Abstract
Purpose
Early and accurate diagnosis of bladder cancer (BCa) will contribute extensively to the management of the disease. The purpose of this review was to briefly describe the conventional imaging methods and other novel imaging modalities used for early detection of BCa and outline their pros and cons.
Methods
Literature search was performed on Pubmed, PMC, and Google scholar for the period of January 2014 to February 2018 and using such words as “bladder cancer, bladder tumor, bladder cancer detection, diagnosis and imaging”.
Results
A total of 81 published papers were retrieved and are included in the review. For patients with hematuria and suspected of BCa, cystoscopy, and CT are most commonly recommended. Ultrasonography, MRI, PET/CT using 18F-FDG, or 11C-choline and recently PET/MRI using 18F-FDG also play a prominent role in detection of BCa.
Conclusion
For initial diagnosis of BCa, cystoscopy is generally performed. However, cystoscopy cannot accurately detect carcinoma in situ and cannot distinguish benign masses from malignant lesions. CT is used in two modes, CT and computed tomographic urography, both for diagnosis and for staging of BCa. However, they cannot differentiate T1 and T2 BCa. MRI is performed to diagnose invasive BCa and can differentiate muscle invasive bladder carcinoma from non-muscle invasive bladder carcinoma. However, CT and MRI have low sensitivity for nodal staging. For nodal staging, PET/CT is preferred. PET/MRI provides a better differentiation of normal and pathologic structures as compared with PET/CT. Nonetheless none of the approaches can address all issues related for the management of BCa. Novel imaging methods that target specific biomarkers, image BCa early and accurately, and stage the disease are warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BCa) presents as the second most common genitourinary malignancy [1,2,3]. Early and accurate diagnosis can reduce mortality [4]. A typical symptom of BCa is painless hematuria [5]. For initial diagnosis, urine analyses are commonly performed. Imaging plays an important role in assessing the extent of BCa for which computed tomography (CT), ultrasonography (US), magnetic resonance imaging (MRI), and molecular imaging modalities such as positron emission tomography/computed tomography (PET/CT), or positron emission tomography/magnetic resonance imaging (PET/MRI) are used [6, 7]. The purpose of this review was to briefly describe the conventional imaging methods, and new imaging modalities used for imaging BCa and outline their pros and cons. In so doing, a literature search for the past 4 years (January 2014–February 2018) was performed on Pubmed, PMC, and Google scholar, using such words as “bladder cancer and bladder tumor detection, diagnosis and imaging”. A total of 81 published papers were found and are included in this review. The approaches described herein to image suspicious BCa are given in Table 1 and are summarized below.
Cystoscopy
Cystoscopy is performed in seven following different ways.
White light cystoscopy
White light cystoscopy (WLC), a widely available technique, that allows visualization of the mucosa within the bladder, is considered a gold standard method for detecting BCa [8]. There are two forms of WLC, rigid and flexible cystoscopy (FC). Rigid cystoscopy provides better image quality, enables working with a large lumen, and provides improved flow. FC on the other hand allows alternative patient positioning, easy passage, and enables examination of all parts of the bladder [8]. Therefore, FC is usually applied for initial assessment of patients. However, FC may miss up to 10% of papillary tumors. Furthermore, its small working channel lumen does not allow resection of BCa [9]. Although technology has improved the WLC image quality significantly, WLC cannot reliably determine flat and carcinoma in situ (CIS) lesions, and cannot distinguish benign lesions from malignant masses. Such a distinction is particularly important when Transurethral Resection of Bladder (TURB) is to be performed [9,10,11]. However, cystoscopy is recommended by national comprehensive cancer network (NCNN) and American urological association (AUA) guidelines for imaging patients with macroscopic hematuria [5, 12].
Other novel endoscopic visualization techniques briefly described below have been developed to overcome some limitations of WLC. Although these methods are helpful for assessment of BCa, they are invasive, time-consuming, and expensive to perform [9, 13].
Computer-assisted cystoscopy
For computer-assisted cystoscopy, standard cystoscopy images are assessed with color segmentation system. This system provided 100% accurate detection of cancerous tissue. However, false-positive (FP) rate is 50% [11]. Therefore, this method is not commonly used.
Photodynamic diagnosis
Photodynamic diagnosis (PDD), in which intravesical photosensitizing agents are introduced into the bladder to accumulate into the tumor cells, provides a helpful evaluation of BCa in the course of TURB. PDD has a higher sensitivity than WLC for detecting CIS and BCa. PDD-guided TURB has a lower recurrence rate than WLC-guided TURB [14]. However, the effect of PDD on progression-free survival is uncertain [9, 13].
Narrow band imaging
Narrow band imaging (NBI) used for increasing the contrast between the BCa and normal mucosa enables imaging of mucosal vascular structures [9]. NBI can be helpful to determine whether TURB is required. However, whether its use is associated with a lower recurrence rate remains to be verified [13, 15].
Confocal laser endomicroscopy
Used for diagnosis and staging of BCa, confocal laser endomicroscopy (CLE) uses fluorescein, administered intravenously or intravesically, and enables high-resolution cellular imaging. CLE is linked with improved surgery results. However, due to limited optical depth penetration, CLE cannot detect disease involving the muscularis propria [9, 16].
Optical coherence tomography
Optical coherence tomography (OCT) which uses infrared light, distinguishes between the layers of the bladder, and provides imaging of tissue and luminal surfaces with high spatial resolution. Urothelium appears in low intensity, lamina propria in slightly high intensity, but with low intensity for muscularis propria. OCT is useful for evaluation of non-muscle invasive bladder carcinoma (NMIBC), CIS, and recurrent tumor [9].
Storz professional image enhancement system
Storz professional image enhancement system (SPIES) employs a camera with four modes to enhance image quality in different clinical situations. The Clara mode enables homogeneous images; the Chroma mode improves the sharpness of the images; and the Spectra A and B modes provide better color contrast. Analysis of outcomes for SPIES-guided TURB remains a work in-progress [13].
Ultrasonography
There are two types of ultrasonography techniques: (a) two-dimensional ultrasonography and (b) contrast-enhanced ultrasonography.
Two-dimensional ultrasonography
Two-dimensional ultrasonography (2D US) is a widely used and recommended method for evaluating hematuria and for staging, particularly in patients who have allergies to intravenous contrast agents and renal failure (Fig. 1) [5, 17,18,19,20,21]. However, according to some publications, 2D US is not recommended for the staging of BCa, because it may not reveal the true local depth of its invasion [18,19,20,21]. Three-dimensional US provides BCa imaging in multiple planes, and may improve the accuracy of staging BCa [19]. A study evaluating the diagnostic accuracy of ultrasound T staging (UTS), in 152 elderly BCa patients, found that UTS results were comparable with those of histological T staging (HTS) [22]. In a 115 patient study, high conformity (75.7%) was found between the UTS and HTS, with 94.5% accuracy for stage T1. In other stages, the accuracy ranged between 84.9 and 91.8%. 2D US is helpful for differentiating the superficial BCa from muscle invasive bladder carcinoma MIBC and can play an important role in planning treatment [22]. A comparative study between US and cystoscopy in 83 patients with low-grade BCa, matched with propensity score, was calculated from clinicopathological variable factors such as age, gender, tumor multiplicity, size, grade, and intravesical treatment. No significant difference in recurrence rate or in recurrent tumor characteristics was noted [20].
Contrast-enhanced ultrasonography
CEUS is a new technique that uses microbubbles as a ultrasonographic contrast agent to determine the grade and stage of BCa [18]. The use of CEUS was investigated to predict T stage and grade of BCa prior to endoscopic resection in 110 patients suspected of BCa. Results were compared with histology. CEUS had the T stage: Ta sensitivity of 75%, specificity of 95%, T1 sensitivity 65%, specificity 85%, and muscle invasion sensitivity 90% with the specificity of 92% [18]. CEUS differentiated high-grade (n = 110) and low-grade (n = 82) urothelial BCa with 86% sensitivity, 90% specificity, 88% accuracy, 92% positive predictive value (PPV), and 82% negative predictive value (NPV). For high-grade and low-grade BCa, the sensitivity was 85%, specificity 89%, accuracy 88%, and PPV 85%, and NPV was 89% [23]. Similar results were found in another study, in 105 patients (55 low grade and 50 high grade). Time-intensity curve parameters for CEUS showed that the tumor microvessel density can be useful for assessing tumor angiogenesis [24]. Even though new US methods have been developed, the role of US for staging BCa is not yet clearly defined [19].
Computed tomography
CT scan is used in two different modes. The first, computed tomographic urography (CTU) can be performed with or without intravenous contrast agent, applied with sufficient phase to exclude a renal tumor and an excretory phase to assess upper urinary tract. CTU provides imaging of urinary system (the kidneys, ureters, bladder, and urethra) and is especially useful for urinary system pathologies. The second mode of CT scan is the conventional CT; this provides examinations of upper–lower abdomen and pelvis. CT is commonly used and is recommended method for staging BCa (Fig. 2) [5, 17, 21, 25, 26].
Transaxial CT image through the bladder obtained at an 8 min delay following intravenous contrast administration. The irregular round filling defect in the right posterior aspect of the bladder (arrow) represents a transitional cell carcinoma. There is thickening of the adjacent bladder wall, but not definite spread beyond the bladder
Computed tomographic urography
In a study of 687 patients, 710 CTU were evaluated to detect BCa. CTU had 91.5% (650/710) accuracy, 86.3% (82/95) sensitivity, 92.4% (568/615) specificity, and 63.6% (82/129) PPV. Some false-positive results (n = 47) were reported due to misinterpretation of images [27].
CTU with enhancement-triggered scan had the highest sensitivity and NPV in corticomedullary phase (CMP). Therefore, CMP is recommended for bladder evaluation in patients with gross hematuria [28, 29]. CTU with enhancement-triggered scan and FC was compared in 435 patients to detect BCa. Both methods detected BCa in 48 patients. CTU had 87% sensitivity, 99% specificity, 91% PPV, and 98% NPV, while FC had 87% sensitivity, 10% specificity, 98% PPV, and 98% NPV [30].
Diagnostic performance of CTU was compared with cystoscopy in 177 patients. CTU performed better with 96.3% sensitivity, 86.4% specificity, 92.8% diagnostic accuracy, 92.9% PPV, and 92.7% NPV. The arterial acquisition phase diagnosed the lesions with the highest accuracy, and demonstrated 93.4% of all lesions [31].
Computed tomography
CT of 231 BCa patients, following radical cystectomy (RC), and pelvic lymphadenectomy, had 93.6% specificity and 52.6% sensitivity. For local staging, the accuracy was 78%. Overstaging was low (6%) [32].
In a study of 206 patients with invasive BCa, increased bladder wall thickness, lymph node (LN) > 5 mm in size, and associated with high risk of death were imaged by CT. Results were helpful for prognostic information and MIBC management [25].
CT is faster and more cost-effective than MRI, but it is associated with the risk of ionizing radiation, high interobserver variability, and can neither differentiate bladder wall muscle layers, nor can it reliably distinguish T1 from T2 disease. Furthermore, its specificity and sensitivity are low for extravesical extension of early stage BCa and small metastatic lesions of BCa. For nodal metastasis in BCa, sensitivity of CT is low. CT may fail to detect nearly 40% of LN metastases [17, 21, 25, 26, 32,33,34,35].
Dual energy spectral CT is a relatively new method that provides multiparameteric imaging of the urinary system. On the monochromatic images, a threshold value of 73.4 Hounsfield unit demonstrated high sensitivity (77.0%) and specificity (82.5%) for differentiating posterior wall BCa from benign prostate hypertrophy [36].
Magnetic resonance imaging
MRI is used for preoperative staging and nodal imaging (Fig. 3) of BCa for management of T2 or more advanced disease. MR urography (MRU), which can be performed without use of contrast agent, is a suitable method for imaging patients with renal failure or those with allergies for iodinated contrast agent. Advanced MRI protocols described below provide functional information and can improve the efficacy for imaging BCa [5, 34, 37].
Morphologic MRI
MRI, unlike CT does not use ionizing radiation, offers superior soft tissue contrast, and provides more anatomical and functional information [19]. MRI also differentiates MIBC from NMIBC, and visualizes extramural invasion, T3b and T4 disease [37].
Bladder distension level during MRI affects interpretation of the images [34]. T1-weighted (T1W)-MRI visualizes perivesical fat tissue infiltration, pelvic lymphadenopathy, and bone metastasis. The detrusor muscle and BCa present similar signal intensity, and compromises the differentiation of bladder wall invasion on T1W-MRI without contrast. T2-weighted (T2W) MRI is superior to T1W-MRI for differentiation of NMIBC from MIBC. Furthermore, T2W-MRI can distinguish urine from the intraluminal BCa [19, 34]. 3 Tesla (3 T) MRI is better than 1.5 Tesla (1.5 T) MRI with regard to resolution, differentiation of tumor from normal tissues, and determination of the depth of tumor invasion [33]. MRI, however, cannot provide detailed tissue characterization and can overestimate the degree of bladder wall invasion after TURB or chemo-radiotherapy [34].
Split-bolus CTU, MR urography (MRU), and FC were compared in 150 patients for diagnosis of BCa which were verified with histopathology. CTU detected tumors with 61.5% sensitivity, 94.9% specificity, 53.3% PPV, and 96.3% NPV, while MRU detected tumors with 79.9% sensitivity and 93.4% specificity, 52.6% PPV and NPV 97.1%. The number of bladder lesions detected using FC was 32, MRU 19, and CTU 15. Split-bolus CTU or MRU cannot replace cystoscopy in patients with hematuria [38].
A review of 24 publications for diagnostic performance for local staging of MIBC with pathologic confirmation showed that 3 T-MRI had higher specificity (93%) than those using 1.5 T-MRI (83%). Studies using multiparametric MRI (conventional + ≥2 functional sequences) showed the highest accuracy with sensitivity of 94% and specificity of 95% [39].
Diffusion-weighted (DW) MRI
DW-MRI is driven by the Brownian motion of water molecules that changes the apparent diffusion coefficient (ADC) value between normal tissue and tumor [19, 34, 40, 41]. The ADC value correlates with cell cycle and proliferative biomarkers [42, 43].
DW-MRI and dynamic contrast-enhanced (DCE)-MRI at 3 T were investigated for aggressiveness of BCa in 59 patients. The combination of ADC and wash-out rate determined the BCa aggressiveness with 96.7% sensitivity, 94.9% specificity, and 95.7% accuracy [44]. In addition, ADC was useful for determining the recurrence and progression risk of BCa [19, 41, 45,46,47]. DWI-MRI, however, helps distinguish benign and malignant bladder lesions, for staging, and for the assessment of efficacy of chemo-radiotherapy treatment [37]. DW-MRI does not require contrast agent; therefore, DW-MRI can be used in patients with renal failure or allergies to contrast agents [40].
Qualitative and quantitative imaging characteristics of MRI and DWI-MRI were compared for detecting pelvic LNs in 36 BCa patients prior to cystectomy. Results were correlated with histopathology. The short axis (> 5 mm) LN imaging had 88% sensitivity and 75% specificity, and the long axis (> 6 mm) LN imaging had 88% sensitivity and 71% specificity. ADC (< 1.35 mm and normalized to muscle) had 75% sensitivity and 68% specificity, and in the absence of fatty hilum, the sensitivity and specificity were 75 and 71%, respectively [41].
Normalized ADC (nADC) of tumor, which was calculated by ADC(tumor)/ADC (reference tissue) using urine in the bladder and from muscles as a reference, was superior to ADC for estimating grade of BCa [48].
The accuracy of differentiating muscle invasion and perivesical fat invasion was found higher with DW-MRI and MRI combined, than MRI alone, for T staging in 160 patients [49]. Correlative values between ADC and clinicopathological parameters, such as tumor diameter, grade, and T stage, were also examined. DWI-MRI provided a better tissue contrast than T1-MRI and T2-MRI.
3TDW-MRI was superior to T2W-MRI for delineating the T stage, for differentiating T1 tumor from T2 or more severe disease, and for showing stalks of BCa [50].
DW-MRI diagnosed BCa with 95% sensitivity and 85% specificity and differentiated MIBC from NMIBC with 85% sensitivity and 90% specificity [51].
For differentiating residual BCa from postoperative changes before a second TURB, in 75 patients, T2W-MRI had 100% sensitivity, only 18% specificity, and 53% accuracy. DCE-MRI had 100% sensitivity, 12% specificity, 50% accuracy and DW-MRI had 92% sensitivity, 82% specificity, and 87% accuracy [52].
The use of DW-MRI was investigated for differentiating recurrent tumor from chronic inflammation and fibrosis after surgery. For detecting recurrent tumors the accuracy, sensitivity, specificity, and PPV of DW-MRI were 92.6, 100, 81.8, and 88.9%, respectively, which were higher than those of DCE-MRI (59.3, 81.3, 27.3, and 54.2%, respectively). The nADC of recurrent tumors was significantly lower than those of postoperative inflammation or fibrosis [53].
Furthermore, DW-MRI visualized small LN metastases in normal-sized LNs that conventional imaging modalities would have missed [54]. ADC is a promising parameter for estimation of BCa stage and grade with high sensitivity and specificity [55]. However, DW-MRI has several disadvantages, including low tumor specificity and poor spatial resolution [56].
Dynamic contrast-enhanced MRI
Dynamic contrast-enhanced MRI (DCE-MRI), which uses paramagnetic contrast agents, is helpful for depicting tumor vascularity, ischemia–necrosis, and mass in the bladder lumen on delayed-phase images. DCE-MRI utilizes T1W sequences providing high-resolution images for detection of BCa. DCE-MRI is helpful for predicting recurrence and chemotherapeutic response [34, 57]. DCE-MRI has strong interobserver agreement and provides high accuracy for distinguishing MIBC from NMIBC [33].
Compared with conventional T2W-MRI alone, the addition of multitransmit 3T-DCE-MRI significantly improved interobserver agreement, and the characterization of BCa especially small malignant tumors and tumors within areas of bladder wall thickening. 3T-DCE-MRI mapping is a promising method to augment BCa imaging beyond the limitations of cystoscopy and CT [33].
The performance of DCE-MRI was evaluated for histological response after chemotherapy on localized urothelial BCa in 12 patients. DCE-MRI was performed prior to chemotherapy to measure the size, thickness, relative enhancement at the arterial and venous phases of each tumor. DCR-MRI helpfully increased selection of patients for localized BCa surgery [58].
Lymphotropic nanoparticle enhanced MRI
Lymphotropic nanoparticle enhanced MRI (LNMRI) uses ultrasmall paramagnetic iron oxide particles. When administered intravenously, they reach LNs through the lymphatics and differentiate benign and malignant LNs. Additional clinical studies are required to determine the role of this method for BCa [59].
Multiparametric MRI
Multiparametric MRI (mpMRI) is composed of T1W-MRI, T2W-MRI, and functional MRI methods, including DCE-MRI and DW-MRI. mpMRI combines anatomic and functional MRI sequences and plays a role for detection, staging, and local recurrence of BCa [59]. In a recent study, efficacy of mpMRI for staging of BCa after TURB in 45 patients was examined. mpMRI was found both sensitive (92%) and specific (84%) method for MIBC detection. The investigators concluded that mpMRI may be helpful for local staging of BCa after TURB [60]. However, to assess its efficacy carefully, clinical studies in large patient groups are required.
PET/CT
18F-FDG-PET/CT
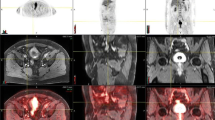
PET/CT is used in oncology for staging, restaging, examining early recurrence, and assessing prognosis. 18Fluorine-2-deoxy-2-fluorodeoxyglucose (18F-FDG), the most commonly used agent in PET/CT imaging [61], is excreted through the kidneys. Therefore, differentiation of bladder pathology or LNs from physiological 18F-FDG activity is difficult (Fig. 4). Forced diuresis may be used to reduce physiological 18F-FDG uptake in the bladder [35, 61].
78-year-old man with large papillary tumor on left lateral wall and high grade, nonpapillary tumor involving the left trigone just lateral to left UO and posterior left bladder neck on cystourethroscopy. Due to concentration of 18F-FDG in the urinary bladder, any increased activity of the thickened bladder wall (arrow) cannot be determined on the PET/CT image of the pelvis a. Asymmetric thickening of the left urinary bladder wall (arrow), on the pelvis CT part of PET/CT imaging (b) and it likely corresponds to known bladder malignancy. Along the left pelvic sidewall, there is a 2.4 × 1.3 cm hypermetabolic lymph node with a maximum SUV of 6.17 (c)
The sensitivity of 18F-FDG-PET/CT was 56% and the specificity was 98% for nodal metastases as confirmed by histology in 78 patients with BCa scheduled for RC (radical cystectomy). PET/CT was more accurate than CT alone for staging BCa [35].
For initial staging after transurethral biopsy (n = 34), PET/CT sensitivity was 87.5%, specificity was 80%, and accuracy was 82%. For CT sensitivity was 66%, specificity 57%, and accuracy was 60%. For restaging BCa (n = 43), PET/CT had 85% sensitivity, 60% specificity, and 70% accuracy. CT, for staging, had 80% sensitivity, 50% specificity, and 58% accuracy. The maximum standardized uptake value (SUVmax) for primary tumor, ranged from 5.7 to 30.4 and for LN metastases ranged from 3.5 to 13.8. Both PET/CT and CT primary tumor detection were 88% and were confirmed by histopathology [62].
18F-FDG-PET/CT and CT with histopathological examination of LNs were compared in 54 locally advanced BCa. PET/CT had 86% specificity, 58% PPV, and 76% NPV, while CT alone had 89% specificity, 64% PPV, and 77% NPV. Both methods had 41% sensitivity for LN metastasis [63].
Role of 18F-FDG-PET/CT as an indicator of response to chemotherapy was investigated in patients with oligometastatic BCa. PET/CT was performed before and after chemotherapy, and results were confirmed with histology. PET/CT predicted histological nodal chemotherapy response in 37 of 43 patients (86%) with LN metastasis following lymphadenectomy. For response, FDG-PET/CT had 100% sensitivity, (37 out of 37), 17% specificity, (1 out of 6), 88% PPV (37 out of 42), and 100% NPV. (1 out of 1) [64].
Preoperative 18F-FDG-PET/CT imaging demonstrated more malignant findings than CT in 47% of high-risk MIBC patients. PET/CT also changed the therapy plan for 27% patients [65].
Extravesical 18F-FDG avid lesions, suspicious for malignancy on PET/CT, were correlated with mortality in 211 patients with MIBC. Data suggested that 18F-FDG may be an independent prognostic indicator of mortality [66].
SUVmax of early dynamic imaging with 18F-FDG was independent of the SUVmax of delayed images. High-grade tumors demonstrated higher SUVmax than low-grade tumors in the early dynamic imaging in pT1 tumors. Non-invasive pTa tumors had significantly lower SUVmax than higher stage tumors during early dynamic imaging [67].
18F-FDG-PET/CT was compared with CT for LN staging in high-grade T1 tumor (n = 9) or MIBC (n = 52). On a patient-based analysis, PET/CT had 47.1% sensitivity, 93.2% specificity, 72.7% PPV, and 82.0% NPV, whereas CT had 29.4% sensitivity, 97.7% specificity, 78.2% PPV, and 78.2% NPV. PET/CT had low sensitivity for LN staging of MIBC and it did not increase diagnostic accuracy of CT for LN metastasis. This can be affected by various factors, such as patient population, extent of LN dissection, and cut-off value of SUV [68].
PET/CT was compared with CT to investigate pelvic LN and distant metastasis in patients with MIBC or high-risk NMIBC. Results were compared with histology. For detecting distant metastasis (n = 207), sensitivity of PET and CT was 54 and 41%, respectively. Both scans had similar specificities of 97 and 98%. For pelvic LN involvement (n = 93), the CT scan had 46% sensitivity and 98% specificity and PET/CT scan had 68% sensitivity and 95% specificity. Therefore, dual imaging should be performed but only in selected patients [69].
Carbon(C)-11-choline-PET/CT
11C-choline-PET/CT is used for staging of BCa. The main advantage of 11C-choline is its low urinary excretion. Its sensitivity is higher than 18F-FDG for showing local relapse of BCa [70].
For relapse of BCa, 11C-choline-PET/CT had 66.7% sensitivity, 84.6% specificity, 76% accuracy, 80% PPV, and 73.3% NPV. For imaging LNs and distant relapse, this method had 90% sensitivity, 93.3% specificity, 92% accuracy, 90% PPV, and 93.3% NPV. No FP or FN was detected [70].
Tabulating overall survival (OS) and cumulative incidence of cancer-specific death (CSD), the prognostic value of 11C-choline-PET/CT was compared with CT and with histology for preoperative staging in 44 BCa patients. The imaging results were in concordance with OS and cumulative incidence of CSD. No statistically significant difference was found in OS and CSD between the patient groups for organ-limited vs. non-organ-limited disease or LN involvement. 11C-choline-PET/CT may play a role to predict prognosis [71].
The role of 11C-choline-PET/CT with Contrast-Enhanced Computed Tomography (CECT) was compared for preoperative LN staging in 26 BCa patients and results were confirmed with pathology. On a patient-based analysis, PET/CT had 42% sensitivity and 84% specificity, while CECT had 14% sensitivity and 89% specificity. On a region-based analysis, PET/CT had 11% sensitivity and 82% specificity, while CECT had 5% sensitivity and 80% specificity. On a lesion-based analysis, PET/CT had 10% sensitivity and 64% specificity and CECT had sensitivity 2% and specificity 63% [72].
11C-choline-PET/CT was compared with histology for preoperative LN evaluation in 59 BCa patients. On a regional-based analysis, PET/CT was positive for primary cancer and/or local relapse in bladder bed in 54.2% of the patients. Pathological LN uptake was found in 23.7% of the patients. For LN metastasis detection, PET/CT had 59% sensitivity, 90% specificity, 71% PPV, 84% NPV, and 81% accuracy [73]. A longer lived 18F-choline has not yet been deeply investigated for imaging BCa.
PET/MRI
A relatively recent modality, PET/MRI provides advantages of the high resolution of MRI and metabolic information of PET. PET/MRI differentiates normal and pathologic structures more clearly than PET/CT and facilitates coregistration of the bladder wall, bladder pathologies, and pelvic LNs. Therefore, PET/MRI is considered to be helpful for the management of BCa [74,75,76].
Diagnostic performance of MRI and 18F-FDG-PET/MRI using a diuresis protocol was compared in 22 BCa patients. At a score of 3, PET/MRI provided higher accuracy for detection of BCa (86 vs. 77%), pelvic LNs (95 vs. 76%), and nonnodal pelvic malignancy (100 vs. 91%). Additional PET was useful for showing exact level of suspicious nodal/nonnodal findings in the pelvis which were equivocal on MRI alone [74].
A PET/MRI study noted improved bladder wall coregistration and slightly increased detection of bladder masses and pelvic LNs [77].
18F-FDG-PET/MRI was investigated for local or metastatic staging of 11 BCa patients. With T1W and T2W images of the pelvis, MRI was helpful for staging, concluding that PET/MRI increased diagnostic utility of PET for the management of MIBC [78].
18F-sodium fluoride PET/CT and bone scintigraphy
18F-Sodium Fluoride (NaF) is a positron emitting radiopharmaceutical used for PET/CT imaging of bone. 18F-NaF-PET/CT has higher sensitivity and better image quality than bone scintigraphy, making it superior for the detection of osseous metastasis of BCa [79, 80]. Bone scintigraphy is recommended for MIBC patients with suspicious bone metastasis [5]. Preoperative bone imaging is associated with improved survival of MIBC, better patient selection for surgery [81].
Conclusion
Great strides have been made for imaging BCa. Nonetheless, current methods suffer from inaccurate detection of BCa and metastatic lesions. Cystoscopy is recommended for patients with painless hematuria. However, cystoscopy cannot accurately show CIS and has difficulty differentiating benign masses from malignant lesions, especially before TURB. Novel cystoscopy techniques are promising for imaging BCa, but further clinical evaluation is needed. US is performed for symptomatic patients to exclude urinary system pathologies. CT is preferred for staging, but cannot differentiate T1 and T2 BCa. MRI can differentiate MIBC from NMIBC and has improved efficacy for local staging. However, MRI is not suitable for patients who have prosthesis or renal failure and is more time-consuming than CT. These results are summarized in Table 2. Both CT and MRI have low sensitivity for nodal staging, where PET/CT plays a leading role. PET/CT is also important for restaging and assessing treatment response. For PET/CT, 11C-choline may be an agent of choice, since it has low urinary excretion, and higher sensitivity for depicting local relapse. However, the half-life of 11C-choline is short, and requires an in house cyclotron to produce it. These results are summarized in Table 3. 18F-NaF is useful for assessment of blastic bone metastasis. Bone scintigraphy is recommended before TURB. PET/MRI provides better differentiation of normal and pathologic structures as compared with PET/CT. PET/MRI is a relatively novel modality to image BCa. However, further studies are needed to evaluate its full potential.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUA:
-
American urological association
- BCa:
-
Bladder cancer
- 11C:
-
11Carbon
- CEUS:
-
Contrast-enhanced ultrasonography
- CECT:
-
Contrast-enhanced computed tomography
- CIS:
-
Carcinoma in situ
- CLE:
-
Confocal laser endomicroscopy
- CMP:
-
Corticomedullary phase
- CSD:
-
Cumulative incidence of cancer-specific death
- CT:
-
Computed tomography
- CTU:
-
Computed tomographic urography
- 2D US:
-
Two-dimensional ultrasonography
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- DW-MRI:
-
Diffusion-weighted magnetic resonance imaging
- FC:
-
Flexible cystoscopy
- 18F-FDG:
-
18Fluorine-2-deoxy-2-fluorodeoxyglucose
- FP:
-
False positive
- HTS:
-
Histological T staging
- LN:
-
Lymph node
- LNMRI:
-
Lymphotropic nanoparticle enhanced MRI
- mpMRI:
-
Multiparametric MRI
- MRI:
-
Magnetic resonance imaging
- MRU:
-
Magnetic resonance urography
- nADC:
-
Normalized ADC
- NaF:
-
Sodium fluoride
- NBI:
-
Narrow band imaging
- NCNN:
-
National comprehensive cancer network
- NMIBC:
-
Non-muscle invasive bladder cancer
- MIBC:
-
Muscle invasive bladder cancer
- NPV:
-
Negative predictive value
- OCT:
-
Optical coherence tomography
- OS:
-
Overall survival
- RC:
-
Radical cystectomy
- PDD:
-
Photodynamic diagnosis
- PET/CT:
-
Positron emission tomography/computed tomography
- PET/MRI:
-
Positron emission tomography/magnetic resonance imaging
- PPV:
-
Positive predictive value
- SPIES:
-
Storz professional image enhancement system
- SUVmax :
-
Maximum standardized uptake value
- 1.5 T:
-
1.5 Tesla
- 3 T:
-
3 Tesla
- TURB:
-
Transurethral resection of bladder
- T1W-MRI:
-
T1-weighted magnetic resonance imaging
- T2W-MRI:
-
T2-weighted magnetic resonance imaging
- UTS:
-
Ultrasound T staging
- US:
-
Ultrasonography
- WLC:
-
White light cystoscopy
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F (2017) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 71:96–108. https://doi.org/10.1016/j.eururo.2016.06.010
National Cancer Institute. Surveillance, epidemiology, and end results program. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 1 Nov 2017
Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, Lotan Y (2014) The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 66:253–262. https://doi.org/10.1016/j.eururo.2014.01.006
Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, Downs TM, Efstathiou JA, Flaig TW, Friedlander T, Greenberg RE, Guru KA, Hahn N, Herr HW, Hoimes C, Inman BA, Jimbo M, Kader AK, Lele SM, Meeks JJ, Michalski J, Montgomery JS, Pagliaro LC, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Preston MA, Sexton WJ, Siefker-Radtke AO, Sonpavde G, Tward J, Wile G, Dwyer MA, Gurski LA (2017) Bladder Cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:1240–1267. https://doi.org/10.6004/jnccn.2017.0156
National Collaborating Center for Cancer. Bladder cancer: diagnosis and management. https://www.ncbi.nlm.nih.gov/books/NBK305022/pdf/Bookshelf_NBK305022.pdf. Accessed 1 Nov 2017
Sherif A, Garske U, de La Torre M, Thörn M (2006) Hybrid SPECT-CT: an additional technique for sentinel node detection of patients with invasive bladder cancer. Eur Urol 50:83–91. https://doi.org/10.1016/j.eururo.2006.03.002
Krajewski W, Zdrojowy R, Wojciechowska J, Kościelska K, Dembowski J, Matuszewski M, Tupikowski K, Małkiewicz B, Kołodziej A (2016) Patient comfort during flexible and rigid cystourethroscopy. Videosurg Miniinv 11:94–97. https://doi.org/10.5114/wiitm.2016.60665
Soubra A, Risk MC (2015) Diagnostics techniques in nonmuscle invasive bladder cancer. Indian J Urol 31:283–288. https://doi.org/10.4103/0970-1591.166449
Degeorge KC, Holt HR, Hodges SC (2017) Bladder cancer: diagnosis and treatment. Am Fam Physician 96:507–514
Gosnell ME, Polikarpov DM, Goldys EM, Zvyagin AV, Gillatt DA (2018) Computer-assisted cystoscopy diagnosis of bladder cancer. Urol Oncol 36:8.e9–8.e15. https://doi.org/10.1016/j.urolonc.2017.08.026
American Urological Association. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults. https://auanet.org/guidelines/asymptomatic-microhematuria-(2012-reviewed-and-validity-confirmed-2016). Accessed 26 Apr 2018
Mari A, Abufaraj M, Gust KM, Shariat SF (2017) Novel endoscopic visualization techniques for bladder cancer detection: a review of the contemporary literature. Curr Opin Urol. https://doi.org/10.1097/MOU.0000000000000459
Lee JY, Cho KS, Kang DH, Do Jung H, Kwon JK, Oh CK, Ham WS, Choi YD (2015) A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer 15:566. https://doi.org/10.1186/s12885-015-1571-8
Hirner L, Stagge E, Rübben H, Schenck M, Eisenhardt A (2016) Narrow band imaging-assisted cystoscopy in bladder tumor follow-up: can more tumors be identified? Urol A 55:370–375. https://doi.org/10.1007/s00120-015-3942-9
Chen SP, Liao JC (2014) Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: a new era of optical diagnosis? Curr Urol Rep 15:437. https://doi.org/10.1007/s11934-014-0437-y
Lee CH, Tan CH, Faria SC, Kundra V (2017) Role of imaging in the local staging of urothelial carcinoma of the bladder. Am J Roentgenol 208:1193–1205. https://doi.org/10.2214/AJR.16.17114
Gupta VG, Kumar S, Singh SK, Lal A, Kakkar N (2016) Contrast enhanced ultrasound in urothelial carcinoma of urinary bladder: an underutilized staging and grading modality. Cent Eur J Urol 69:360–365. https://doi.org/10.5173/ceju.2016.893
McKibben MJ, Woods ME (2015) Preoperative imaging for staging bladder cancer. Curr Urol Rep 16:22. https://doi.org/10.1007/s11934-015-0496-8
Niwa N, Matsumoto K, Hayakawa N, Ito Y, Maeda T, Akatsuka S, Masuda T, Nakamura S, Tanaka N (2015) Comparison of outcomes between ultrasonography and cystoscopy in the surveillance of patients with initially diagnosed TaG1-2 bladder cancers: a matched-pair analysis. Urol Oncol 33:386.e15–386.e21. https://doi.org/10.1016/j.urolonc.2015.04.018
Srivastava A, Douglass LM, Chernyak V, Watts KL (2017) Advances in imaging in prostate and bladder cancer. Curr Urol Rep 18:69. https://doi.org/10.1007/s11934-017-0718-3
Tadin T, Sotosek S, Rahelić D, Fuckar Z (2014) Diagnostic accuracy of ultrasound T-staging of the urinary bladder cancer in comparison with histology in elderly patients. Coll Antropol 38:1123–1126
Li Q, Tang J, He E, Li Y, Zhou Y, Wang B (2017) Differentiation between high- and low-grade urothelial carcinomas using contrast enhanced ultrasound. Oncotarget 8:70883–70889. https://doi.org/10.18632/oncotarget.20151
Guo S, Xu P, Zhou A, Wang G, Chen W, Mei J, Xiao F, Liu J, Zhang C (2017) Contrast-enhanced ultrasound differentiation between low- and high-grade bladder urothelial carcinoma and correlation with tumor microvessel density. J Ultrasound Med 36:2287–2297. https://doi.org/10.1002/jum.14262
Schmid SC, Zahel T, Haller B, Horn T, Metzger I, Holzapfel K, Seitz AK, Gschwend JE, Retz M, Maurer T (2016) Prognostic value of computed tomography before radical cystectomy in patients with invasive bladder cancer: imaging predicts survival. World J Urol 34:569–576. https://doi.org/10.1007/s00345-015-1654-9
Raman SP, Fishman EK (2014) Bladder malignancies on CT: the underrated role of CT in diagnosis. Am J Roentgenol 203:347–354. https://doi.org/10.2214/AJR.13.12021
Trinh TW, Glazer DI, Sadow CA, Sahni VA, Geller NL, Silverman SG (2017) Bladder cancer diagnosis with CT urography: test characteristics and reasons for false-positive and false-negative results. Abdom Radiol 43:663–671. https://doi.org/10.1007/s00261-017-1249-6
Helenius M, Dahlman P, Magnusson M, Lönnemark M, Magnusson A (2014) Contrast enhancement in bladder tumors examined with CT urography using traditional scan phases. Acta Radiol 55:1129–1136. https://doi.org/10.1177/0284185113513762
Helenius M, Dahlman P, Lonnemark M, Brekkan E, Wernroth L, Magnusson A (2016) Comparison of post contrast CT urography phases in bladder cancer detection. Eur Radiol 26:585–591. https://doi.org/10.1007/s00330-015-3844-7
Helenius M, Brekkan E, Dahlman P, Lönnemark M, Magnusson A (2015) Bladder cancer detection in patients with gross haematuria: computed tomography urography with enhancement-triggered scan versus flexible cystoscopy. Scand J Urol 49:377–381. https://doi.org/10.3109/21681805.2015.1026937
Capalbo E, Kluzer A, Peli M, Cosentino M, Berti E, Cariati M (2015) Bladder cancer diagnosis: the role of CT urography. Tumori 101:412–417. https://doi.org/10.5301/tj.5000331
Horn T, Zahel T, Adt N, Schmid SC, Heck MM, Thalgott MK, Hatzichristodoulou G, Haller B, Autenrieth M, Kübler HR, Gschwend JE, Holzapfel K, Maurer T (2016) Evaluation of computed tomography for lymph node staging in bladder cancer prior to radical cystectomy. Urol Int 96:51–56. https://doi.org/10.1159/000440889
Nguyen HT, Pohar KS, Jia G, Shah ZK, Mortazavi A, Zynger DL, Wei L, Clark D, Yang X, Knopp MV (2014) Improving bladder cancer imaging using 3-T functional dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 49:390–395. https://doi.org/10.1097/RLI.0000000000000022
Malayeri AA, Pattanayak P, Apolo AB (2015) Imaging muscle-invasive and metastatic urothelial carcinoma. Curr Opin Urol 25:441–448. https://doi.org/10.1097/MOU.0000000000000208
Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G, Konety BR (2016) The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol 34:1229–1237. https://doi.org/10.1007/s00345-016-1772-z
Chen A, Liu A, Liu J, Tian S, Wang H, Liu Y (2016) Application of dual-energy spectral CT imaging in differential diagnosis of bladder cancer and benign prostate hyperplasia. Medicine (Baltimore) 95:e5705. https://doi.org/10.1097/MD.0000000000005705
Hoosein MM, Rajesh A (2014) MR imaging of the urinary bladder. Magn Reson Imaging Clin N Am 22:129–134. https://doi.org/10.1016/j.mric.2014.01.001
Gandrup KL, Løgager VB, Bretlau T, Nordling J, Thomsen HS (2015) Diagnosis of bladder tumours in patients with macroscopic haematuria: a prospective comparison of split-bolus computed tomography urography, magnetic resonance urography and flexible cystoscopy. Scand J Urol 49:224–229. https://doi.org/10.3109/21681805.2014.981203
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2017) Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: a systematic review and meta-analysis. Eur J Radiol 95:46–55. https://doi.org/10.1016/j.ejrad.2017.07.021
Yoshida S, Koga F, Masuda H, Fujii Y, Kihara K (2014) Role of diffusion-weighted magnetic resonance imaging as an imaging biomarker of urothelial carcinoma. Int J Urol 21:1190–1200. https://doi.org/10.1111/iju.12587
Wollin DA, Deng FM, Huang WC, Babb JS, Rosenkrantz AB (2014) Conventional and diffusion-weighted MRI features in diagnosis of metastatic lymphadenopathy in bladder cancer. Can J Urol 21:7454–7459
Kobayashi S, Koga F, Kajino K, Yoshita S, Ishii C, Tanaka H, Saito K, Masuda H, Fujii Y, Yamada T, Kihara K (2014) Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging 39:172–178. https://doi.org/10.1002/jmri.24148
Sevcenco S, Haitel A, Ponhold L, Susani M, Fajkovic H, Shariat SF, Hiess M, Spick C, Szarvas T, Baltzer PA (2014) Quantitative apparent diffusion coefficient measurements obtained by 3-tesla MRI are correlated with biomarkers of bladder cancer proliferative activity. PLoS One 9:e106866. https://doi.org/10.1371/journal.pone.0106866
Zhou G, Chen X, Zhang J, Zhu J, Zong G, Wang Z (2014) Contrast-enhanced dynamic and diffusion-weighted MR imaging at 3.0 T to assess aggressiveness of bladder cancer. Eur J Radiol 83:2013–2018. https://doi.org/10.1016/j.ejrad.2014.08.012
Kikuchi K, Shigihara T, Hashimoto Y, Miyajima M, Haga N, Kojima Y, Shishido F (2017) Apparent diffusion coefficient on magnetic resonance imaging (MRI) in bladder cancer: relations with recurrence/progression risk. Fukushima J Med Sci 63:90–99. https://doi.org/10.5387/fms.2017-05
Suo ST, Chen XX, Fan Y, Wu LM, Yao QY, Cao MQ, Liu Q, Xu JR (2014) Histogram analysis of apparent diffusion coefficient at 3.0 T in urinary bladder lesions: correlation with pathologic findings. Acad Radiol 21:1027–1034. https://doi.org/10.1016/j.acra.2014.03.004
Yoshida S, Koga F, Kobayashi S, Tanaka H, Satoh S, Fujii Y, Kihara K (2014) Diffusion-weighted magnetic resonance imaging in management of bladder cancer, particularly with multimodal bladder-sparing strategy. World J Radiol 6:344–354. https://doi.org/10.4329/wjr.v6.i6.344
Wang HJ, Pui MH, Guo Y, Li SR, Liu MJ, Guan J, Zhang XL, Feng Y (2014) Value of normalized apparent diffusion coefficient for estimating histological grade of vesical urothelial carcinoma. Clin Radiol 69:727–731. https://doi.org/10.1016/j.crad.2014.03.001
Yamada Y, Kobayashi S, Isoshima S, Arima K, Sakuma H, Sugimura Y (2014) The usefulness of diffusion-weighted magnetic resonance imaging in bladder cancer staging and functional analysis. J Cancer Res Ther 10:878–882. https://doi.org/10.4103/0973-1482.138225
Ohgiya Y, Suyama J, Sai S, Kawahara M, Takeyama N, Ohike N, Sasamori H, Munechika J, Saiki M, Onoda Y, Hirose M, Gokan T (2014) Preoperative T staging of urinary bladder cancer: efficacy of stalk detection and diagnostic performance of diffusion-weighted imaging at 3 T. Magn Reson Med Sci 13:175–181. https://doi.org/10.2463/mrms.2013-0104
Zhai N, Wang YH, Zhu LM, Wang JH, Sun XH, Hu XB, Li X, Yu T, Wang XL, Meng N, Yan QC, Li XJ, Luo YH (2015) Sensitivity and specificity of diffusion-weighted magnetic resonance imaging in diagnosis of bladder cancers. Clin Invest Med 38:E173–E184
Nakamura Y, Yoshida S, Tanaka H, Inoue M, Ito M, Kijima T, Yokoyama M, Ishioka J, Matsuoka Y, Saito K, Fujii Y, Kihara K (2017) Potential utility of diffusion-weighted magnetic resonance imaging in diagnosis of residual bladder cancer before second transurethral resection. Urol Int 98:298–303. https://doi.org/10.1159/000456722
Wang HJ, Pui MH, Guo Y, Yang D, Pan BT, Zhou XH (2014) Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging 39:135–141. https://doi.org/10.1007/s00261-013-0038-0
Thoeny HC, Froehlich JM, Triantafyllou M, Huesler J, Bains LJ, Vermathen P, Fleischmann A, Studer UE (2014) Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted mr imaging. Radiology 273:125–135. https://doi.org/10.1148/radiol.14132921
Sevcenco S, Ponhold L, Heinz-Peer G, Fajkovic H, Haitel A, Susani M, Shariat SF, Szarvas T, Baltzer PA (2014) Prospective evaluation of diffusion-weighted MRI of the bladder as a biomarker for prediction of bladder cancer aggressiveness. Urol Oncol 32:1166–1171. https://doi.org/10.1016/j.urolonc.2014.04.019
Lin WC, Chen JH (2015) Pitfalls and limitations of diffusion-weighted magnetic resonance imaging in the diagnosis of urinary bladder cancer. Transl Oncol 8:217–230. https://doi.org/10.1016/j.tranon.2015.04.003
Nguyen HT, Jia G, Shah ZK, Pohar K, Mortazavi A, Zynger DL, Wei L, Yang X, Clark D, Knopp MV (2015) Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging 41:1374–1382. https://doi.org/10.1002/jmri.24663
Chakiba C, Cornelis F, Descat E, Gross-Goupil M, Sargos P, Roubaud G, Houédé N (2015) Dynamic contrast enhanced MRI-derived parameters are potential biomarkers of therapeutic response in bladder carcinoma. Eur J Radiol 84:1023–1028. https://doi.org/10.1016/j.ejrad.2015.02.026
de Haas RJ, Steyvers MJ, Fütterer JJ (2014) Multiparametric MRI of the bladder: ready for clinical routine? Am J Roentgenol 202:1187–1195. https://doi.org/10.2214/AJR.13.12294
van der Pol CB, Der Van, Shinagare AB, Tirumani SH, Preston MA, Vangel MG, Silverman SG (2018) Bladder cancer local staging: multiparametric MRI performance following transurethral resection. Abdom Radiol. https://doi.org/10.1007/s00261-017-1449-0
Lakhani A, Khan SR, Bharwani N, Stewart V, Rockall AG, Khan S, Barwick TD (2017) FDG PET/CT pitfalls in gynecologic and genitourinary oncologic imaging. Radiographics 37:577–594. https://doi.org/10.1148/rg.2017160059
Chakraborty D, Mittal BR, Kashyap R, Mete UK, Narang V, Das A, Bhattacharya A, Khandelwal N, Mandal AK (2014) Role of fluorodeoxyglucose positron emission tomography/computed tomography in diagnostic evaluation of carcinoma urinary bladder: comparison with computed tomography. World J Nucl Med 13:34–39. https://doi.org/10.4103/1450-1147.138572
Aljabery F, Lindblom G, Skoog S, Shabo I, Olsson H, Rosell J, Jahnson S (2015) PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urol 15:87. https://doi.org/10.1186/s12894-015-0080-z
Kollberg P, Almquist H, Bläckberg M, Cwikiel M, Gudjonsson S, Lyttkens K, Patschan O, Liedberg F (2017) [18 F]Fluorodeoxyglucose-positron emission tomography/computed tomography response evaluation can predict histological response at surgery after induction chemotherapy for oligometastatic bladder cancer. Scand J Urol 51:308–313. https://doi.org/10.1080/21681805.2017.1321579
Kollberg P, Almquist H, Bläckberg M, Cronberg C, Garpered S, Gudjonsson S, Kleist J, Lyttkens K, Patschan O, Liedberg F (2015) [(18F)]Fluorodeoxyglucose-positron emission tomography/computed tomography improves staging in patients with high-risk muscle-invasive bladder cancer scheduled for radical cystectomy. Scand J Urol 49:296–301. https://doi.org/10.3109/21681805.2014.990053
Mertens LS, Mir MC, Scott AM, Lee ST, Fioole-Bruining A, Vegt E, Vogel WV, Manecksha R, Bolton D, Davis ID, Horenblas S, van Rhijn BW, Lawrentschuk N (2014) 18F-fluorodeoxyglucose–positron emission tomography/computed tomography aids staging and predicts mortality in patients with muscle-invasive bladder cancer. Urology 83:393–398. https://doi.org/10.1016/j.urology.2013.10.032
Sharma A, Mete UK, Sood A, Kakkar N, Gorla AK, Mittal BR (2017) Utility of early dynamic and delayed post-diuretic 18F-FDG PET/CT SUV max in predicting tumour grade and T-stage of urinary bladder carcinoma: results from a prospective single centre study. BJR 90:20160787. https://doi.org/10.1259/bjr.20160787
Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim CS (2015) FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol 22:3150–3156. https://doi.org/10.1245/s10434-015-4369-7
Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, Evison F, Dasgupta P, O’Brien T, Khan MS (2014) Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int 114:389–395. https://doi.org/10.1111/bju.12608
Graziani T, Ceci F, Lopes FL, Chichero J, Castellucci P, Schiavina R, Bianchi L, Chondrogiannis S, Colletti PM, Costa S, Rubello D, Fanti S (2015) 11C-choline PET/CT for restaging of bladder cancer. Clin Nucl Med 40:e1–e5. https://doi.org/10.1097/RLU.0000000000000573
Maurer T, Horn T, Souvatzoglou M, Eiber M, Beer AJ, Heck MM, Haller B, Gschwend JE, Schwaiger M, Treiber U, Krause BJ (2014) Prognostic value of 11C-choline PET/CT and CT for predicting survival of bladder cancer patients treated with radical cystectomy. Urol Int 93:207–213. https://doi.org/10.1159/000357686
Brunocilla E, Ceci F, Schiavina R, Castellucci P, Maffione AM, Cevenini M, Bianchi L, Borghesi M, Giunchi F, Fiorentino M, Chondrogiannis S, Colletti PM, Rubello D, Fanti S, Martorana G (2014) Diagnostic accuracy of (11)C-choline PET/CT in preoperative lymph node staging of bladder cancer: a systematic comparison with contrast-enhanced CT and histologic findings. Clin Nucl Med 39:e308–e312. https://doi.org/10.1097/RLU.0000000000000342
Ceci F, Bianchi L, Graziani T, Castellucci P, Pultrone C, Eugenio B, Martorana G, Colletti PM, Rubello D, Fanti S, Schiavina R (2015) 11C-choline PET/CT and bladder cancer: lymph node metastasis assessment with pathological specimens as reference standard. Clin Nucl Med 40:e124–e128. https://doi.org/10.1097/RLU.0000000000000604
Rosenkrantz AB, Friedman KP, Ponzo F, Raad RA, Jackson K, Huang WC, Balar AV (2017) Prospective pilot study to evaluate the incremental value of PET information in patients with bladder cancer undergoing 18F-FDG simultaneous PET/MRI. Clin Nucl Med 42:e8–e15
Partovi S, Robbin MR, Steinbach OC, Kohan A, Rubbert C, Vercher-Conejero JL, Kolthammer JA, Faulhaber P, Paspulati RM, Ros PR (2014) Initial experience of MR/PET in a clinical cancer center. J Magn Reson Imaging 39:768–780. https://doi.org/10.1002/jmri.24334
Rosenkrantz AB, Friedman K, Chandarana H, Melsaether A, Moy L, Ding YS, Jhaveri K, Beltran L, Jain R (2016) Current status of hybrid PET/MRI in oncologic imaging. Am J Roentgenol 206:162–172. https://doi.org/10.2214/AJR.15.14968
Rosenkrantz AB, Balar AV, Huang WC, Jackson K, Friedman KP (2015) Comparison of coregistration accuracy of pelvic structures between sequential and simultaneous imaging during Hybrid Pet/MRI in patients with bladder cancer. Clin Nucl Med 40:637–641. https://doi.org/10.1097/RLU.0000000000000772
Civelek AC, Apolo A, Agarwal P, Evers R, Bluemke D, Malayeri A (2016) 18F-FDG PET-MRI in the management of muscle invasive bladder cancer: challenges in imaging and solutions. J Nucl Med 57:1292
Jadvar H, Desai B, Conti PS (2015) Sodium 18F-fluoride PET/CT of bone, joint and other disorders. Semin Nucl Med 45:58–65. https://doi.org/10.1053/j.semnuclmed.2014.07.008
Mick CG, James T, Hill JD, Williams P, Perry M (2014) Molecular imaging in oncology: 18 F-sodium fluoride pet imaging of osseous metastatic disease. Am J Roentgenol 203:263–271. https://doi.org/10.2214/AJR.13.12158
McInnes MD, Siemens DR, Mackillop WJ, Peng Y, Wei S, Schieda N, Booth CM (2016) Utilisation of preoperative imaging for muscle-invasive bladder cancer: a population-based study. BJU Int 117:430–438. https://doi.org/10.1111/bju.13034
Acknowledgements
Dr. Thakur (PI), thanks NIH/NCI RO1CA157372, Deans Translational Science Award and the Sidney Kimmel Cancer Center Research award for their support in part. Dr. Ebru Salmanoglu is the visiting scholar to the Thakur laboratories and thanks The Scientific and Technological Research Council of Turkey (TÜBİTAK) for their fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Ebru Salmanoglu, Ethan Halpern, Edouard J. Trabulsi, Sung Kim, and Mathew L. Thakur have no of conflicts of interest.
Rights and permissions
About this article
Cite this article
Salmanoglu, E., Halpern, E., Trabulsi, E.J. et al. A glance at imaging bladder cancer. Clin Transl Imaging 6, 257–269 (2018). https://doi.org/10.1007/s40336-018-0284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-018-0284-9