Abstract
Hyperglycemia is a common occurrence in patients undergoing cardiac surgery. Evidence suggests it is associated with increased perioperative mortality and complications. Preoperatively, HbA1c and fasting blood glucose are reliable methods for identifying patients at increased risk for both hyperglycemia and perioperative morbidity. Perioperative glycemic control by insulin therapy provides considerable benefit to cardiac surgery patients at a cellular and clinical level. Optimal target for blood glucose concentrations as well as the optimal protocol for its delivery remains controversial due to wide variability of studied populations, methodology, and outcomes. Review of available literature suggests that moderate glycemic control (100–140 mg/dL, or 140–180 mg/dL) is at least equivalent, if not superior to intensive control (80–110 mg/dL) in patients undergoing cardiac surgery, however, special populations may benefit from intensive control.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Hyperglycemia is common in patients undergoing cardiac surgery, and likely represents a maladaptive response influenced by factors associated with the perioperative period, including co-existing diabetes and the stress response to surgery. Myocardial ischemia and infarction, fluid and vasopressor administration, and exposure to the cardiopulmonary bypass circuit also contribute. When one considers the increase in the incidence of diabetes and obesity in the population at risk, perioperative hyperglycemia may become an increasingly common condition [1].
Hyperglycemia in the perioperative or postoperative ICU period is associated with a host of detrimental effects, particularly with respect to the cardiovascular system. At a cellular level, these include an imbalance of myocardial oxygen supply and demand, maladaptive diversion of glucose from dependent organs, endothelial dysfunction, platelet aggregation, and impaired immune function [1, 2]. Hyperglycemia is associated with poorer clinical outcomes including increased short and long-term mortality, impaired wound healing, and most notably deep sternal wound infections, increased hospital and ICU length of stay, cognitive dysfunction, renal dysfunction, increased transfusions, and increased costs to the healthcare system [2, 3]. Perioperative glycemic control with insulin therapy has therefore been studied in an effort to determine if its control would improve clinical outcomes by tempering its detrimental effects.
The exact metric which best reflects glycemic control, target blood sugar concentrations and the method and protocol for insulin delivery continues to be controversial, as existing studies have widely varied methodologies. This chapter, therefore, attempts to address the following question- Is there a uniform postoperative glycemic control target and a preferred protocol known to improve outcomes in patients undergoing cardiac surgery?
Search Strategy
We performed a literature search of English language publications to identify published data on perioperative glycemic control in adult cardiothoracic surgical patients in accordance with the PICO outline (Table 29.1). PubMed, EMBASE, and Cochrane Library databases were searched. Terms searched include “cardiothoracic,” “thoracic surgery,” “cardiac surgery,” “aortic valve replacement,” “coronary artery bypass,” “heart valve prosthesis implantation,” “postoperative period,” “postoperative care,” “postoperative,” “post-operative,” and “glycemic control.” Duplicates and articles with pediatric subjects were excluded. Regarding optimal glycemic target in postoperative cardiac surgical patients, nine studies resulted. Of these, there were five randomized controlled trials, two prospective cohort studies, and two retrospective case-control studies. Data was assessed using the GRADE system.
Results
Pathophysiology of Hyperglycemia in Cardiac Surgery Patients
Hyperglycemia has a variety of deleterious effects on the heart, all of which appear to mediate increases in morbidity and mortality during the care of the critically ill, and specifically, the postoperative cardiac surgery patient. Experimental evidence in animal models implicates hyperglycemia as a factor associated with increased infarct size after an ischemic insult [4]. In human cardiomyocytes, hyperglycemia abolishes the protective effect of ischemic [4, 5] and anesthetic preconditioning [6, 7] and furthermore, exacerbates the injury associated with reperfusion [8]. At a cellular level, hyperglycemia has been linked with greater degrees of hypophosphatemia [9] and lactemia [10]. Clinically, hyperglycemia may induce further myocardial damage in diabetics undergoing coronary artery bypass, evidenced by decreased troponin I release with tight glycemic control [11]. Diabetics may suffer more myocardial hypertrophy due to longstanding effects of hyperglycemia, and similarly, diabetics undergoing surgical or trans-catheter aortic valve replacement for severe aortic stenosis exhibit poorer left ventricular mass regression following correction [12]. Hyperglycemia also potentiates vasospasm as it interferes with endothelin mediated relaxation [13, 14]. Additionally, fluctuations in blood sugar concentrations may increase oxidative stress, mediating an additional mechanism for endothelial cell dysfunction [15].
Adverse Effects of Preoperative Hyperglycemia
HbA1c
HbA1c is an important marker for long term glucose control in the diabetic population. In hyperglycemia, a vulnerable NH2 moiety of the hemoglobin molecule becomes irreversibly glycosylated, an event that lasts the duration of that red cell, 90–120 days. This glycosylation event occurs commonly in all of us, not just diabetic patients, but normally accounts for <6% of our Hemoglobin. Any value over 6.5% can be used for the diagnosis of diabetes; in the poorly controlled diabetic, the percent glycosylation can affect upwards of 10–12% of hemoglobin. Importantly, the HbA1c concentration represents, in effect, a window into the average glucose control of a patient over the previous 3–4 months, i.e. the lifespan of a red cell. The American Diabetes Association recommends that diabetics target a HbA1c level of <6.5% to mitigate the complications associated with their disease [16].
The relationship between an elevated HbA1c level and postoperative complications in cardiac surgery have addressed the issue almost solely in coronary artery bypass surgery (CABG) patients. Interestingly, the specific results are mixed, but in a recent systematic review, Tennyson et al. evaluated 11 publications which they felt represented the best evidence on the subject [17]. In that paper, only five studies were prospective, none were randomized, and there were no attempts to look at the data in a propensity matched way. Nonetheless, in all prospective studies there was a strong signal for increased complications for elevated HbA1c levels. The lack of significance seen in some of the studies was, in general, the result of small numbers of subjects. The fourfold increase in mortality seen by Halkos et al. [18] for HbA1c >8.5% was striking, as was the correlation those authors found between increased risk of morbidity and mortality for every percent increase in HbA1c above 6%. In the most recent paper addressing this topic, Narayan et al. [19] performed a retrospective look at close to 4700 patients, three quarters of whom had an off-pump approach. He found a 25% increase in respiratory complications and a more than twofold increase in deep sternal wound complications in that population with a preoperative HbA1c >6.5%. The observed 36% increase in mortality was at the p = 0.08 level.
There are no intervention trials addressing elevated HbA1c levels, nor have there been any attempts in the literature at propensity matching to isolate the HbA1c concentration as an independent risk factor for adverse outcomes after CABG. The most we can say at this moment is that there is a strong signal for an association. Justification for the postponement of surgery to lower the HbA1c concentration similarly has no evidentiary basis. Even were that to exist, 1 month of superb glucose control, yielding a concentration of 6% during that period would only have a partial effect on the overall HbA1c concentration, never lowering it more than 10–15% of the difference between the observed value and 6%. Thus, at least at present, there is only theoretical justification for postponing surgery for patients exhibiting recent, poorly controlled diabetes.
Admission Hyperglycemia and Coronary Artery Bypass Surgery
Given the experimental evidence alluded to in the introduction to this chapter, it is not surprising that the outcomes of any presentation of an acute coronary syndrome are much worse in the presence of hyperglycemia. Furthermore, the commonly prescribed oral hypoglycemic sulfonylureas, so frequently taken by diabetic patients, inhibit myocardial KATP channels, a structure intrinsically involved with the protective mechanisms of preconditioning, thereby worsening any ischemic insult. As a result, the ACC/AHA guidelines advise strict glucose control for all patients admitted with an acute coronary syndrome [20]. Similar reasoning provided the foundation for the Surgical Care Improvement Project emphasis on perioperative glucose control in postop coronary artery bypass patients [21].
To date, the focus of perioperative glucose control in the cardiac surgery patient has been on the intra- and postoperative phases of care. Few data exist regarding the effect of admission hyperglycemia on this group of patients. In 2001, Zindrou et al. [22] found in female patients who did not carry a diagnosis of diabetes, but had an admission glucose concentration >110 mg/dl, a fourfold increase in coronary artery surgery mortality. Surprisingly, this increase in mortality was not seen in men, at any given admission glucose level. In a more recent study, Thiele et al. [23] looked at 240 emergency coronary bypass patients, and found on multivariable analysis an independent effect of admission hyperglycemia on mortality, with a mortality increase of 16% for every 10 mg/dl increment in admission blood sugar for patients admitted with a blood sugar concentration >120 mg/dl.
Ascribing a causal effect to an elevated admission glucose may not be appropriate as the elevated blood glucose concentration may simply reflect the severity of illness of the patient. Nevertheless, we have referenced many important deleterious effects of acute hyperglycemia [7, 20, 22,23,24,25,26,27,28], and so it might be reasonable to implicate hyperglycemia as causal. However, this attribution of causation to admission hyperglycemia should be tentative, as, for example, the stress associated with an acute coronary syndrome may in and of itself cause a sympathetic mediated rise in serum glucose, and thereby account for an associative but not causal role of hyperglycemia and increased mortality and morbidity in this setting.
In summary, although admission hyperglycemia could be a marker for critical illness and thereby simply be associated with poor outcomes, the significant evidence for toxic effects of hyperglycemia at the cellular and biochemical level argue for controlling admission glucose prior to surgery, if possible.
Intraoperative and Postoperative Hyperglycemia
Intraoperative or postoperative hyperglycemia is associated with increased mortality and morbidity. Doenst et al. found hyperglycemia (defined as glucose>360 mg/dl) occurring during cardiopulmonary bypass to be an independent risk factor for mortality. In addition, patients demonstrating hyperglycemia above this level during cardiopulmonary bypass carried an increased incidence of preoperative risk factors including reduced LVEF, CHF, cardiogenic shock, renal failure, previous cardiac surgery, or indication for emergency surgery [29]. Ghandi et al. similarly showed intraoperative hyperglycemia to be an independent risk factor for perioperative complications in a dose-dependent manner such that for every 20 mg/dl increase in blood glucose above 100 mg/dl patients suffered a 34% increase in perioperative complications [30]. Fish et al. determined a comparable relationship during the postoperative period finding that for every 30 mg/dl increase in serum glucose, hospital length of stay increased by 1 day. In addition, postoperative blood glucose exceeding 250 mg/dl was associated with a tenfold increase in complications, primarily cardiac or infectious [31]. Multiple subsequent studies corroborate postoperative hyperglycemia with adverse cardiovascular outcomes [32, 33].
Glycemic Control in the Perioperative Period
Glycemic Control in ICU Patients
Although the detrimental effects of hyperglycemia are well established, the optimal practice for perioperative glycemic control remains somewhat controversial. Results of large randomized controlled trials evaluating optimal glycemic target in critically ill patients is summarized in Table 29.2. In 2001, Van den Berghe et al. challenged the longstanding notion that hyperglycemia occurs as a tolerated component of the stress response. In that study, they demonstrated a 4% absolute mortality reduction in mechanically ventilated surgical ICU patients randomized to an intensive insulin regimen, targeting blood glucose level between 80 and 110 mg/dl, compared with “conventional” management, in which blood glucose was treated only when above 200 mg/dl [34]. This specific study formed the basis for the many major healthcare agency guidelines advising tight glucose control in the critically ill. However, the results of the Van den Berghe study have not been replicated, and, in fact, several large multicenter trials since have produced contradictory results [35,36,37,38]. In the NICE-SUGAR trial, mixed medical surgical ICU patients with an ICU length of stay anticipated to be >3 days were randomly assigned to intensive glucose control (BG 81–108 mg/dl) vs. conventional control (BG<180 mg/dL), The intensively controlled cohort demonstrated an increase in mortality as well as severe hypoglycemic events, with no difference in ICU or hospital length of stay, days on mechanical ventilation, or initiation of renal replacement therapy [36]. The Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, similarly demonstrated an increased risk of adverse events related to hypoglycemia in critically ill septic patients treated with insulin targeting a blood sugar between 80 and 110 mg/dl vs. 180–200 mg/dl [37]. In the GluControl trial, mixed medical-surgical ICU patients treated with intensive insulin therapy (80–110 mg/dL) showed no clinical benefit but did demonstrate an increase in hypoglycemic events. Of note, this last study was stopped prematurely for study protocol violations, and was therefore underpowered [38]. Jacobi et al. recommends treatment of hyperglycemia >150 mg/dl with a maintenance target glucose <150 mg/dl and absolutely <180 mg/dl, with caution to avoid hypoglycemia, especially in certain vulnerable populations [39]. As it currently stands, the updated guidelines by the American Diabetic Association of Clinical Endocrinologists recommend targeting a blood glucose of 140–180 mg/dl in ICU patients [40].
Glycemic Control in Postoperative Cardiac Surgery Patients
Early glucose-insulin-potassium (GIK) solution trials in cardiac surgery patients showed a benefit of insulin therapy despite the occurrence of hyperglycemia in non-diabetics [41], suggesting a pleotropic and protective effect of insulin itself [42]. Lazar et al. went on to investigate the effect of glycemic control with GIK solutions by randomizing 141 diabetic patients undergoing CAB to either “tight” glycemic control with GIK (target glucose 125–200 mg/dl) vs. standard (<250 mg/dl) using intermittent SQ insulin before surgery to 12 h postop. GIK patients demonstrated lower blood glucose (mean 138 mg/dl) with an associated reduced incidence of postoperative atrial fibrillation, wound infections, hospital length of stay, 2-year survival, and recurrent ischemia [43].
The Portland Diabetic Project similarly established the benefit of glycemic control and insulin therapy by following 14,051 diabetic patients undergoing coronary bypass surgery treated either with SQ insulin (1987–1991 protocol) or continuous insulin infusion (1992–2001 protocol). In the continuous insulin infusion group, the glycemic target was periodically lowered according to protocol for goal 150–200 mg/dl during 1991–1998, 125–175 mg/dl during 1999–2001, and 100–150 mg/dl from 2001 on [44]. The group treated by continuous insulin infusion demonstrated improved glucose control as well as reduced mortality (2.5% vs. 5.3%), deep sternal wound infections, and hospital length of stay [45]. In 2007, D’Alessandro et al. further corroborated the benefit of glucose targeted insulin therapy by decreasing mortality in intensively treated diabetics. In this study, 300 diabetic patients undergoing CAB were risk-stratified by Euroscore. Patients exposed to glycemic control (initiation of intravenous insulin therapy for blood glucose >120 mg/dl) demonstrated reduced mortality compared with their Euroscore expected mortality, with the greatest reduction seen in moderate-high risk patients [46].
However, since the initial Van den Berghe trial, no study has shown improved mortality with insulin therapy that targets a blood glucose <110 mg/dl compared with moderate control (<180–200 mg/dl), although few studies suggest improved morbidity and cellular physiology [47, 48]. Even when “tight” control is relaxed to <140–160 mg/dl, few additional studies support an improvement in early mortality compared with targeting a blood glucose of simply less than 180 mg/dl [49, 50]. Improved morbidity has also been described with a glucose target <140–160 mg/dl vs. <180 mg/dl, though infrequently, and includes a diminution in postoperative cognitive dysfunction, postoperative atrial fibrillation, sternal wound infections, duration of mechanical ventilation, and degree of inotropic support [50,51,52].
Most evidence suggests equivalent outcomes among cardiac surgery patients treated with a moderate target (<180–200 mg/dl) vs a tighter one (<140–160 mg/dl) [53,54,55,56,57,58,59,60]. In 2007, Ghandi et al. established superiority of moderate glycemic control (defined as <200 mg/dl) vs. intensive control (80–100 mg/dl), as a result of an increase in mortality and stroke in patients treated with a lower blood glucose target [53]. Bhamidipati et al. investigated the effect of a target <140 mg/dl vs a target <180 mg/dl in patients undergoing isolated valve procedures, and showed equivalent mortality and rate of major complications [54]. That same year, those same investigators additionally examined patients undergoing isolated CAB, and demonstrated a superiority of <180 mg/dL over tighter as well as more liberal insulin regimens, with improvement in mortality as well as morbidity [55]. In 2015, Umpierrez et al. executed the GLUCOCABG trial, in which 303 patients undergoing coronary artery bypass were randomized to receive either intensive (100–140 mg/dl) or conservative (141–180 mg/dl) postoperative glycemic control. Although there was a statistically significant different in mean blood glucose among the two groups (132 vs. 154 mg/dl), there were no significant differences in any of the measured composite endpoints, including mortality, wound infection, pneumonia, bacteremia, respiratory failure, acute kidney injury, or major adverse cardiovascular events. Hypoglycemia did not occur at a statistically greater rate in the 100–140 mg/dl group [56]. Note that intensive therapy in the more recent studies no longer targets 80–110 mg/dl, but rather levels higher than that, so as not to expose patients to the morbid risk of hypoglycemia.
Interestingly, post-hoc analysis of the GLUCOCABG study showed that among nondiabetics, the 100–140 mg/dl insulin therapy group experienced improved clinical endpoints, suggesting the need for further investigation to support more intensive therapy aimed at the lower glucose targets in nondiabetics undergoing CAB [56]. Similarly, Greco et al. merged patient data from the Cardiothoracic Surgical Trials Network and University Health Consortium, and found that among patients undergoing cardiothoracic surgery (isolated valve, isolated CAB, or CAB/valve surgery), complications from hyperglycemic events were more common in non-diabetics, and furthermore, additional hospital costs associated with hyperglycemia were only seen in that patient group [61].
In cardiac surgery patients, glycemic control (and insulin therapy) consistently improves clinical outcomes and lessens morbidity, although the optimal target remains controversial. Results of studies evaluating optimal glycemic target in postoperative cardiac surgery patients are summarized in Table 29.3. The exact target range has not been defined, nor has the issue of different targets for the diabetic vs the non-diabetic been resolved, although perhaps the non-diabetic population might benefit by tighter control. As it currently stands, the STS recommends a blood glucose targeted at <180 mg/dl in all patients, but stricter glycemic targets (<150 mg/dl) in high-risk patients, defined as those with a >3 day anticipated ICU length of stay, ventilator dependence, vasopressor use, and mechanical circulatory support [62].
Recommendations
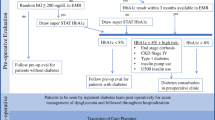
Hyperglycemia in perioperative cardiac surgical patients is common, and has been linked to an increased rate of mortality and perioperative morbidity. Diabetics and patients with unrecognized impaired glucose metabolism suffer worse outcomes and should be identified preoperatively through screening by HbA1c levels as well as fasting blood glucose measurements.
-
Perform preoperative screening utilizing HbA1c in all patients (evidence quality low; weak recommendation)
-
Initiate a glycemic control protocol with continuous intravenous insulin therapy at the induction of anesthesia (evidence quality low; weak recommendation)
-
Continue intravenous insulin therapy for all patients through the first night of surgery and transition to subcutaneous insulin on the first postoperative day, maintaining control for the first 3 days postoperatively (evidence quality moderate; weak recommendation)
-
Target moderate- glycemic control (blood glucose 140–180 mg/dl) in most patients (evidence quality moderate; weak recommendation)
-
Consider strict glycemic targets (blood glucose 100–140 mg/dl) in nondiabetics or high-risk patients (evidence quality low; weak recommendation)
Personal View of the Data
The ill effects of hyperglycemia on the cardiac surgery patient are well recognized at the biochemical, cellular, and patient based level. Although initial enthusiasm for control of blood glucose concentrations to levels between 80 and 110 mg/dl has waned, evidence supports the prevention of hyperglycemia above the range of 160–180 mg/dl. We believe all patients will benefit from preoperative screening and improved glucose control if indicated and time permits. Admission hyperglycemia should be treated prior to surgery, aiming for a level below 120 mg/dl. Intra- and postoperative blood sugar concentrations should initially be with intravenous insulin and changed to subcutaneous insulin after the first postoperative day, when no longer critically ill, targeting blood sugars <160–180 mg/dl. Further research is necessary, however, to define glycemic targets in vulnerable populations, the optimal glucose metric for measurement, and glucose delivery protocol.
References
Lazar HL. How important is glycemic control during coronary artery bypass? Adv Surg. 2012;46:219–35.
Girish G, Agarwal S, Satsangi DK, Tempe D, Dutta N, Pratap H. Glycemic control in cardiac surgery: rationale and current evidence. Ann Card Anaesth. 2014;17(3):222–8.
Breithaupt T. Postoperative glycemic control in cardiac surgery patients. Proc (Bayl Univ Med Cent). 2010;23(1):79–82.
Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol. 2000;278(4):H1218–24.
Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC. Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am J Physiol. 1998;275(2 Pt 2):H721–5.
Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology. 2002;96(1):183–8.
Raphael J, Gozal Y, Navot N, Zuo Z. Hyperglycemia inhibits anesthetic-induced postconditioning in the rabbit heart via modulation of phosphatidylinositol-3-kinase/Akt and endothelial nitric oxide synthase signaling. J Cardiovasc Pharmacol. 2010;55(4):348–57.
Verma S, Maitland A, Weisel RD, Li SH, Fedak PW, Pomroy NC, et al. Hyperglycemia exaggerates ischemia-reperfusion-induced cardiomyocyte injury: reversal with endothelin antagonism. J Thorac Cardiovasc Surg. 2002;123(6):1120–4.
Garazi E, Bridge S, Caffarelli A, Ruoss S, Van der Starre P. Acute cellular insulin resistance and hyperglycemia associated with hypophosphatemia after cardiac surgery. A & A Case Rep. 2015;4(2):22–5.
Qu C, Zhang R, Xiang K. Blood glucose control in the perioperative stage of cardiac value replacement influences levels of blood lactic acid. Chin J Tissue Eng Res. 2012;16(53):9955–9.
Brown JR, Furnary AP, Mackenzie TA, Duquette D, Helm RE, Paliotta M, et al. Does tight glucose control prevent myocardial injury and inflammation? J Extra-Corpor Technol. 2011;43(3):144–52.
Nakamura T, Toda K, Kuratani T, Miyagawa S, Yoshikawa Y, Fukushima S, et al. Diabetes mellitus impairs left ventricular mass regression after surgical or transcatheter aortic valve replacement for severe aortic stenosis. Heart Lung Circ. 2016;25(1):68–74.
Gross ER, LaDisa JF Jr, Weihrauch D, Olson LE, Kress TT, Hettrick DA, et al. Reactive oxygen species modulate coronary wall shear stress and endothelial function during hyperglycemia. Am J Physiol Heart Circ Physiol. 2003;284(5):H1552–9.
Koltai MZ, Hadhazy P, Posa I, Kocsis E, Winkler G, Rosen P, et al. Characteristics of coronary endothelial dysfunction in experimental diabetes. Cardiovasc Res. 1997;34(1):157–63.
Egi M, Shimizu K, Toda Y, Takenouchi S, Morita K. Perioperative glucose variability and oxidative stress in postoperative critically ill patients. Crit Care Med. 2010;38:A69.
American Diabetes Association. Erratum. Glycemic targets. Sec. 6. In standards of medical care in diabetes-2017. Diabetes Care. 2017;40(Suppl. 1):S48–56. Diabetes Care. 2017 Jul;40(7):985, er07a. Epub 2017 May 18.
Tennyson C, Lee R, Attia R. Is there a role for HbA1c in predicting mortality and morbidity outcomes after coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg. 2013;17(6):1000–8.
Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136(3):631–40.
Narayan P, Kshirsagar SN, Mandal CK, Ghorai PA, Rao YM, Das D, et al. Preoperative glycosylated hemoglobin: a risk factor for patients undergoing coronary artery bypass. Ann Thorac Surg. 2017;104:606–12.
Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60(7):645–81.
How-to guide: prevent surgical site infections [Internet]. 2012. Available from: www.ihi.org.
Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care. 2001;24(9):1634–9.
Thiele RH, Hucklenbruch C, Ma JZ, Colquhoun D, Zuo Z, Nemergut EC, et al. Admission hyperglycemia is associated with poor outcome after emergent coronary bypass grafting surgery. J Crit Care. 2015;30(6):1210–6.
Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36(7):2185–91.
Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–701.
Oliver MF. Fatty acids and the risk of death during acute myocardial ischaemia. Clin Sci (Lond). 2015;128(6):349–55.
Davi G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322(25):1769–74.
Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101(19):2247–51.
Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130(4):1144.
Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862–6.
Fish LH, Weaver TW, Moore AL, Steel LG. Value of postoperative blood glucose in predicting complications and length of stay after coronary artery bypass grafting. Am J Cardiol. 2003;92(1):74–6.
Frioud A, Comte-Perret S, Nguyen S, Berger MM, Ruchat P, Ruiz J. Blood glucose level on postoperative day 1 is predictive of adverse outcomes after cardiovascular surgery. Diabetes Metab. 2010;36(1):36–42.
Giakoumidakis K, Nenekidis I, Brokalaki H. The correlation between peri-operative hyperglycemia and mortality in cardiac surgery patients: a systematic review. Eur J Cardiovasc Nurs. 2012;11(1):105–13.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67.
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61.
NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108–18.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39.
Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–48.
Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251–76.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–69.
Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1997;113(2):354,60; discussion 360–2.
Hasegawa A, Iwasaka H, Hagiwara S, Koga H, Hasegawa R, Kudo K, et al. Anti-inflammatory effects of perioperative intensive insulin therapy during cardiac surgery with cardiopulmonary bypass. Surg Today. 2011;41(10):1385–90.
Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–502.
Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–21.
Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(Suppl 2):21–33.
D’Alessandro C, Leprince P, Golmard JL, Ouattara A, Aubert S, Pavie A, et al. Strict glycemic control reduces EuroSCORE expected mortality in diabetic patients undergoing myocardial revascularization. J Thorac Cardiovasc Surg. 2007;134(1):29–37.
Caroleo S, Onorati F, Rubino A, Calandese F, De Munda C, Renzulli A, et al. Intensive versus conventional insulinotherapy after elective and on-pump myocardial revascularization: a prospective and randomized study. Intensive Care Med. 2009;35:S107.
Asida SM, Atalla MM, Gad GS, Eisa KM, Mohamed HS. Effect of perioperative control of blood glucose level on patient’s outcome after anesthesia for cardiac surgery. Egypt J Anaesth. 2013;29(1):71–6.
Giakoumidakis K, Eltheni R, Patelarou E, Theologou S, Patris V, Michopanou N, et al. Effects of intensive glycemic control on outcomes of cardiac surgery. Heart Lung. 2013;42(2):146–51.
Song B, Jiang P, Wang Z. Clinical effects of strict control versus conventional control of blood glucose on perioperative cardiac surgery: a meta-analysis. Chin J Evid Based Med. 2012;12(10):1229–34.
Kurnaz P, Sungur Z, Camci E, Sivrikoz N, Orhun G, Senturk M, et al. The effect of two different glycemic management protocols on postoperative cognitive dysfunction in coronary artery bypass surgery. Bras J Anestesiol. 2017 2017/05;67(3):258–65.
Wahby EA, Abo Elnasr MM, Eissa MI, Mahmoud SM. Perioperative glycemic control in diabetic patients undergoing coronary artery bypass graft surgery. J Egypt Soc Cardio-Thorac Surg. 2016 2016/08;24(2):143–9.
Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–43.
Bhamidipati CM, LaPar DJ, Mehta GS, Kern JA, Kron IL, Ailawadi G. Tight glucose control is not superior to permissive hyperglycemia following valve surgery. J Am Coll Cardiol. 2011 2011/04;57(14):E1394.
Bhamidipati CM, LaPar DJ, Stukenborg GJ, Morrison CC, Kern JA, Kron IL, et al. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141(2):543–51.
Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. 2015;38(9):1665–72.
Mulla I, Schmidt K, Cashy J, Wallia A, Oakes DJ, Andrei A-, et al. Comparison of glycemic and surgical outcomes in cardiac surgery patients after change in glycemic targets. Diabetes. 2014;63:A233.
Liou HL, Shih CC, Chung KC, Chen HI. Comparison of the effect of intensive versus conventional insulinotherapy in patients with cardiac surgery after cardiopulmonary bypass. Chin J Physiol. 2013;56(2):101–9.
McDonnell ME, Alexanian SM, Junqueira A, Cabral H, Lazar HL. Relevance of the Surgical Care Improvement Project on glycemic control in patients undergoing cardiac surgery who receive continuous insulin infusions. J Thorac Cardiovasc Surg. 2013;145(2):590,4; discussion 595–7.
Leibowitz G, Raizman E, Brezis M, Glaser B, Raz I, Shapira O. Effects of moderate intensity glycemic control after cardiac surgery. Ann Thorac Surg. 2010;90(6):1825–32.
Greco G, Ferket BS, D’Alessandro DA, Shi W, Horvath KA, Rosen A, et al. Diabetes and the association of postoperative hyperglycemia with clinical and economic outcomes in cardiac surgery. Diabetes Care. 2016;39(3):408–17.
Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cha, S., Whitman, G.J. (2019). Glycemic Control Does Matter in the Cardiac Surgery Patient. In: Lonchyna, V. (eds) Difficult Decisions in Cardiothoracic Critical Care Surgery. Difficult Decisions in Surgery: An Evidence-Based Approach. Springer, Cham. https://doi.org/10.1007/978-3-030-04146-5_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-04146-5_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04145-8
Online ISBN: 978-3-030-04146-5
eBook Packages: MedicineMedicine (R0)