Abstract
Bariatric surgery in patients with type 2 diabetes has been shown to improve glycemic control and reduce need for glucose-lowering medications. Some of these improvements occur in the early postoperative period prior to any weight loss. These early reductions in circulating glucose can be attributed to primarily perioperative caloric restriction and prolonged fasting. Inpatient glycemic targets for patients undergoing bariatric surgery are similar to those recommended for other surgical procedures as a way of minimizing risk for complications. There is evidence that achieving perioperative and postoperative glycemic targets can improve the ability to achieve remission of type 2 diabetes following gastric bypass surgery. This review provides recommendations regarding glycemic goals, strategies for achieving these goals with minimal risk for hypoglycemia, and an examination of the data suggesting an association between perioperative glycemic management and diabetes remission following bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical characteristics of patients with type 2 diabetes (T2D) undergoing bariatric surgery are inherently different from those receiving other surgeries. Unlike gastrointestinal (GI) surgeries for acute processes (e.g., cholecystitis, appendicitis, small bowel obstruction, incarcerated hernia), bariatric surgery is performed electively. Patients with obesity and T2D have a quantity and distribution of adiposity causing pathologic levels of visceral and ectopic fat, insulin resistance, and impaired beta cell function [1].
Despite the growing evidence demonstrating efficacy of bariatric surgical procedures for patients with T2D and class 1 obesity (Body Mass Index (BMI) 30–35 kg/m2), the recommended candidates have class 2 or greater categories of obesity (BMI ≥ 35 kg/m2) [2•]. These patients report difficulty losing weight or maintaining prior weight loss achieved using lifestyle interventions, medications, or commercial weight loss programs. Some patients may have previously tried or received prescriptions for very low-calorie (<800 kcal/day) or ketogenic (<50 g net carbohydrate/day) diets that mimic the metabolic effects of prolonged fasting (discussed below), but they are in the minority. Attempting one of these diet strategies is not a prerequisite for surgery.
Bariatric surgery often results in significant and lasting weight loss with associated improvements in glycemic control in patients with T2D. The degree of glycemic improvement varies with the type of procedure performed and the preoperative diabetes severity [3, 4••]. Normalization of glucose levels with remission of T2D has been observed with the several types of bariatric surgical procedures, including the previously utilized biliopancreatic diversion, adjustable gastric banding, the increasingly popular sleeve gastrectomy, and the most frequently performed Roux-en-Y gastric bypass (RYGB) [5–8].
As reported in the surgery literature as far back as 1987, improvements in glycemic control following bariatric surgical procedures are often observed in the early postoperative period before any significant weight loss has had time to occur [9, 10]. The mechanism for this acute change is proposed to be diet intake alone, supported by the section “Mechanisms of Diabetes Improvement” below. For this reason, providers should alter glycemic management regimens by decreasing doses of usual diabetes medications (including insulin) as a way of avoiding postoperative hypoglycemia, as recommended in joint guidelines from the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic and Bariatric Surgery (AACE/TOS/ASMBS) [2•]. In our clinical experience, this can result in an inappropriate over-reliance on sliding-scale regular- or rapid-acting insulin in the hospital and following discharge, with potential for clinically significant hyperglycemia and complications [11••, 12]. Here, we will review the literature and guidelines for perioperative glycemic management of the patient with diabetes undergoing bariatric surgery and propose a standard of care based on current evidence. The premise of this review is that early attention to preadmission and post-discharge glycemic control has the potential to improve beta cell function and improve long-term remission rates of T2D.
Perioperative Glycemic Management of the Patient With Diabetes Undergoing Bariatric Surgery
Preoperative Glycemic Goals
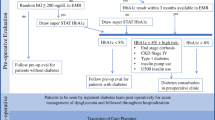
The AACE/TOS/ASMBS Bariatric Surgery Clinical Practice Guideline recommends that patients with T2D achieve a preoperative hemoglobin A1C (A1C) of ≤6.5–7.0 %, a fasting blood glucose level of ≤110 mg/dl, and a 2-h postprandial blood glucose concentration of ≤140 mg/dl as a grade A best evidence level [2•]. An A1C of 7–8 % is recommended for patients with long-standing diabetes, advanced diabetes-related complications, and extensive comorbid conditions or those who are unable to safely achieve lower targets. Clinical judgment is advised for patients with A1C values >8 % [2•]. In such cases, allowing a bariatric surgical procedure to take place has the potential to contribute to improved glycemic control by permitting morbid obesity patients to resume exercise and more reasonable eating patterns. This guideline does not provide specific recommendations for diabetes medications to use preoperatively but recommends discontinuation of insulin secretagogues and modification of insulin doses (see discussion below) in the immediate postoperative period [2•].
While there is some evidence linking preoperative glycemic control with a risk for postoperative complications, the available evidence is more suggestive than absolute [4••, 13–15]. One large retrospective review of 468 patients with T2D who underwent RYGB procedures grouped them according to preoperative A1C (<6.5, 6.5–7.9, and >8 %) and found that those with the lower A1C levels experienced lower levels of postoperative hyperglycemia, more weight loss, and a greater likelihood of diabetes remission at 1 year. Postoperative hyperglycemia was independently associated with increased morbidity from wound infections and acute renal failure [4••]. This report was followed by GLUCOSURG-pre which randomly assigned 34 patients with T2D with a mean A1C of 10 % at study entry to optimized versus non-optimized diabetes therapy for 3 months prior to RYGB. There were no differences in the length of stay or surgical complications. The mean pre-op A1C achieved remained suboptimal at 8.4 and 9.7 %, respectively. These preoperative A1C values negate the ability to make any conclusions regarding the efficacy or non-efficacy of intensified preoperative glycemic management, as these levels (>8 %) were associated with higher morbidity in the earlier retrospective study [4••, 16••]. Also, all patients underwent 2 weeks of 800 kcal/day diets before surgery and were treated with metformin and titrated glargine to fasting blood glucose (BG) goal after discharge [16••]. The beneficial effects of non-standard therapies in both arms may overcome any difference achieved by improving glycemic control in the 2.5 months prior. In institutions that do not use the pre-op 800 kcal/day diets or postoperative glycemic control protocols, interventions that attempt to achieve an A1C <6.5 or <8 may confer benefit, but to date, no randomized control trail has been done to determine this.
At our institution, a Bariatric Surgery Perioperative Management Protocol has been implemented as a quality improvement collaboration between the division of endocrinology and the division of minimally invasive bariatric and general surgery. The protocol (Table 1) recommends measurement of an A1C within 3 months of planned bariatric surgery, modification of the diabetes regimen as needed, with referral to endocrinology for initiation of insulin for patients with more severe degrees of hyperglycemia defined as an A1C >9 %.
Postoperative Glycemic Goals
The inpatient glycemic goal for bariatric surgery patients is similar to what is recommended for other patients with diabetes undergoing surgical procedures, which is to maintain glucose levels between 140 and 180 mg/dl [2•, 17, 18]. A prospective cohort of over 11,000 patients from 47 hospitals admitted for elective colorectal or bariatric surgical procedures demonstrated that a BG >180 mg/dl in the early postoperative period was associated with a higher risk of infections (2.9 vs 1.0 %, P < 0.001), in-hospital mortality (0.22 vs 0.09 %, P < 0.001), and need for reoperative intervention (3.1 vs 1.6 %, P < 0.001). Patients in this cohort who were treated with insulin on the day of surgery did not experience this increase risk of adverse outcomes [11••].
In the GLUCOSURG-post study, 35 patients with T2D treated with metformin and insulin were randomly assigned to 2 weeks of intensive (target fasting glucose 99–117 mg/dl) versus conservative (target fasting glucose 117–135 mg/dl) for 15 days following RYGB [18]. Patients in both groups were instructed to call in their fasting glucose levels to a metabolic physician on a daily basis for recommendations regarding insulin adjustments. Mean glucose levels during this time period were approximately 18 mg/dl lower in the intensified group (P < 0.001), with no observed differences in length of stay, surgical complications, hypoglycemia events, or need for glucose-lowering medications at 1 year. An important difference in the two randomized groups (n = 17 and 18) was that the intense treatment group had an average diabetes duration of 5 years longer than that of the conservative treatment group [16••]. As duration of T2D has long been known to be a strong determinant of diabetes remission rate, this alone could nullify the interpretation that earlier tight control has no long-lasting benefits.
Diabetes Medications
Preoperative Adjustments to Diabetes Medications
The perioperative glycemic management of a patient with T2D undergoing bariatric surgery is similar to that provided to a patient with T2D on a 72-h fast. The goal is to provide sufficient insulin to regulate hepatic glucose output, stimulate peripheral glucose uptake, and maintain glycemic targets while avoiding hypoglycemia.
Patients taking oral and non-insulin injectable diabetes medications are advised to take their usual doses the day before surgery if their diet is not significantly changed [19]. Recommendations for adjustments to basal insulin are based on the patient’s home regimen. Insulin-treated patients will almost always require continuation of all or part of their basal insulin dose prior to and during the surgical procedure as a way of regulating hepatic glucose production and preventing hyperglycemia [19]. Patients treated with basal-heavy insulin regimens, defined as doses of Neutral Protamine Hagedorn (NPH), glargine, or detemir of >0.6–1.0 units/kg/day with low-dose or no premeal insulin, may require dose reductions of ≥50 % as a way of avoiding perioperative hypoglycemia [20].
In a study by Datta et al. from 2007, glargine insulin started at a dose at 0.3 U/kg/day immediately post RYGB resulted in a mean postoperative BG of 134 versus 154 mg/dl in the group randomly assigned to sliding-scale regular (SSR) insulin with no difference in the incidence of hypoglycemia [21]. This suggests that administering a dose of basal insulin of ≤0.3 U/kg/day the night before surgery is safe for patients on equivalent or higher doses prior to surgery.
Patients receiving home basal insulin doses of ≤0.3 units/kg/day can have their dose decreased by 0–50 % depending on the type of basal insulin being used. The pharmacokinetics of NPH insulin with a peak time of action at 6–10 h following administration supports dose reductions of 25 to 50 % of usual doses [22], while the pharmacokinetics of detemir can vary from a flat profile at lower doses (<0.3 units/kg/day) to a more pronounced peak action time at higher doses, again supporting recommendations for dose modifications in some patients during periods of fasting [23].
At our institution, a protocol for perioperative glycemic management for same-day surgery (not bariatric surgery) recommended 50 % reductions in basal insulin doses the night before surgery resulting in 74 % of patients achieving a goal pre-op BG 70–199 mg/dl (2 % was below and 24 % above) [19]. Postoperative hyperglycemia occurred in 44 and 9 % of patients with and without preoperative hyperglycemia, respectively. This suggested that 50 % was too large of a reduction in basal insulin dose, prompting modification of the same-day surgery pre-op instructions to allow 75–100 % of basal insulin dose to be given for patients receiving glargine [19].
Perioperative Glycemic Management
All oral and non-insulin injectable diabetes medications should be discontinued on the day of admission for the bariatric surgical procedure. Patients with diabetes can have a capillary blood glucose (CBG) measured on arrival in the presurgical holding area. Patients with a BG <70 mg/dl can be treated according to established hypoglycemia treatment protocols, while those with a BG ≥200 mg/dl can be treated with a rapid-acting analog dosed according to low or moderate correction insulin scales [19]. A preferred alternative would be to use an intravenous (IV) insulin protocol, particularly for patients with type 1 diabetes or more severe degrees of hyperglycemia [19, 24].
General anesthesia is associated with increases in counterregulatory hormones and insulin resistance, often increasing insulin requirements [25, 26]. The responsibility for glucose monitoring with administration of SQ or IV (preferred) doses of regular or rapid-acting insulin analogs in the operating room will fall to the anesthesiologist [19, 27, 28]. Once a patient is transferred to the recovery room, responsibility is usually transitioned from anesthesiology to the admitting service with or without consultation from medicine or endocrinology.
Postoperative Glycemic Management
Patients dosed with basal insulin prior to surgery will usually require the first postoperative dose of basal insulin the evening or morning following surgery in combination with correction insulin doses (basal plus regimen), depending on when this was taken earlier [14]. Doses of basal can be maintained or even cautiously increased if BG levels remain persistently >180 mg/dl. For patients with a BG <140 mg/dl, basal insulin doses can be gradually decreased in anticipation of lower basal insulin requirement during prolonged fasting or caloric restriction. In cases where BG falls below 100 mg/dl, the dose of basal insulin should be more aggressively decreased to prevent hypoglycemia [17, 18].
Patients treated with diet or non-insulin therapies prior to admission can be treated in the postoperative period with low to moderate doses of correction insulin administered every 4 to 6 h, depending on whether the insulin used is rapid-acting insulin analog or regular insulin. If glycemic targets are not met with correction insulin alone, basal insulin can be added to the regimen and adjusted as needed to achieve BG between 140 and 180 mg/dl [21, 28]. As previously noted, Datta et al. showed that glargine insulin initiated at a dose of 0.3 U/kg in combination with correction insulin resulted in significantly better glycemic control than SSR alone without increasing risk for hypoglycemia [21].
A more conservative approach for patients with significant hyperglycemia (defined as persistent glucose over 180 mg/dl) is to initiate basal insulin at a dose of 0.1 U/kg. This low dose minimizes any concern for hypoglycemia in a fasting obese patient with diabetes. The RABBIT 2-Surgery study utilized a dosing algorithm for initiation of basal insulin at a dose of 0.15–0.25 U/kg in combination with premeal insulin for patients who were eating or with correction insulin in patients who were not and adjusted on a daily basis according to results of bedside blood glucose monitoring performed four times a day [14]. The patients in this earlier study underwent primarily cancer, abdominal, or vascular procedures which differed from the prolonged fasting that occurs in bariatric surgery patients.
Although additional studies would be useful in determining insulin doses that are both appropriate and safe, there is wide variability in insulin requirements among different patients, necessitating sound clinical judgment in making dosing decisions for any one patient. At our institution, the Bariatric Surgery Perioperative Management Protocol guides the early initiation of basal insulin at a dose of 0.1 U/kg for patients with a CBG >180 mg/dl during treatment with correction-scale regular or rapid insulin every 4 or 6 h. The goal is to maintain a BG <180 mg/dl during the immediate postoperative hospital stay while also avoiding hypoglycemia. An interim analysis of this approach demonstrated a decrease in the frequency of inpatient hypoglycemia following implementation of this protocol (manuscript in review).
Special Consideration: Patients With Severe Insulin Resistance
Many morbidly obese individuals with T2D require high insulin doses to maintain even modest degrees of glycemic control. Patients requiring high doses of insulin out of proportion to their weight may present for bariatric surgery as a way of reducing insulin requirement with improvements in insulin sensitivity through surgical weight loss. Patients using over 250 units of insulin/day are often prescribed U-500 insulin to achieve control with less volume of injection [29••]. Estimating the initial basal and ongoing insulin requirements in this group of patients is challenging. While there are no studies guiding therapy in this specific group of patients, one suggested approach would be to make conservative reductions in basal insulin (whether analog or U-500) the night before surgery and avoid administration of SQ insulin the morning of surgery, with initiation of a continuous IV insulin infusion adjusted hourly by anesthesia to keep BG 140–180 mg/dl. Subsequent doses of SQ insulin can be based on a stable adequate insulin infusion rate and then adjusted to maintain a target BG <180 mg/dl.
Glycemic Management Following Hospital Discharge
Fenske et al. sequentially compared non-standardized diabetes management followed by a standardized glycemic management protocol over a period of 1 year in patients with T2D following RYGB [29••]. The protocol included prescriptions for regular-strength metformin 1000 mg twice daily if there were no contraindications and once daily glargine insulin dosed according to the total correction insulin requirement during the 24 h prior to discharge. Dose adjustments in the outpatient setting were made by the provider on a daily basis until the target fasting BG 100–120 mg/dl was achieved for three consecutive days [29••]. Following this, patients self-adjusted insulin doses and were encouraged to call for BG values <81 or >126 mg/dl. No other medications were used. Insulin was stopped only when the protocol directed a dose of zero units. Metformin was stopped for an A1C <5.7 % in patients treated with metformin alone at 3 months postoperatively or later. Implementation of the protocol resulted in larger reductions in A1C and fasting glucose, with complete remission of diabetes in 50 % of subjects at 1 year (compared to 6 % with standard therapy). At our institution, we have implemented the same metformin and glargine titration protocol in response to these results [29••].
Schauer et al. describe an alternative postoperative glycemic management protocol in the STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial [30, 31]. STAMPEDE compared the efficacy of bariatric surgery versus medical management as a means of improving glycemic control or achieving diabetes remission with a target A1C <6.0 % [30, 31]. The protocol for achieving glycemic control included the use of maximal tolerated doses of metformin, followed by the stepwise addition of pioglitazone, a glucagon-like peptide-1 (GLP-1) receptor analog (exenatide/liraglutide), and basal insulin (starting dose 10 or 0.2 units/kg), with progression to a basal bolus insulin (BBI) regimen if needed. The 1-year diabetes remission rate was 42 and 37 %, respectively, in the RYGB and sleeve gastrectomy groups compared to 12 % in the medically treated group.
This study did not specifically address whether intensification of glycemic management contributed to diabetes remission in the surgical groups. It did achieve an impressive complete remission rate in RYGB patients considering that the group’s mean preoperative duration of diabetes (8.2 years), insulin use (44 %), and A1C (9.3 %) all predict low rates of complete remission.
Glycemic Control and Diet in Patients Undergoing Bariatric Surgery
Some bariatric surgical programs recommend 6 months of a physician-supervised weight loss program prior to any surgical intervention (http://www.upmc.com/Services/bariatrics). We feel this time period provides the opportunity to intensify glycemic management to achieve glycemic goals before surgery. Adjustment of diabetes medications is usually required according to reductions in calorie and carbohydrate consumption and level of glycemic control at the time a patient enters into one of these programs.
Other programs put selected patients on a very low-calorie diet (VLCD) of <800 kcal/day for days to weeks before surgery as a way of acutely reducing the size of the liver, making a laparoscopic technique easier and safer [32]. A VLCD can be achieved with meal replacements or a strict diet of portion-controlled lean proteins and non-starchy vegetables. In either situation, the benefits of significant calorie and carbohydrate restriction precede admission. Dose reduction or discontinuation of diabetes medications that can cause hypoglycemia is required with initiation of these diets to prevent severe hypoglycemia.
The majority of bariatric surgical procedures are now performed laparoscopically. Patient management in the immediate postoperative period focuses on pain control, early ambulation, and establishing the tolerability of oral intake of liquids before hospital discharge which usually occurs on postoperative day 2 or 3 if there are no extenuating circumstances.
The calorie quantity and macronutrient distribution of the post-bariatric surgery diet is a major factor in the metabolic changes that occur postoperatively. Dextrose-free IV fluids are usually administered for the first 24 h before patients progress to a sugar-free clear liquid diet once the integrity of the surgical anastomosis is confirmed. Consumption of only non-caloric beverages during this early postoperative period is presumed to mimic the metabolic effects observed during complete fasting of the same duration. Though it has not been studied in this population, we suspect that the use of dextrose-containing IV fluids can increase plasma glucose and insulin requirements, delaying the anticipated transition to the physiology of the prolonged fasting state.
Once there is confirmation that oral liquid intake is tolerated, the patient is discharged on a non-caloric clear liquid diet with gradual progression to a full-liquid diet or liquid protein supplements for the next 2 weeks. Patients are usually advanced to pureed proteins in the following weeks. As the diet is advanced over the next 2 months, patients are advised to target a minimum protein intake level of 60 g or 1.5 g/kg ideal body weight per day. They are encouraged to eat protein first to ensure they reach their minimum and as a way of limiting the more calorie-dense fat and carbohydrate-containing foods. Patients are also counseled to avoid foods that may cause the early dumping syndrome which is characterized by symptoms of tachycardia, abdominal pain, nausea, and diarrhea within 15 min of consuming high-glycemic index carbohydrates. This can be prevented by avoiding sugary beverages, concentrated sweets, and other high-glycemic index carbohydrates [33, 34].
Early phases of the post-bariatric surgery diet are similar to what has been described with low-calorie ketogenic diets with consumption of <800 kcal/day. As the diet progresses to the final phase without food consistency restrictions, patients may initially consume ~1200 kcal/day with >40 % of calories from carbohydrates (>120 g/day) [35]. This and other such data come from patient self-reports.
A food pyramid was developed and published for the post-gastric bypass patient, detailing the appropriate proportions of food groups and macronutrient distributions. A patient following these recommendations would be eating a low-glycemic load diet, with good sources of protein and fiber [33]. Although not relevant to hospital glycemic control, the diet course after discharge is crucial to the prescribing of discharge medications for diabetes and outpatient follow-up arrangements.
Mechanisms of Diabetes Improvement
There is the question as to whether the glycemic improvements observed following bariatric surgery are similar to what would happen if the patient was placed on the same dietary restrictions without surgery. In one study, 11 obese subjects with T2D undergoing RYGB were compared to 14 subjects matched for BMI, A1C, and duration of diabetes and admitted to an inpatient research unit for an average of 21 days [36••]. Subjects in both groups received a VLCD of 500 kcal/day with similar macronutrient content. Those receiving a VLCD experienced similar improvements in insulin sensitivity and secretion as measured by a frequently sampled IV glucose tolerance test (FSIGTT) as well as in body weight, fasting glucose, and fructosamine [36••]. In another study, 10 obese patients with T2D were studied 6 to 15 weeks prior to RYGB and again for a 10-day period that began 2 days immediately prior to the bariatric surgical procedure [37]. The dietary protocol used during each inpatient study period was identical, including a day (day 3) of no caloric intake. Weight loss was greater during the preoperative study period (7.3 vs 4 kg, P = 0.01), with similar improvements in fasting, area under the curve (AUC), and maximal glucose levels.
In a third study, patients with normoglycemia or T2D undergoing biliopancreatic diversion (n = 9 in each group) were studied on postoperative days 3–5 with a standardized meal tolerance test and compared to obese subjects with T2D treated with identical caloric restriction but no surgery [38]. Again, similar improvements were observed in HOMA-IR when comparing the surgical to the calorie-restricted groups.
In a non-surgical study, reversal of diabetes with normalization of beta cell function was reported in 11 obese subjects with T2D following 8 weeks of a 600 kcal VLCD. Normalization of plasma glucose was observed after 1 week of restricted energy intake. Improvements were also noted in rates of hepatic glucose production, first-phase insulin responses, and hepatic and pancreatic triacylglycerol content [39].
Together, these studies suggest that short-term supervised calorie-restricted diets have the ability to promote weight loss and improve glycemic control to a similar extent to what is achieved with RYGB; however, the ability to sustain this level of caloric restriction over longer periods of time free from supervision is limited. No studies have been done attempting to match the postoperative calorie and macronutrient content with and without bariatric surgery for longer than 3 weeks.
Incretins such as GLP-1 and gastric inhibitory polypeptide (GIP) are considered to play a significant role in the weight loss and diabetes improvement after gastric bypass, making it a more effective surgery than adjustable gastric banding. Laferrère et al. studied incretin levels in diabetic patients after gastric bypass versus matched non-surgical controls both at 10 kg weight loss [40]. Surgery patients were on a 600–800 kcal/day 70-g protein diet with food and supplements and reached 10 kg weight loss in 32 days, while non-surgical patients were on a 1000 kcal/day total meal replacement diet and reached 10 kg weight loss in 55 days. In a 3-h 50 g oral glucose tolerance test (OGTT) at 10 kg weight loss, glucose AUC and glucose levels at 120 min were significantly lower after GBP compared to diet, and the pattern of secretion of insulin showed recovery of the early phase. Stimulated levels of incretins increased significantly after surgery by a factor of 6 for GLP-1 and 1.5 for GIP. On the contrary, after diet, GLP-1 levels tended to decrease (P, not significant) and GIP did not change significantly. Notably, 4 out of 9 gastric bypass patients experienced stomach cramping and discomfort, nausea, sweating, flushing, and palpitations 5–20 min into the OGTT [40].
The above study confirms that gastric bypass patients 1 month postoperatively have increases in GLP-1 and GIP after OGTT, improved glucose AUC, and first-phase insulin response compared to matched weight loss controls, proving that these changes are not explained by the amount of weight loss alone. The study does not prove causation, recently brought into doubt by early postoperative studies using the GLP-1 receptor antagonist Exendin 9-39 [41]. It does, however, demonstrate how important matching diet is in acute weight loss and diabetes remission trials. Losing weight on a 600–800 kcal/day diet is likely to have significantly different effects on glycemic control and beta cell function than losing weight on 1000 kcal/day diet taking 23 days longer in non-surgical patients (see Wing et al.’s work with 400 vs 1000 kcal/day) [42]. Also, the gut hormonal response to an OGTT, which is not tolerated by half of the study patients, likely does not represent the hormonal milieu of the ambulatory patient eating a tolerated and recommended diet of sugar-free liquids and pureed proteins in the first month after surgery.
So, while incretins likely play a role in long-term satiety, weight loss, glucose control, and even beta cell function, studies to date have not shown an acute effect during the early postoperative diet compared to diet-matched controls. Other proposed mechanisms for weight loss and diabetes improvements after bariatric surgeries include gut microbiota and enterohepatic pathways of bile acids [43]. No studies comparing different diabetes medication after bariatric surgery have been conducted to show that one class of agents is better than another due to the mechanisms of the specific surgery performed. Therefore, we recommend that all bariatric surgery patients be treated with diabetes agents appropriate for their current diet, regardless of the surgery performed.
Conclusion
Patients with diabetes who undergo bariatric surgery require the same attention to glycemic control during the perioperative and late postoperative time periods as any other patient. Basal insulin can be used when needed to achieve inpatient glycemia <180 mg/dl. Diabetes medications at discharge can be adjusted according to the hypocaloric protein-sparing diet that is prescribed for the first month following surgery. Metformin alone or in combination with basal insulin titrated to outpatient target fasting BG of 100–120 mg/dl has a low risk for hypoglycemia. The attention to glycemic control in the preoperative and postoperative time periods has the potential to favorably influence diabetes remission rates. Until more studies are performed investigating this practice, attention to glycemic control minimizes risk for adverse surgical outcomes similar to what is observed with other surgical procedures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Snel M, Jonker JT, Schoones J, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814.
Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21 Suppl 1:S1–27. These guidelines provide a level of evidence-based recommendations, including perioperative diabetes management, agreed upon by three leading international societies.
Ardestani A, Rhoads D, Tavakkoli A. Insulin cessation and diabetes remission after bariatric surgery in adults with insulin-treated type 2 diabetes. Diabetes Care. 2015;38:659–64.
Perna M, Romagnuolo J, Morgan K, et al. Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis. 2012;8:685–90. This analysis demonstrates the association between pre-op glycemic control, hospital hyperglycemia, and postoperative complications in bariatric surgery patients.
Scheen AJ, Letiexhe M, Rorive M, et al. Bariatric surgery: 10-year results of the Swedish Obese Subjects Study. Rev Med Liege. 2005;60:121–5.
Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150:931–40.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. discussion 50-2.
Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987;206:316–23.
Kwon S, Thompson R, Dellinger P, et al. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257:8–14. This large multicenter study quantifies the associated effects of inpatient hyperglycemia after bariatric surgery and demonstrates the amelioration of those effects when patients receive insulin treatment in the hospital.
Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157:545–52.
Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783–8.
Umpierrez GE, Smiley D, Jacobs S, et al. Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes Undergoing General Surgery (RABBIT 2 Surgery). Diabetes Care. 2011;34:256–61.
Letourneau J, Bui H, Schricker T, et al. HbA1c: a prognostic biomarker in the surgical and critically ill patient population. J Cardiothorac Vasc Anesth. 2013;27:760–4.
Chuah LL, Miras AD, Papamargaritis D, et al. Impact of perioperative management of glycemia in severely obese diabetic patients undergoing gastric bypass surgery. Surg Obes Relat Dis. 2015;11:578–84. The first and only RCT studying the effect of attempting pre- and postoperative glycemic control on short-term and 1-year outcomes. The study is small with confounders but demonstrates that many questions remain unanswered and will require larger well-designed trials comparing interventions to current standard practices.
Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–69.
Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2012;97:16–38.
DiNardo M, Donihi AC, Forte P, et al. Standardized glycemic management and perioperative glycemic outcomes in patients with diabetes mellitus who undergo same-day surgery. Endocr Pract. 2011;17:404.
Rubin DJ, Rybin D, Doros G, et al. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care. 2011;34:1723–8.
Datta S, Qaadir A, Villanueva G, et al. Once-daily insulin glargine versus 6-hour sliding scale regular insulin for control of hyperglycemia after a bariatric surgical procedure: a randomized clinical trial. Endocr Pract. 2007;13:225–31.
Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644–9.
Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28:1107–12.
Sobel SI, Augustine M, Donihi AC, et al. Safety and efficacy of a peri-operative protocol for patients with diabetes treated with continuous subcutaneous insulin infusion who are admitted to same day surgery. Endocr Pract. 2015;21(11):1269–76.
Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110:478–97.
Joshi GP, Chung F, Vann MA, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111:1378–87.
Lowery JB, Donihi AC, Korytkowski MT. U-500 insulin as a component of basal bolus insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2012;14:505–7.
Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38 Suppl:S4.
Fenske WK, Pournaras DJ, Aasheim ET, et al. Can a protocol for glycaemic control improve type 2 diabetes outcomes after gastric bypass? Obes Surg. 2012;22:90–6. This is the first study that evaluated the effect of a postoperative glycemic control intervention on diabetes remission rate. This study opens the door to the concept of adjuvant therapy for diabetes remission. Further studies to determine the best glycemic targets and medication/diet regimens to maximize postoperative diabetes remission rate and duration can be justified based on its clear and optimistic findings.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. New Engl J Med. 2014;370:2002–13.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New Engl J Med. 2012;366:1567–76.
Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg. 2011;146:1300–5.
Moize VL, Pi-Sunyer X, Mochari H, et al. Nutritional pyramid for post-gastric bypass patients. Obes Surg. 2010;20:1133–41.
Parkes E. Nutritional management of patients after bariatric surgery. Am J Med Sci. 2006;331:207–13.
Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:640–6.
Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes. 2013;62:3027–32. Demonstrating that short-term glycemic and metabolic improvements after gastric bypass can be attributed to diet change alone, this paper reverses the early-held belief that the physiology of the surgical procedure itself was an instant surgical cure for many patients with T2D.
Lingvay I, Guth E, Islam A, et al. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care. 2013;36:2741–7.
Plourde CE, Grenier-Larouche T, Caron-Dorval D, et al. Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity. 2014;22:1838–46.
Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506.
Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metabol. 2008;93(7):2479–85.
Vetter ML, Wadden TA, Teff KL, et al. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434–46.
Wing RR, Blair EH, Bononi P, et al. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17(1):30–6.
Argyropoulos G. Bariatric surgery: prevalence, predictors, and mechanisms of diabetes remission. Curr Diab Rep. 2015;15(4):15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Rometo and Mary Korytkowski declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article contains references to studies with human subjects performed by Dr. Mary Korytkowski.
Each of these studies was performed following a review by the University of Pittsburgh Institutional Review Board and/or by the Quality Improvement Committee of the University of Pittsburgh Medical Center
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Rometo, D., Korytkowski, M. Perioperative Glycemic Management of Patients Undergoing Bariatric Surgery. Curr Diab Rep 16, 23 (2016). https://doi.org/10.1007/s11892-016-0718-6
Published:
DOI: https://doi.org/10.1007/s11892-016-0718-6