Abstract
Purpose of review
In this review, the current literature of imaging of bladder pain syndrome and interstitial cystitis (BPS/IC) will be addressed. Topics include BPS/IC, cystoscopy, computed tomography, and magnetic resonance image (MRI).
Recent findings
There are no randomized clinical trials on imaging of BPS/IC. Recently, contrast-enhanced MRI could detect the brain alterations and the changes in bladder permeability, and detection of the latter is enhanced by intravesical injection of contrast agents.
Summary
MRI could advance the understanding of pathological changes in the brain and the bladder of BPS/IC patients. Especially, contrast-enhanced MRI has a potential to become a diagnostic tool although more evidences are necessary for clarifying the efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
International continence society (ICS) defines bladder pain syndrome/interstitial cystitis (BPS/IC) as a symptom complex with the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology [1••]. Cystoscopy is one of the tools for the diagnosis of BPS/IC, and Hunner lesion, which presents as a circumscript, reddened mucosal area with small vessels radiating towards a central scar, with a fibrin deposit or coagulum attached to this area [2], is the typical finding of IC. However, the significant number of BPS/IC patients does not have the Hunner lesion, which is sometimes difficult to be confirmed by cystoscopy; therefore, the examination is not a reliable tool for the diagnosis [3]. Furthermore, it has been considered that typical findings of BPS/IC patients do not exist in imaging with computed tomography (CT) or magnetic resonance imaging (MRI), while these imaging techniques are useful for detecting other diseases such as bladder cancer and urinary stones. Thus, until recently, there is scarce evidence of imaging of BPS/IC in clinical settings for the diagnosis and or underlying pathophysiology [4]. However, just recently, several studies have reported on the efficacy of MRI for detecting the findings suggestive of BPS/IC. BPS is considered to be maintained by alterations in the central nervous system. For example, functional MRI could detect an increment of gray matter volume in several brain regions of BPS/IC patients [5]. Moreover, intravesical injection of contrast medium in MRI could help to detect changes in bladder permeability, which is specific to BPS/IC [6, 7]. Therefore, this review introduces the current development of imaging of BPS/IC.
Pathophysiology of Bladder Pain Syndrome/Interstitial Cystitis
Bladder wall is composed of several layers including urothelium, lamina propria, smooth muscles, and serosa. These compartments play roles in voiding, storage, and barrier functions.
In BPS/IC patients, the functional loss of urothelium is considered to be an important factor in pathophysiology. Especially, Hunner lesion found in BPS/IC patients indicates impaired restoration of normal urothelium due to unknown irreparable mechanisms [4]. By contrast, non-Hunner-type BPS, which comprises the significant portion of BPS/IC, has the relatively unaltered urothelium with sparse inflammatory lesions [8]. Histologically, mast cells, inflammatory cells, and cytokines are often detected in BPS/IC bladders; however, these are not found to be specific markers of BPS/IC. Recently, Maeda et al. have shown that inflammatory changes are often seen in BPS/IC patients with Hunner lesion. Substantial lymphoplasmacytic inflammation was observed in 93% of BPS/IC with Hunner lesion, whereas only 8% of BPS/IC without Hunner lesion were inflamed [9]. Also, plasmacytic infiltration was more prominent in BPS/IC with Hunner lesion compared with non-Hunner BPS/IC.
Several factors are considered to be involved in the pathogenesis of BPS/IC [10]. As mentioned above, damaged and leaky urothelium is observed in the BPS/IC bladder, which increases permeability of irritative substances in urine such as urea and potassium, resulting in bladder pain and urinary frequency [11]. Decrement of urothelial growth factor such as heparin-binding epidermal growth factor-like growth factor or antiproliferative factor reduces the urothelial proliferation [12, 13]. Impaired tight junction proteins such as ZO-1, connexin 43, and connexin 45 are also reported to increase bladder permeability [14, 15]. The release of nitric oxide and altered expression of uroplakins are also considered to be the factor of impaired urothelial barrier function in the bladder [16, 17]. Immunological mechanism has also been considered to play roles in pathophysiology of BPS/IC. Several chemokines and cytokines are upregulated in patients with BPS/IC [18, 19]. Furthermore, altered sensory nerve activity may induce the persistence of chronic pelvic pain [20]. At present, pathophysiology of BPS/IC is considered as not dominated by a single factor but is multifactorial, and unfortunately these mechanisms have not been well established for the clinical use such as biomarkers for diagnosis and treatment.
Cystoscopy
Cystoscopy detects the characteristic findings of BPS/IC including Hunner lesion as well as glomerulations with cascade bleeding after hydrodistension. Hunner lesion is a reddish mucosa without the normal capillary structure associated with converging vessels, covering fibrin clots or scars in the vicinity [21••]. Glomerulations have been considered to be an important finding for the diagnosis of bladder-centric BPS/IC; however, according to recent studies, they have a limited association with BPS/IC [22]. Also, the incidence of Hunner lesion varies between 5 and 57% among studies [2]. Thus, cystoscopic diagnosis is often difficult and inconsistent for common urologists [23], while it is useful to rule out other diseases such as bladder cancer. It has been reported that cystoscopic examination assisted by narrow band images may help to find Hunner lesion and other bladder-specific changes in BPS/IC [24•], although the procedure has not been popular. Further development is needed to standardize the cystoscopy-based diagnostic methods, including the cystoscopy atlas, for identification of Hunner lesion and other bladder-specific pathological changes in BPS/IC.
Computed Tomography
CT has often been used in detection of urogenital diseases such as cancer and stones. CT could be used to produce three-dimensional images of the internal body by recording the x-rays. Recent advancement in CT such as a multidetector enables acquisition of multiplanar imaging to obtain sagittal, coronal, and axial images. Dual-source CT using two rotating tubes allows tissue differentiation of tissues as well as renal stones [25]. The technology could detect the unenhanced lesion of bladder cancer. Urothelial tumor was identified on arterial phase, nephrographic-excretory phase, and both combined phases with sensitivity of 91.9%, 83.4%, and 97.3%, respectively [26]. However, these reports did not prove the efficacy of CT to accurately detect the tumor stage including the cancer invasion into the bladder muscle layer. A case report of BPS/IC showed efficacy of the immunosuppressive drug on bladder thickening by CT image [27]. However, at present, CT lacks the sensitivity to detect the changes in the bladder wall and the tissue composition associated with fibrosis compared with MRI (Fig. 1). Therefore, currently, CT is not considered as the diagnostic tool for BPS/IC.
Magnetic Resonance Imaging
MRI is also routinely used for diseases of the urinary tract owing to the progress in this area of technology. MRI obtains the excellent signal contrast resolution especially in the soft tissue compared with CT imaging. The mechanisms of imaging MRI are as follows: the patient is placed on a framework, which produces a magnet field of sufficient strength, and then the free water protons in the patient orient themselves in the equilibrium along the magnetic field’s longitudinal axis. A radio frequency (RF) antenna or coil is placed over the body, which transmits the RF pulses to flip the orientation of protons in the imaged organ of patient. When the RF pulse stops, the magnetic dipoles of water protons return to the equilibrium orientation in the process called relaxation and the moment of magnetic dipoles through the space enclosed by the receiver coils is captured as signal for generating the MR image. The captured signal depends on the tissue characteristics, the physics of the pulse sequence, and the speed of T1 and T2 relaxation time to generate T1 or T2 weighted images. The T1-weighted images are generated by the time taken to return to equilibrium in the z-axis. The T2-weighted images are generated by the time taken to return to equilibrium in the transversal axis. In general, accumulation of fluids in organ appears darker and brighter in T1-weighted and T2-weighted images, respectively. In addition, gadolinium (Gd) is used as a contrast agent in MRI, which shortens the relaxation times of water, resulting in an increase in the enhancement of signal in T1-weighted images.
MRI is advantageous over CT in imaging of the bladder because of the increased signal contrast between bladder muscles and urothelium. This allows for differentiation between invasive and superficial bladder cancer with an accuracy of 85% [28]. The advantage may have the efficacy in evaluating pathological changes in the thin bladder urothelium, which is typical in BPS/IC with Hunner lesion [29•].
Dynamic Contrast-Enhanced MRI
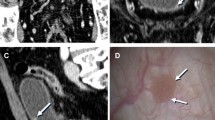
DCE-MRI refers to T1-weighted imaging with Gd-based contrast agents, which can assess vascular permeability and perfusion of the organs such as prostate by obtaining multiple image acquisitions [30]. Rapid image acquisition typical of DCE-MRI is not meant to obtain clear anatomic images; rather, it is used to assess the blood flow and vascular permeability throughout organs over time. The advantage makes it possible to evaluate the staging of bladder cancer (Fig. 2). Rabie et al. compared the DCE-MRI imaging with pathological findings of bladder cancer [31•]. Kappa agreement coefficient between imaging and pathology was 0.7, which was statistically significant. Accuracy of DCE-MRI is dependent on the bladder cancer stage and useful for detecting the tumor progression and the tumor invasion depth [31•]. Hassanien et al. also showed the usefulness of DCE-MRI on evaluation the invasion of bladder cancer [32]: Overall accuracy in tumor staging was 89.5%. In addition, they also stated that DCE-MRI was superior to detect angiogenesis mediated growth and tissue invasion of bladder cancer [32].
Dynamic contrast-enhanced MRI and CT images of bladder cancer. A. Dynamic contrast MRI (coronal image). B. Enhanced CT (coronal image). C. Magnified image of red dotted square in A. D. magnified image of red dotted square in B. Dynamic contrast MRI represents the clear outline of bladder wall compared with the enhanced CT
MRI-Based Diagnosis of BPS/IC
To the best of our knowledge, the investigation of MRI for the BPS/IC was firstly focused on the brain. In a multicenter study, high-resolution T1-weighted MRI [5] and functional MRI (fMRI) of the brain have been used to detect alterations in central pain processing of well-phenotyped BPS/IC patients [33••]. Compared with healthy controls, pain, mood (anxiety), and urological symptoms of BPS/IC, patients were associated with a notably elevated volume of gray matter in the right primary somatosensory cortex, the superior parietal lobule bilaterally, and the right supplementary motor area. [5].
MRI could also detect the pelvic floor hypertonicity including shortened levator ani muscles, increased posterior puborectalis angles, and decreased puborectal distances, which may aggravate pelvic pain in patients with chronic pelvic pain syndrome (CPPS) including BPS/IC [34•].
More recently, Charlanes examined whether diffusion-weighted magnetic resonance imaging (DWMRI) could contribute to the diagnosis of BPS/IC. In this study, BPS/IC patients showed high signal intensity of the bladder wall with a sensitivity of 28% and a specificity of 88%, whereas the control patients showed no signal intensity of the bladder with a sensitivity of 96% and a specificity of 29% [35].
These reports indicate that MRI could detect the alteration in the central nervous system and peripheral organs of the lower urinary tract in patients with BPS/IC, and that it might be useful for the diagnosis and treatment. However, these studies were all retrospective studies with a small sample size; thus, further clinical studies are essential.
The Possibility of Contrast-Enhanced MRI for the Diagnosis of BPS/IC
Bladder permeability is one of the considered etiologies of BPS/IC and holds the potential to become a biological tool for the diagnosis of this disease. Towner et al. examined the usefulness of MRI for detection of increased bladder permeability in experimental models of cystitis induced by intravesical administration of protamine sulfate [6]. Twenty-four hours after instillation, diagnostic contrast-enhanced magnetic resonance imaging (CE-MRI) approach involved administration of Gd-DTPA (0.034-mM Gd-DTPA diluted to 800 μl in saline), administered via an intravesical catheter, for visualization of permeability of the bladder urothelium (bladder contrast images). The enhanced contrast (7 min following Gd-DTPA administration) was able to establish that there was bladder urothelium leakage of the Gd-DTPA contrast agent following protamine sulfate-induced damage of the glycosaminoglycan (GAG) layer in T1-weighted (T1w) horizontal MR images of rat bladders (399.7 ± 68.7% change in MRI signal intensity for protamine sulfate-exposed rats, compared with 39.2 ± 12.2 for controls; p < 0.0001). Based on these animal experiments, they investigated the efficacy of CE-MRI on patients with BPS/IC to evaluate changes in permeability of the bladder urothelium after GD-DTPA intravesical instillation [36•]. Quantitative assessment of MRI signal intensities indicated a significant increase in signal intensity within anterior bladder regions compared with posterior regions in BPS/IC patients, and significant increases in signal intensities within anterior bladder regions and kurtosis (descriptor of shape of probability distribution) and skewness (measure of asymmetry of probability distribution) associated with contrast enhancement in total bladders for BPS/IC patients compared with controls. Regarding symptomatology, BPS/IC cases differed significantly from controls for the questionnaire of lower urinary tract symptoms such as SF-36, Pelvic Pain, Urgency, and Frequency (PPUF) questionnaire and Interstitial Cystitis Problem Index (ICPI) with no overlap in range of scores for each group, indicating that CE-MRI provides an objective, quantifiable measurement of bladder permeability that could be used to stratify bladder pain patients and monitor therapy.
Using similar, but improved methods, our group recently investigated the feasibility of intravesical instillation of contrast mixture (4-mM gadobutrol and 5-mM ferumoxytol) to segment the bladder wall from the bladder lumen in a rat cystitis model [37•]. Gadobutrol, whose molecular weight of 604.71 Da, reaches the extracellular space in the lamina propria to produce T1 contrast, whereas ferumoxytol, superparamagnetic iron oxide nanoparticles, increased contrast of the bladder wall by shortening the T2 in the bladder lumen. Hyperintensity in the bladder wall combined with hypointensity in the lumen is consistent with the increased diffusion of the dissolved Gd-DTPA and simultaneous localization of the larger nanoparticles of ferumoxytol in the lumen. The hyperintense signal in the bladder wall was significantly increased in rat cystitis models compared with normal rats [37•]. Furthermore, the efficacy of the same compounds to visualize the human bladder wall we investigated in 4 BPS/IC patients and 2 controls [7] (Fig. 3). The contrast mixture could increase the contrast on MRI without inducing bladder pain or discomfort. A fourfold increase in the bladder wall contrast-to-noise ratio and very small pixel size with few artifacts significantly allowed accurate determination of bladder wall thinning. The contrast mixture significantly shortened the relaxation time in Hunner-type BPS/IC compared with non-Hunner BPS/IC and control patients, indicating that the compounds could achieve the artifact-free differential contrast and spatial resolution of human bladder wall, which is suitable for measuring bladder wall thickness. These results are promising; however, the sample size is small, and further studies with a large number of BPS/IC patients are needed to establish the evidence.
Quantitative measurement of gadolinium diffusion in the bladder of BPS/IC patients. T1-weighted fast low angle shot (FLASH) images with constant repetition time (TR) of 5.5 milliseconds (ms) at flip angle (FA) of 6° (A, C, and E) and 14° (B, D, and E) demonstrate that gadobutrol-mediated signal enhancement (visible in the right tube containing gadobutrol 4 mM alone) is suppressed by the presence of ferumoxytol (5 mM) in the novel contrast mixture (NCM) tube, as the gadobutrol concentration of 4 mM is the same in both tubes (A and B). T1-weighted FLASH images demonstrates that greater separation of gadobutrol into the bladder wall away from the NCM instilled in the bladder lumen occurs in Hunner-type BPS/IC patients (E and F) than in non-Hunner BPS/IC patient (C and D), which is evident from the dramatic increase in signal intensity in Panel F relative to Panel D at FA of 14°. Catheter used for instillation is shown by C in panel C–H. Constant TR of 5.5 ms at different FAs achieves the stable steady-state conditions necessary for the differences in signal intensity of the same slice to become a function of T1 relaxation time as indicated by the color panel in Panel H. Greater shortening of T1 relaxation time (blue color) in Hunner-type BPS/IC patients is consistent with higher diffusion of gadobutrol into the expanded extracellular matrix of the thickened bladder wall of IC/BPS patients. This figure was modified from a figure in our previous publication [29•]
Conclusion
Recent development of imaging methodologies could obtain clear images of alterations in the bladder and the brain in patients with BPS/IC. These findings could also help understand the pathophysiology of BPS/IC. T1-weighted MRI and fMRI can inform the change in some brain regions associated with chronic pelvic pain of BPS/IC. Contrast-enhanced MRI of the bladder wall has the potential to objectively separate three types of BPS/IC: Hunner-type BPS/IC, non-Hunner BPS/IC, and hypersensitive BPS/IC bladder. The objective classification may establish the tailored treatments for BPS/IC, which could reduce the subjective symptoms. However, more studies are essential to accumulate the evidence for the usage of imaging techniques in clinical settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78. The terminology has been widely used as a standard in worldwide.
Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(Suppl 1):26–34.
Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–70.
Yoshimura N, Oguchi T, Yokoyama H, Funahashi Y, Yoshikawa S, Sugino Y, et al. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21(Suppl 1):18–25.
Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2015;193(1):131–7.
Towner RA, Smith N, Saunders D, Van Gordon SB, Wisniewski AB, Tyler KR, et al. Contrast enhanced magnetic resonance imaging as a diagnostic tool to assess bladder permeability and associated colon cross talk: preclinical studies in a rat model. J Urol. 2015;193(4):1394–400.
Tyagi P, Janicki J, Moon CH, Kaufman J, Chermansky C. Novel contrast mixture achieves contrast resolution of human bladder wall suitable for T1 mapping: applications in interstitial cystitis and beyond. Int Urol Nephrol. 2018;50(3):401–9.
Johansson SL, Fall M. Clinical features and spectrum of light microscopic changes in interstitial cystitis. J Urol. 1990;143(6):1118–24.
Maeda D, Akiyama Y, Morikawa T, Kunita A, Ota Y, Katoh H, et al. Hunner-type (classic) interstitial cystitis: a distinct inflammatory disorder characterized by pancystitis, with frequent expansion of clonal B-cells and epithelial denudation. PLoS One. 2015;10(11):e0143316.
Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology. 2004;63(3 Suppl 1):85–92.
Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg Gynecol Obstet. 1990;171(6):493–6.
Keay S, Warren JW, Zhang CO, Tu LM, Gordon DA, Whitmore KE. Antiproliferative activity is present in bladder but not renal pelvic urine from interstitial cystitis patients. J Urol. 1999;162(4):1487–9.
Kim J, Keay SK, Freeman MR. Heparin-binding epidermal growth factor-like growth factor functionally antagonizes interstitial cystitis antiproliferative factor via mitogen-activated protein kinase pathway activation. BJU Int. 2009;103(4):541–6.
Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171(4):1554–8.
Heinrich M, Oberbach A, Schlichting N, Stolzenburg JU, Neuhaus J. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS One. 2011;6(6):e20792.
Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol. 2005;173(2):625–9.
Zeng Y, Wu XX, Homma Y, Yoshimura N, Iwaki H, Kageyama S, et al. Uroplakin III-delta4 messenger RNA as a promising marker to identify nonulcerative interstitial cystitis. J Urol. 2007;178(4 Pt 1):1322–7 discussion 1327.
Ogawa T, Homma T, Igawa Y, Seki S, Ishizuka O, Imamura T, et al. CXCR3 binding chemokine and TNFSF14 over expression in bladder urothelium of patients with ulcerative interstitial cystitis. J Urol. 2010;183(3):1206–12.
Corcoran AT, Yoshimura N, Tyagi V, Jacobs B, Leng W, Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31(1):241–6.
Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron. 2012;73(3):435–44.
•• Homma Y, Ueda T, Tomoe H, Lin AT, Kuo HC, Lee MH, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int J Urol. 2016;23(7):542–9. The guideline first stated the concept of hypersensitive bladder.
Wennevik GE, Meijlink JM, Hanno P, Nordling J. The role of glomerulations in bladder pain syndrome: a review. J Urol. 2016;195(1):19–25.
Yamada Y, Nomiya A, Niimi A, Igawa Y, Ito T, Tomoe H, et al. A survey on clinical practice of interstitial cystitis in Japan. Transl Androl Urol. 2015;4(5):486–90.
• Ueda T, Nakagawa M, Okamura M, Tanoue H, Yoshida H, Yoshimura N. New cystoscopic diagnosis for interstitial cystitis/painful bladder syndrome using narrow-band imaging system. Int J Urol. 2008;15(12):1039–43. Narrow band imaging techniques enhances the bladder images in cystoscopy.
Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30(4):1037–55.
Hansen C, Becker CD, Montet X, Botsikas D. Diagnosis of urothelial tumors with a dedicated dual-source dual-energy MDCT protocol: preliminary results. AJR Am J Roentgenol. 2014;202(4):W357–64.
Kaneko G, Nishimoto K, Ito Y, Uchida A. The combination therapy of prednisolone and tacrolimus for severe painful bladder syndrome/interstitial cystitis. Can Urol Assoc J. 2012;6(2):E46–9.
Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol. 2005;184(1):121–7.
• Tyagi P, Moon CH, Janicki J, Kaufman J, Chancellor M, Yoshimura N, et al. Recent advances in imaging and understanding interstitial cystitis. F1000 Research 2018;7 (F1000 Faculty Rev):1771. Summary of recent advances in MRI-based diagnosis of urothelila pathophysiology of BPS/IC.
Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198(6):1277–88.
• Rabie E, Faeghi F, Izadpanahi MH, Dayani MA. Role of dynamic contrast-enhanced magnetic resonance imaging in staging of bladder cancer. J Clin Diagn Res. 2016;10(4):TC01–5. Gadolinium enhanced MRI increases accuracy in staging bladder cancer.
Hassanien OA, Abouelkheir RT, Abou El-Ghar MI, Badawy ME, El Gamal SA, El-Hamid MA. Dynamic contrast-enhanced magnetic resonance imaging as a diagnostic tool in the assessment of tumour angiogenesis in urinary bladder cancer. Can Assoc Radiol J. 2019;70:254–63.
•• Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192(3):947–55. The pathophysiology of BPS/IC is associated with some brain lesions.
• Ackerman AL, Lee UJ, Jellison FC, Tan N, Patel M, Raman SS, et al. MRI suggests increased tonicity of the levator ani in women with interstitial cystitis/bladder pain syndrome. Int Urogynecol J. 2016;27(1):77–83. MRI techniques help indentify changes in levator ani muscle tonicity in BPS/IC patients.
Charlanes A, Boudghene F, Chesnel C, Ciofu C, Le Breton F, Jousse M, et al. Diffusion-weighted magnetic resonance imaging: a new tool for the diagnosis of bladder pain syndrome/interstitial cystitis. Urol Int. 2019;102(1):109–12.
• Towner RA, Wisniewski AB, Wu DH, Van Gordon SB, Smith N, North JC, et al. A feasibility study to determine whether clinical contrast enhanced magnetic resonance imaging can detect increased bladder permeability in patients with interstitial cystitis. J Urol. 2016;195(3):631–8. Conrast Enhanced MRI could monitor bladder urothelial permeability.
• Tyagi P, Janicki JJ, Hitchens TK, Foley LM, Kashyap M, Yoshimura N, et al. Novel contrast mixture improves bladder wall contrast for visualizing bladder injury. Am J Physiol Renal Physiol. 2017;313(2):F155–62. Conrast mixture could help to detect changes in bladder permeability in MRI.
Acknowledgments
Authors’ research has been supported by grants from National Institutes of Health (R42DK108397) (P Tyagi) and the Department of Defense (W81XWH-12-1-0565) (N Yoshimura, MB Chancellor).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
T Ogawa, O Ishizuka, P Tyagi, and CJ Chermansky have no conflict of interests. MB Chancellor has conflict of interests related to Allergan, Astellas, Cook, Lipella, Medtronic, Pfizer, and Targacept. N Yoshimura has conflict of interests related to Astellas and Kyorin. T Ueda has conflict of interests related to Kyorin.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on BPS/Interstitial Cystitis
Rights and permissions
About this article
Cite this article
Ogawa, T., Tyagi, P., Ishizuka, O. et al. Recent Developments in Imaging in BPS/IC. Curr Bladder Dysfunct Rep 14, 301–307 (2019). https://doi.org/10.1007/s11884-019-00556-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-019-00556-1