Abstract
Purpose of Review
Current guidelines on the management of patients with dyslipidemias recommend specific risk-dependent low-density lipoprotein cholesterol (LDL-C) treatment goals. Recently, several randomized clinical trials have investigated further lowering of LDL-C in addition to statin therapy using novel therapeutic approaches and examined their effects on cardiovascular (CV) risk. This review summarizes newly available data on efficacy and safety of lowering LDL-C beyond statin therapy and below current treatment targets.
Recent Findings
In patients at very high risk for CV events, a significant residual risk remains when failing to achieve significant LDL-C reduction on maximally tolerated statin therapy alone. Further lowering of LDL-C, even beyond current treatment targets, has been shown to be safe and was associated with a further reduced CV risk reduction. The relative risk reduction per change in LDL-C levels has been observed to be consistent even in patient populations achieving extremely low levels of LDL-C.
Summary
In patients at very high CV risk, further lowering of LDL-C beyond statin therapy and present treatment targets has been observed to further reduce CV risk, which may be foremost relevant for patients at a particular high absolute CV risk, e.g., for patients with progressive and/or very extensive coronary disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic cardiovascular disease (CVD) remains a leading cause of premature death and morbidity. In Europe, CVD causes more than 4 million deaths each year, accounting for up to 45% of all deaths [1]. Most of these deaths are attributable to coronary heart disease (CHD) [2], although improved treatment of cardiovascular (CV) risk factors as well as the establishment of a more effective therapeutic strategy in the setting of acute coronary syndromes has resulted in a decrease of age-adjusted mortality of CHD [3]. Dyslipidemias represent a leading modifiable risk factor for CVD. In the INTERHEART case-control study with 27,098 participants, dyslipidemia, as defined by an elevated apolipoproteinB to apolipoproteinA1 ratio, was associated with a particular high mortality odds ratio (3.25), i.e., higher when compared to other CV risk factors [4].
The observation that increased low-density lipoprotein cholesterol (LDL-C) represents a causal risk factor for atherosclerotic CVD is now well accepted [5]. An independent positive association between LDL-C and CVD can be confirmed by epidemiological data, extending even to very low levels of LDL-C [6]. Genetic studies have shown that, in patients with familial hyperlipoproteinemia (FH), lifetime exposure to elevated LDL-C is associated with an excessive risk of CVD [7], while the overall CV risk is considerably low in populations with genetically determined low levels of LDL-C [8].
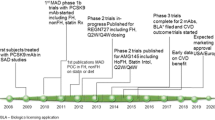
The therapeutic concept of reducing CV risk by lowering LDL-C with statin therapy has been proven successful in numerous clinical studies [9]. Later, the results of the IMPROVE-IT trial by using ezetimibe therapy showed that, in CHD patients, lowering LDL-C below levels achieved by statin therapy alone resulted in improved CV outcomes [10]. Also, additional therapies with monoclonal antibodies inhibiting proprotein convertase subtilisin-kexin type 9 (PCSK9) and a long-acting RNA interference agent inhibiting the synthesis of PCSK9 have proven to substantially lower LDL-C beyond statin therapy [11,12,13,14], and well below current treatment targets [15, 16]. Moreover, the findings of the FOURIER and the ODYSSEY OUTCOMES trials showed, for the first time, that inhibition of PCSK9, on the background of statin therapy, not only lowers LDL-C but also significantly reduces the risk of CV events in patients at very high CV risk [17, 18]. Since these data strongly support the “the lower the better” hypothesis for LDL-C, the question arises as to whether lowering of LDL-C beyond statin therapy and the currently recommended targets should be considered in patients at particular high CV risk. Additionally, even though the relationship between increased levels of LDL-C and risk of CV morbidity and mortality is well established, the safety of extremely low LDL-C levels also needs to be considered.
Current Treatment Goals
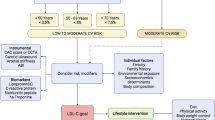
Randomized controlled clinical trials have shown that, in patients with established CVD, lowering of LDL-C with statins reduces the risk for CV events [9]. Consequently, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines, published in 2014, recommended a high-intensity statin therapy in high-risk patients in order to achieve a reduction of LDL-C by at least 50% [16], whereas the former European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines, published in 2011, recommended reducing LDL-C to a goal of < 1.8 mmol/L (< 70 mg/dL) in very high-risk patients [19]. Both of these recommendations are derived from the consideration that a CV-protective effect of lowering LDL-C is, in addition to the individual CV risk, primarily determined by the absolute reduction of LDL-C [5].
There has been an extensive discussion about the recommendation of risk-driven statin therapy without specific, predefined LDL-C treatment goals (“fire and forget”) in ACC/AHA guidelines. Additional LDL-C-lowering therapies beyond statins were not adequately established at that time [20]. When additional LDL-C-lowering options became available, this concern was addressed by an ACC consensus statement, published in 2016, evaluating the role of non-statin therapy for lowering LDL-C and recommending to consider the use of ezetimibe in very high-risk patients who had remaining elevated LDL cholesterol levels (may consider LDL-C < 1.8 mmol/L (< 70 mg/dL)) on maximally tolerated statin therapy alone [21].
Conversely, there were concerns that suggesting a sole, fixed target LDL-C level of < 1.8 mmol/L (< 70 mg/dL), as proposed by the 2011 ESC/EAS guidelines, might result in an inadequate lowering of LDL-C by statin therapy in high-risk patients with only slightly elevated levels of LDL-C at baseline. Hence, the current European guidelines, published in 2016, recommend reducing LDL-C to a goal of < 1.8 mmol/L (< 70 mg/dL) or by at least 50% if the baseline LDL-C is between 1.8 and 3.5 mmol/L (70–135 mg/dL) in these patients [15]. Current ACC/AHA and ESC/EAS guideline recommendations on lowering LDL-C in patients at very high CV risk are summarized in Table 1.
Benefits of a Targeted Approach to Lowering of LDL-C
An important reason for the divergence from pre-existing guidelines [19] and the move away from specific LDL-C targets in the 2013 ACC/AHA guidelines was the limited evidence for CV benefit of additional LDL-C-lowering therapies beyond statins, and the consideration that in randomized controlled trials (RCTs) there were no specific LDL-C goals. The National Lipid Association, however, noticed that treatment goals represent a useful approach to ensure that the intensity of an LDL-C-lowering therapy is matched to the individual risk for CVD [22].
Having an on-treatment target is a familiar and helpful aspect of clinical practice in CV medicine [23,24,25]. An on-treatment target can aid in communication between doctors and patients and can help improve compliance. Providing targets in well-defined treatment paradigms, as established in the treatment of hypertension [24, 25], for example, also can make monitoring response to treatment more convenient.
The strongest argument for a LDL-C-lowering goal strategy exists for patients with a high CV risk and high pretreatment levels of LDL-C [26]: an unmonitored approach to lowering LDL-C (“fire and forget”) might result in a high residual CV risk when high-risk patients fail to achieve significant reductions of LDL-C, keeping in mind the large patient-to-patient variability in response to statins [27].
Another argument for the presence of specific treatment targets is their potential to decrease barriers for access and reimbursement, since these barriers will likely result in underutilization of effective LDL-C-lowering treatments.
Residual CV Risk Despite Low Levels of LDL-C
As evidenced by meta-analyses of statin trials, an on-treatment residual CV risk can be observed in high-risk patients, with a 5-year major event rate of 22% among patients with prior CVD [9, 28]. In the Treating to New Targets Trial, 8.7% of patients with stable CHD experienced a major event over 5 years, despite receiving 80 mg of atorvastatin daily and having an on-treatment LDL-C of 1.8–2.6 mmol/L (70–135 mg/dL) only [29]. Pooled data from statin trials highlight LDL-C as a marker of residual risk even in LDL-C level < 2.0 mmol/L (77 mg/dL) [9, 30].
Evidence of a substantial, and clinically significant, residual CV risk despite already relatively low levels of LDL-C also comes from clinical trials investigating CV outcomes of further LDL-C-lowering therapies in addition to statins. In the IMPROVE-IT trial, ezetimibe or placebo was added to 40 mg of simvastatin in patients after an acute coronary syndrome (ACS). The study had a median follow-up of 6 years. A primary endpoint (composite of cardiovascular death, non-fatal myocardial infarction (MI), unstable angina requiring rehospitalization, coronary revascularization (≥ 30 days after randomization), or non-fatal stroke) occurred in 32.7% of patients in the simvastatin-ezetimibe group, even though the median time-weighted average LDL-C level during the study was only 1.4 mmol/L (53.7 mg/dL) in this group [10]. In the FOURIER trial, 27,564 patients with evident atherosclerotic disease and an LDL-C ≥ 70 mg/dL despite statin therapy (preferably high-intensity statin therapy), with or without ezetimibe, were randomized to receive either the PCSK9 inhibitor evolocumab or matching placebo as subcutaneous injections. The mean duration of follow-up was 2.2 years. Since patients were already treated with statins (and additional LDL-C-lowering therapy using ezetimibe in 5% of the patients), baseline LDL-C was 2.4 mmol/L (92 mg/dL) only. At 48 weeks of treatment with evolocumab, the median level of LDL-C was as low as 0.8 mmol/L (30 mg/dL), correlating to a mean 59% reduction of LDL-C as compared with placebo. Still, in the group of patients treated with evolocumab, the primary endpoint (CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) occurred in 9.8%, while 5.9% experienced a key secondary endpoint (CV death, MI, or stroke) [17].
Efficacy of Lowering LDL-C Below Current Targets in High-Risk Patients
Statins substantially reduce CV risk, but, as mentioned above, a relevant CV risk remains in patients at very high CV risk, especially in those who remain to have high LDL-C levels even on a maximum tolerated dose of statin therapy [31]. Also, side effects of statin therapy such as statin-associated muscle symptoms are rather rare, but they become relevant by reducing adherence to statin treatment [32, 33]. While the residual CV risk in patients on a maximally tolerated statin therapy may further be reduced by focusing also on other CV disease risk factors related to lifestyles, blood pressure control, and detection and management of dysglycemia, the aspects mentioned above, among others, have led to trials investigating additional LDL-C-lowering therapeutic approaches and their effects on CV events.
In IMPROVE-IT, the addition of ezetimibe to simvastatin in patients after ACS resulted in a significantly lower median time-weighted average LDL-C as compared with simvastatin monotherapy (1.4 mmol/L (53.7 mg/dL) vs. 1.8 mmol/L (69.5 mg/dL), P < 0.001). The further reduction of LDL-C translated into significantly fewer primary endpoints in the simvastatin-ezetimibe group (P = 0.016) with no evidence of adverse effects caused by further reducing LDL-C [10]. Likewise, in FOURIER, the further reduction of LDL-C to a median level of 0.8 mmol/L (30 mg/dL) using the PCSK9 inhibitor evolocumab significantly reduced the primary efficacy endpoint by 15% (9.8 vs. 11.3%, P < 0.001) and the key secondary efficacy endpoint by 20% (5.9 vs. 7.4%, P < 0.001) in patients with established CVD. Injection-site reactions were more common with evolocumab, but with regard to other adverse events, there was no significant difference between the study groups [17].
In SPIRE-1 and SPIRE-2, a total of 27,438 patients with either a history of CVD or FH or with a high risk for CVD were randomized to either the PCSK9 inhibitor bococizumab or matching placebo as subcutaneous injections. Because of high rates of the development of neutralizing antidrug antibodies, resulting in an attenuation of the LDL-C-lowering effect, the SPIRE trials were stopped early. Still, in SPIRE-2, the addition of bococizumab, as compared with placebo at 52 weeks of treatment, resulted in a 1.5 mmol/L (57.3 mg/dL) absolute difference in LDL-C. After a median follow-up of 1.0 year, treatment of bococizumab significantly reduced the incidence of the primary composite outcome (CVD, MI, stroke, or hospitalization for unstable angina requiring urgent coronary revascularization) as well as the secondary composite outcome (CVD, MI, or stroke) (P = 0.02 and P < 0.007, respectively) [34].
Recently, Ference et al. compared the efficacy of PCSK9 inhibitors and statins for reducing the risk of CV events by comparing the results of the FOURIER and SPIRE trials with the results of the Cholesterol Treatment Trialists Collaboration (CTTC) meta-analysis of statin trials. The authors found that the magnitude of risk reduction in the PCSK9 inhibitor trials was exactly what would have been expected based on the CTTC meta-analysis on statin trials when the effects of PCSK9 inhibitors and statins are compared by total duration of therapy and during each year of treatment: per 1 mmol/L (38.7 mg/dL) reduction in LDL-C, treatment with a PCSK9 inhibitor reduced multiple different CV events by 11–16% in the first year of treatment, which is nearly identical to the 4–16% reduction in risk seen during the first year of treatment in the statin trials. Likewise, in the FOURIER trial, treatment with evolocumab reduced multiple different CV events by 18–23% per 1 mmol/L (38.7 mg/dL) reduction in LDL-C during the second year of treatment, which is again nearly identical to the 22–25% reduction in risk seen during the second year of treatment in the statin trials [35].
Current ESC/EAS guidelines have already mentioned PCSK9 inhibition as a class IIb recommendation in patients at very high CV risk, with persistent high levels of LDL-C despite treatment with maximally tolerated statin dose, in combination with ezetimibe or in patients with statin intolerance [15]. Additionally, an ESC/EAS task force recently provided a consensus document, discussing an optimized clinical use of PCSK9 inhibiting antibodies in patients at very high CV risk or familial hypercholesterolemia [36, 37•]. In summary, the task force recommends to consider PCSK9 inhibition in:
-
Patients at very high CV risk with additional risk indicators, who have an LDL-C > 100 mg/dL despite recommended maximally tolerated statin plus ezetimibe treatment
-
Patients at very high CV risk without additional risk indicators, who have an LDL-C > 140 mg/dL despite recommended maximally tolerated statin plus ezetimibe
-
FH patients without CVD, but with additional risk factors, who have an LDL-C > 140 mg/dL despite recommended maximally tolerated statin plus ezetimibe
-
FH patients without CVD and without additional risk factors, who have an LDL-C > 180 mg/dL despite recommended maximally tolerated statin plus ezetimibe
Although current European and US recommendations highlight the use of PCSK9 inhibitors to further lower LDL-C in specific very high-risk patients [21, 36, 37•], uptake of PCSK9 inhibitors has been limited [38]. Several cost-effectiveness analyses of PCSK9 inhibitors have been published, producing widely varying and potentially conflicting results [39,40,41,42,43,44,45,46]. Just recently, Annemans et al. have reported a “highest risk–highest benefit” concept to optimize cost-effectiveness not only on an individual basis, but also for society [47]. In this concept, the highest risk categories include:
-
Polyvascular disease
-
CVD with comorbidities such as diabetes with end-organ damage or chronic kidney disease
-
FH with a CV event
Applying the “highest risk–highest benefit” concept in daily practice might help promote cost-effective and reasonable utilization of PCSK9 inhibitors in high-risk patients while preventing inefficiency of costs in patients at lower risk for CV events.
In a recent meta-analysis for statin and non-statin treatments of dyslipidemia, Sabatine et al. investigated efficacy and safety of further lowering LDL-C in patients with very low levels of LDL-C. While, for statins, a 22% reduction of major vascular events per 1 mmol/L (38.7 mg/dL) reduction of LDL-C was observed in a subgroup of the patients from the CTTC meta-analysis with a mean LDL-C in the control arm of 1.7 mmol/L (65.7 mg/dL), an analysis of three trials of non-statin LDL-C-lowering therapies added to statins with a median LDL-C in the control arms ranging from 1.6 to 1.8 mmol/L (63 to 70 mg/dL) revealed an almost identical 21% reduction of major vascular events per 1 mmol/L (38.7 mg/dL) reduction of LDL-C. Combining the data of statin and non-statin trials, the authors found a 21% reduction of major vascular events per 1 mmol/L (38.7 mg/dL) reduction of LDL-C [48].
Table 2 summarizes those randomized controlled trials in which LDL-C levels below targets recommended by current guidelines were achieved.
Safety of Lowering LDL-C Below Current Targets in Very High-Risk Patients
In the meta-analysis by Sabatine et al. mentioned above, lowering LDL-C to very low levels was not associated with an increased risk of serious adverse events, myalgias, myositis, elevation in the level of aminotransferases, new-onset diabetes, hemorrhagic stroke, or cancer, even in patients with very low levels of LDL-C [48]. Another meta-analysis demonstrated an association of treatment with statins and a small increase in the risk of diabetes [49••]. Furthermore, Mendelian randomization studies show that genetic variants mimicking PCSK9 and statins are associated with a similar increased risk of diabetes per unit change in LDL-C [50]. Additional analyses of the PCSK9 inhibitor trials stratified by fasting glucose level should provide more insight into whether the effect of PCSK9 inhibition on the risk of new-onset diabetes is clinically relevant. However, it must be emphasized that both the naturally randomized genetic evidence and the numerous statin trials clearly suggest that the beneficial effect of lowering LDL-C by either statins or PCSK9 inhibitors far exceeds any potential risk of new-onset diabetes [49••, 50].
Achievement of Current LDL-Cholesterol Treatment Goals: Real-World Data
The benefits of LDL-C-lowering therapies seen in studies and recommended in the guidelines will only be implemented in real life if patients are treated accordingly and adhere to the prescribed LDL-C-lowering strategy.
As discussed above, lowering LDL-C to the goal of < 1.8 mmol/L (< 70 mg/dL) and achieving ≥ 50% LDL-C reduction when this goal cannot be reached is a class IA recommendation in current guidelines [15, 16, 21], but only 35–40% of very high-risk patients in registries achieve their LDL-C treatment goals [51]. The EUROASPIRE investigators gathered CHD patients’ data from 79 centers in 24 European countries. A total of 6,648 patients were surveyed 6–36 months after hospitalization for a CHD event (coronary artery bypass grafting, percutaneous coronary intervention, acute myocardial infarction, acute myocardial ischemia). At the time of the interview, statin therapy had been discontinued in 11.6% and only 19.3% of all CHD patients had LDL-C levels below the target value of 1.8 mmol/L (70 mg/dL) [52].
Conclusions
Multiple epidemiological, genetic, and clinical studies have shown a continuous relation between LDL-C and risk for CV events. The benefit of lowering LDL-C has been proven by numerous randomized clinical trials. Currently, LDL-C treatment goals are recommended, but, since the relationship between LDL-C and CV risk is continuous, these goals are also provided for educational purposes. Furthermore, LDL-C treatment goals have neither been challenged nor verified in randomized trials. However, in clinical practice, LDL-C treatment goals are a strong incentive for both the patient and the physician, representing a useful metric of therapeutic success and preventing underutilization of further LDL-C-lowering therapies such as ezetimibe and PCSK9 inhibitors in patients at particular high CV risk. Since the efficacy of LDL-C-lowering therapies to reduce CV risk depends on the magnitude of their LDL-C-lowering effect, the greatest CV risk reduction can be achieved by intensively lowering LDL-C in patients at very high CV risk with very high levels of LDL-C. In clinical trials, lowering of LDL-C below targets currently recommended has proven to be efficacious and safe, and can be cost-effective in selected patients at particular high CV risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–98.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr Cardiol Rep. 2012;14(6):709–20.
Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303(6797):276–82.
Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol. 2004;160(5):421–9.
Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631–9.
Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51.
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL Cholesterol. N Engl J Med. 2017;376(15):1430–40.
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99.
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9.
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
European Association for Cardiovascular P, Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818.
Martin SS, Abd TT, Jones SR, Michos ED, Blumenthal RS, Blaha MJ. 2013 ACC/AHA cholesterol treatment guideline: what was done well and what could be done better. J Am Coll Cardiol. 2014;63(24):2674–8.
Writing C, Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-Cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68(1):92–125.
Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8(5):473–88.
Authors/Task Force M, Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–87.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C.
Davies JT, Delfino SF, Feinberg CE, Johnson MF, Nappi VL, Olinger JT, et al. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights. 2016;9:13–29.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78.
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–35.
Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90.
Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1(6):e001800.
Munkhaugen J, Sverre E, Otterstad JE, Peersen K, Gjertsen E, Perk J, et al. Medical and psychosocial factors and unfavourable low-density lipoprotein cholesterol control in coronary patients. Eur J Prev Cardiol. 2017;24(9):981–9.
Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur Heart J. 2015;36(17):1012–22.
Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527–39.
Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists collaboration. Eur Heart J. 2018;39(27):2540–5.
Landmesser U, Chapman MJ, Farnier M, Gencer B, Gielen S, Hovingh GK, et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38(29):2245–55.
• Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Boren J, et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J. 2018;39(14):1131–43 This consensus statement provides practical guidance for the use of proprotein convertase subtilisin/kexin type 9 inhibitiors in high-risk patients.
Sabatine MS. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: comparing and contrasting guidance across the Atlantic. Eur Heart J. 2017;38(29):2256–8.
Arrieta A, Page TF, Veledar E, Nasir K. Economic evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLoS One. 2017;12(1):e0169761.
Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(10):1069–78.
Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39(6):313–20.
Hernandez I. Revisiting outcomes-based pricing propositions for the PCSK9 inhibitor evolocumab. JAMA Intern Med. 2017;177(9):1388–90.
Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743–53.
Kazi DS, Penko J, Coxson PG, Moran AE, Ollendorf DA, Tice JA, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER Trial. JAMA. 2017;318(8):748–50.
Toth PP, Danese M, Villa G, Qian Y, Beaubrun A, Lira A, et al. Estimated burden of cardiovascular disease and value-based price range for evolocumab in a high-risk, secondary-prevention population in the US payer context. J Med Econ. 2017;20(6):555–64.
Villa G, Lothgren M, Kutikova L, Lindgren P, Gandra SR, Fonarow GC, et al. Cost-effectiveness of evolocumab in patients with high cardiovascular risk in Spain. Clin Ther. 2017;39(4):771–86 e3.
Annemans L, Packard CJ, Briggs A, Ray KK. ‘Highest risk-highest benefit’ strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur Heart J. 2018;39(27):2546–50.
Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol. 2018;3:823–8.
•• Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72 This consensus stament summarizes the consistent evidence coming from numerous epidemiological, clinical, and genetic studies that low-density lipoproteins unequivocally cause cardiovascular disease.
Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–61.
Sinning D, Landmesser U. Effective low-density lipoprotein-lowering therapy: implementation in clinical practice. Eur J Prev Cardiol. 2017;24(3_suppl):71–6.
Reiner Z, De Backer G, Fras Z, Kotseva K, Tokgozoglu L, Wood D, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries--findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243–50.
Author information
Authors and Affiliations
Contributions
DS and UL contributed to the conception or design of the work. DS drafted the manuscript. UL critically revised the manuscript. Both gave final approval and agree to be accountable for all aspects of work ensuring both integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of Interest
David Sinning has received lecture honoraria or advisory honoraria from Amgen, Sanofi, and Berlin-Chemie. Ulf Landmesser has received lecture or advisory honoraria from Amgen, Sanofi, Medicines Company, and Berlin-Chemie.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lipid Abnormalities and Cardiovascular Prevention
Rights and permissions
About this article
Cite this article
Sinning, D., Landmesser, U. Is There a Need to Revise Goals in the Management of Dyslipidemias?. Curr Cardiol Rep 21, 51 (2019). https://doi.org/10.1007/s11886-019-1128-6
Published:
DOI: https://doi.org/10.1007/s11886-019-1128-6