dyskinesia and childhood epilepsy [4], [13••]. In a French study, patients who were being tested for associated HM genes (CACNA1A, APT1A2, SCN1A) were also tested for the PRRT2 variant. Thirty patients (n = 860) were identified with PRRT2 variations while testing negative for the other three genes. This was more common than the SCN1A variant which was seen in 15% of patients compared to 17% of patients with a PRRT2 mutation. Sixteen patients had pure HM while others also had associated epilepsy, learning disability, idiopathic hypersomnia, and abnormal movements, which represents a similar phenotype to other genes identified. Some patients reported improvement with lamotrigine and acetazolamide [13••].
Another gene that is hypothesized to be implicated is SLC1A3, which codes for a glial glutamate transporter protein that leads to truncated protein. This mutation is also associated with episodic ataxia type 6. A recent case report describes a 24-year-old female with history of PCOS and later diagnosed adrenocortical carcinoma with SHM is found to have a mutation in SLC1A3 without any other mutations identified in CACNA1A, ATP1A2, and SCN1A genes. On exam, she is found to have interictal nystagmus and magnetic resonance imaging is notable for mild cerebellar atrophy. She ultimately responds well to acetazolamide 250 mg twice a day. The authors surmise that SLC1A3 variants may be similar to those in CACNA1A mutations [14••].
Pathophysiology
The pathophysiology of HM is similar to that of typical migraine with aura, though more severe and occurring at a lower threshold [4•]. There is a slow march of symptoms as seen with typical migraine with aura, usually starting with visual symptoms and then progressing to sensory, motor, and eventually aphasia. Given the slow spread of these symptoms spreading throughout the cerebral cortex, it is thought that this phenomenon is due to a neuronal and glial depolarization causing CSD [15].
SHM differs from migraine with aura in that with the former, there is always more than one aura symptom, usually all four auras, whereas the latter is typically associated with visual symptoms only. Aura with HM is also often associated with posterior circulation symptoms suggesting spread to the brain stem or bilateral hemispheres. Additionally, the duration of symptoms is usually longer, typically more than 60 min, compared to classic migraine with aura [16].
FHM is known to have a strong genetic component, as noted previously. However, these genetic mutations account for about 75% of all FHM and thus there is still a large subset of these patients for which a clear pathophysiologic mechanism has not been identified [15]. In FHM1, it has been established that a missense mutation leads to enhanced glutamatergic transmission thus lowering the threshold for cortical spreading depolarization. Namely, in FHM1 mutant mice, there is evidence that neurons activate surrounding astrocytes and microglia which may be contributing to this pathway of CSD [17].
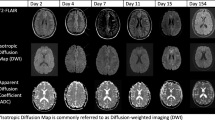
Prior studies have showed metabolic abnormalities in patients with HM evidenced by magnetic resonance spectroscopy (MRS), which showed reduced brain parenchyma fraction, N-acetyl aspartate, and glutamate and increased myo-inositol in the cerebellum, as compared to healthy control groups [18]. A case report in an 8-year-old patient with a prolonged attack of SHM, later found to have been carrying a missense mutation of the ATP1A2 gene, is evaluated with repeated neuroimaging subsequent to the attack. The patient’s initial magnetic resonance imaging (MRI) brain was negative, but MRI scans days 4 and 11 after the attack showed progressive left hemispheric cortical swelling. MR spectroscopy on day 15 showed a decrease in the N-acetylaspartate/creatinine ratio in the left hemisphere and single-photon emission computerized tomography scan on day 27 showed significant left hemispheric hypoperfusion. This case report in combination with prior studies demonstrates that in addition to cerebellar atrophy noted in some patients, there is also neuronal dysfunction at a more microscopic level in patients with HM [19].
Diagnostic Testing
There are no confirmatory laboratory or radiologic tests to diagnose HM. Diagnosis of HM is based largely on clinical history and judgment using the ICHD criteria. Given the severity of symptoms in HM as well as the numerous mimickers, it is important to pursue a thorough work-up in these cases. Important differentials to consider, but not limited to, include cerebrovascular disease, seizures, Sturge-Weber syndrome, pulmonary arteriovenous malformation, Moya-Moya disease, CREST syndrome, and lupus [20]. Additionally, cerebral amyloid angiopathy, even in the absence of bleeding, can cause transient focal neurologic deficits, known as amyloid spells. Further differentials include neoplasm, alternating hemiplegia of childhood, central nervous system infections, and hereditary metabolic conditions such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS), and hereditary hemorrhagic telangiectasias [4•].
There was one recently reported case of a patient with a history of SHM with left hemiparesis who had an episode of hemiparesis that persisted beyond the headache. He was found to have new right cerebral edema and was diagnosed with neuronal intranuclear inclusion disease (NIID) by brain biopsy. While both diseases are rare, the authors postulate that cerebral hypoperfusion related to HM may have led to the accumulation of eosinophilic inclusions [20].
There are some reported diagnostic findings that can be seen with HM. EEG can show asymmetric slowing in the contralateral hemisphere to the hemiparesis [21]. Cerebrospinal fluid (CSF) studies may show increased protein, though some studies have also reported pleocytosis.
Imaging in patients with FHM1 or SHM1 has shown evidence of cerebellar atrophy, but for most patients imaging studies are normal, especially between attacks [22]. As previously noted, during hemiplegic attacks, there has been evidence of MRI findings of cortical thickening and alternation between hypoperfusion and hyperperfusion as well as T2/FLAIR hyperintensity in the affected hemisphere contralateral to the motor weakness [23]. Additionally, there may also be enhancement on MRI with gadolinium administration, possibly due to breakdown of the blood-brain barrier [4•]. In one case report, a 43-year-old male with a history of migraine presented with right-sided headaches, left-sided hemiplegia, and altered mental status. Despite a thorough work-up including serum, CSF, and brain biopsy, no significant findings were found and he was ultimately diagnosed with SHM. The MRI showed diffuse cortical enhancement of the right hemisphere on both FLAIR and DWI sequences, suggestive of blood-brain barrier disruption. The authors suggest this could be a marker for the severity of a migraine [23]. These abnormal findings are usually reversed after resolution of the attack. It must also be noted that these MRI changes may not be seen early on in the attack, as evidenced by prior case reports [19].
Molecular genetic testing can be used to help further classify FHM which includes single gene testing, a multigene panel, and exome sequencing. Single gene testing should be pursued in patients who have nystagmus, ataxia, epilepsy, development delay, or paroxysmal tonic upgaze. Exome sequencing is most commonly used [24].
Treatment
Thus far, there have been no randomized control studies evaluating medications for the treatment of HM, both acute and preventative therapies. Providers are currently reliant on small case reports and retrospective studies to decide how to best manage these patients. We will discuss some of the more recent findings, but medications that have been used for acute treatment include verapamil, nimodipine, ketamine, triptans, pulse steroids, and hypertonic saline. Medications that have been used for preventative treatment include verapamil, acetazolamide, flunarizine, lamotrigine, OnabotulinumtoxinA, calcitonin gene-related peptide (CGRP) antagonists, and propranolol [4•].
Triptans
Triptans are one of the most commonly used rescue medications for migraine. They are 5-HT1B/1D agonists that lead to vasoconstriction and inhibit the release of vasoactive peptides. Among several absolute contraindications, HM is also listed due to the concern of vasoconstriction worsening symptoms and increasing the risk of ischemic stroke [4•]. Given the severity of HM symptoms, there have been retrospective studies to assess the efficacy and safety of this class. In a retrospective Finnish study (n = 76), patients with HM had an average response of 6.8 (on a scale of 1 to 10, 10 being excellent response). About half the patients used the triptan during the aura phase. There were no reports of ischemic stroke or myocardial infarction [25]. Of note, one patient who treated herself with rizatriptan had prolonged symptoms of hemiparesis, dizziness, ataxia, and visual disturbances lasting 3 months with normal imaging. Given the tolerability and efficacy seen in this small trial, the authors surmise there may be a different mechanism of action of HM that does not involve vasoconstriction. In a multi-center retrospective analysis, 80 patients with either HM (n = 13) or basilar migraine (n = 67) were treated with dihydroergotamine (DHE) and/or triptan. At follow-up visits, there were no reported adverse effects of stroke or MRI [26]. Again, this study is limited given its small sample size but suggests that DHE and triptans may be considered without risk of ischemic vascular events. Given the change in ideology of the pathophysiology of migraine, primarily modulated by CSD, and the above studies, it may be worth reconsidering the current black box contraindication of the use of triptans in HM [27].
OnabotulinumtoxinA
In a retrospective cohort study from 1994 to 2017, 11 patients with HM and frequent aura symptoms (at least 2 per month) were treated with OnabotulinumtoxinA (OnabotA) using a modified protocol. Nine patients noted a decrease in frequency, severity, and/or duration of the aura. While other changes to medications may have also simultaneously been made during this period, the findings warrant the consideration of using OnabotA for the prevention of HM. The authors hypothesize that OnabotA is mediating CSD through CGRP given OnabotA has been shown to decrease interictal levels [28].
Nimodipine
One recent case report described successfully treating a 13-year-old patient with FHM2 with intravenous nimodipine after a prolonged attack. MRI of the brain during the attack showed right temporoparietal cortical edema. The patient was initially treated with dexamethasone and levetiracetam. On day 8 of persistent symptoms, she was started on nimodipine with symptom improvement in 12 h. After 6 days of treatment, she was back to baseline. Authors hypothesize nimodipine may be treating the underlying mechanism of HM as it relates to CSD, cerebral vasoconstriction, and edema compared to altering the structural mutation [29].
CGRP
Thus far there have been no case reports or studies looking at the utility of CGRP monoclonal antibody or small-molecule CGRP antagonists for the treatment of HM.
In a Danish study, 11 patients with FHM with no identified genes received human alpha CGRP. They were compared to 11 healthy control patients, who also received human alpha CGRP. No difference was found between the two groups in terms of reported migraine-like attacks between two groups. Only 1 FHM patient experienced a migraine attack. Similar rates (8/11 FHM patients and 7/11 control patients) experienced a headache post-infusion. Adverse effects reported included flushing, palpitations, and heat sensation [30]. A similar study was completed in patients with genetically confirmed FHM (n = 9; 2 FHM2 and 7 FHM1). There were no reports of aura after infusion and there were similar rates of reported migraine/migraine-like headaches with no statistical significance (2/9 in FHM group and 1/10 in control group) [31]. The authors surmise that both studies suggest CGRP is not related to the pathophysiology of HM [30, 31].
Ketamine
In an unblinded study, 11 patients with HM were treated with intranasal ketamine (25 mg), as needed, with 5 patients reporting an improvement in severity and duration of aura symptoms. Given that CSD is mediated, in part, by the release of glutamate and aspartate which activate NMDA receptors, ketamine, an NMDA antagonist, may be modulating this process in some patients [4•] [32].
While many of these studies show favorable results with the use of various medications, they are all small studies and often retrospective making it difficult to draw any meaningful conclusions. There is a large need for further studies and trials in the treatment of HM, both acute and preventative.
Conclusion
HM is a rare subtype of migraine with aura. Patients may have additional atypical aura symptoms not limited to seizures, cognitive impairment, and cerebellar symptoms. Recent studies have identified new mutations involved in the three genes classically associated with FHM. Additionally, two new genes that are likely also implicated include PPRT2 and SLC1A3. Given the severity of symptoms with hemiparesis, the differential should remain broad at presentation and work-up should include an MRI in addition to EEG and CSF studies in the appropriate clinical context. Given the low prevalence, studies regarding treatment are limited but recent data suggests possible benefits with triptans, OnabotA, nimodipine, and ketamine. Further studies with larger sample sizes would be beneficial to draw stronger conclusions.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Olesen J. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia. 2018;38(1):1–211.
Thomsen LL, Eriksen MK, Romer SF, Andersen I, Ostergaard E, Keiding N, Olesen J, Russell MB. An epidemiological survey of hemiplegic migraine. Cephalalgia. 2002;22(5):361–75.
Moore BA, Hale WJ, Nabity PS, Koehn TR, McGeary D, Peterson AL. A retrospective, epidemiological review of hemiplegic migraines in a military population. Mil Med. 2019;184(11-12):781–7.
• Di Stefano V, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, Napoli C, Pietrobon D, Brighina F, Parisi P. Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. 2020;91(7):764–71. Detailed review of differential diagnosis and studied treatment options.
Thomsen LL, Ostergaard E, Olesen J, Russell MB. Evidence for a separate type of migraine with aura: sporadic hemiplegic migraine. Neurology. 2003;60(4):595–601.
Vincent M, Hadjikhani N. The cerebellum and migraine. Headache. 2007;47(6):820–33.
Trahan EM, Mercado JM. Familial hemiplegic migraines and baseline neuropsychological testing: a case report. Headache. 2019;59(6):917–23.
Sprouse Blum AS, Couperus CJ, Rosen BJ, Haskin-Leahy LF, Shapiro RE. Familial “diplegic” migraine–description of a family with a novel CACNA1A mutation. Headache. 2020;60(3):600–6.
Antonaci F, Ravaglia S, Grieco GS, Gagliardi S, Cereda C, Costa A. Familial hemiplegic migraine type 2 due to a novel missense mutation in ATP1A2. J Headache Pain. 2021;22(1):1–6.
Tang W, Zhang M, Qiu E, Kong S, Li Y, Liu H, Dong Z, Yu S. A Chinese family with familial hemiplegic migraine type 2 due to a novel missense mutation in ATP1A2. Cephalalgia. 2019;39(11):1382–95.
Maksemous N, Smith RA, Sutherland HG, Maher BH, Ibrahim O, Nicholson GA, Carpenter EP, Lea RA, Cader MZ, Griffiths LR. Targeted next generation sequencing identifies a genetic spectrum of DNA variants in patients with hemiplegic migraine. Cephalalgia Rep. 2019;11(2):2515816319881630.
Bertelli S, Barbieri R, Pusch M, Gavazzo P. Gain of function of sporadic/familial hemiplegic migraine-causing SCN1A mutations: use of an optimized cDNA. Cephalalgia. 2019;39(4):477–88.
•• Riant F, Roos C, Roubertie A, Barbance C, Hadjadj J, Auvin S, Baille G, Beltramone M, Boulanger C, Cahn A, Cata F. Hemiplegic migraine associated with PRRT2 variations: a clinical and genetic study. Neurology. 2022;98(1):e51–61. Study related to newly identified gene variant, PRRT2.
•• Paucar M, Granberg T, Lagerstedt-Robinson K, Waldenlind E, Petersson S, Nordin L, Svenningsson P. SLC1A3 variant associated with hemiplegic migraine and acetazolamide-responsive MRS changes. Neurology. Genetics. 2020;6(4). https://doi.org/10.1212/NXG.0000000000000474Case report describing new SCL1A3 variant.
Kumar A, Samanta D, Emmady PD, Arora R. Hemiplegic migraine. 2018.
Thomsen LL, Olesen J. Sporadic hemiplegic migraine. Cephalalgia. 2004;24(12):1016–23.
Magni G, Boccazzi M, Bodini A, Abbracchio MP, van den Maagdenberg AM, Ceruti S. Basal astrocyte and microglia activation in the central nervous system of familial hemiplegic migraine type I mice. Cephalalgia. 2019;39(14):1809–17.
Dichgans M, Herzog J, Freilinger T, Wilke M, Auer DP. 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology. 2005;64(4):608–13.
Toldo I, Cecchin D, Sartori S, Calderone M, Mardari R, Cattelan F, Laverda AM, Drigo P, Battistella PA. Multimodal neuroimaging in a child with sporadic hemiplegic migraine: a contribution to understanding pathogenesis. Cephalalgia. 2011;31(6):751–6.
Wang R, Nie X, Xu S, Zhang M, Dong Z, Yu S. Interrelated pathogenesis? Neuronal intranuclear inclusion disease combining with hemiplegic migraine. Headache. 2020;60(2):382–95.
Gastaut JL, Yermenos E, Bonnefoy M, Cros D. Familial hemiplegic migraine: EEG and CT scan study of two cases. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1981;10(4):392–395. https://doi.org/10.1002/ana.410100414
Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011;10(5):457–70.
Pellerin A, Marois C, Mezouar N, Mokhtari K, Leclercq D, Law-Ye B. Neuronal injuries evidenced by transient cortical magnetic resonance enhancement in hemiplegic migraine: a case report. Cephalalgia. 2019;39(2):323–5.
Jen JC . Familial Hemiplegic Migraine. GeneReviews® [Internet] 2021. https://www.ncbi.nlm.nih.gov/books/NBK1388/
Artto V, Nissilä M, Wessman M, Palotie A, Färkkilä M, Kallela M. Treatment of hemiplegic migraine with triptans. Eur J Neurol. 2007;14(9):1053–6.
Mathew PG, Krel R, Buddhdev B, Ansari H, Joshi SG, Spinner WD, Klein BC. A retrospective analysis of triptan and dhe use for basilar and hemiplegic migraine. Headache. 2016;56(5):841–8.
Mathew PG, Klein BC. Getting to the heart of the matter: migraine, triptans, DHE, ditans, CGRP antibodies, first/second-generation gepants, and cardiovascular risk. Headache. 2019;59(8):1421–6.
Chen TY, Garza I, Dodick DW, Robertson CE. The effect of OnabotulinumtoxinA on aura frequency and severity in patients with hemiplegic migraine: case series of 11 patients. Headache. 2018;58(7):973–85.
Dannenberg F, Prager C, Schmidt F, Tietze A, Bittigau P, Kaindl AM. Intravenous nimodipine treatment for severe episode of ATP1A2 hemiplegic migraine. Pediatr Neurol. 2020;1(112):71–2.
Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache. 2011;51(4):544–53.
Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene–related peptide does not cause the familial hemiplegic migraine phenotype. Neurology. 2008;71(11):841–7.
Kaube H, Herzog J, Käufer T, Dichgans M, Diener HC. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology. 2000;55(1):139–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Arathi Nandyala: none.
Tulsi Shah: none.
Jessica Ailani:
-Stock/stock options: CtrlM (2022)
-Editorial Boards/Steering Committee: Medscape, NeurologyLive, Current Pain and Headache (Editor, Unusual Headache Syndromes), SELF magazine (medical editor)
-Leadership or fiduciary role in other board society, committee or advocacy group: American Headache Society (unpaid)
-Participation on a Data Safety Monitoring Board or Advisory Board: Aeon (migraine) and Gore (migraine)
-Consulting fees: Abbvie, Amgen, Axsome, Biohaven, BioDeliveryScientificInternational (2022), Eli-Lilly, GlaxoSmithKline, Lundbeck, Linpharma, Impel, Miravio, Pfizer, Neurolief, Neso, Satsuma, Theranica, Teva
-Grants or contracts from any entity: Clinical Trials (Grant to institution)—Abbvie (2021), Biohaven (2021), Eli-Lilly (2021), Satsuma (2022), Zosano (2021)
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nandyala, A., Shah, T. & Ailani, J. Hemiplegic Migraine. Curr Neurol Neurosci Rep 23, 381–387 (2023). https://doi.org/10.1007/s11910-023-01277-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01277-z